Transplantation of donor bone marrow or umbilical cord blood partially corrected the collagen deficiency in five of seven children with recessive dystrophic epidermolysis bullosa who underwent the experimental therapy, greatly ameliorating their severe symptoms by improving their skin and mucosal integrity, according to a recent report in the Aug. 12 issue of the New England Journal of Medicine.
In the phase II clinical trial, six of the patients were alive at 130-799 days after the procedure; their rates of recovery and ultimate outcomes varied. Two showed rapid and dramatic clinical improvement in wound healing and mucocutaneous blistering, two showed marked improvement, and one showed slow, modest improvement. Another patient had a recurrence of blistering after 2 months of substantial improvement, said Dr. John E. Wagner of the University of Minnesota Health Center, Minneapolis, and his associates.
One of the patients who responded to the transplantation died from opportunistic infections on day 183. And one of the patients died before bone marrow infusion could be performed, from complications of the conditioning immunomyeloablative chemotherapy.
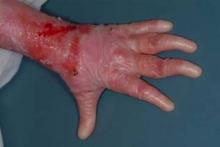
Epidermolysis bullosa (EB) refers to a group of inherited skin diseases characterized by painful erosions and blisters on skin and mucosal membranes, induced by mild trauma. Recessive dystrophic EB is one of the most severe forms of the disease, present at birth and often eventually resulting in esophageal strictures, mutilating scars, local and systemic infections, joint contractures, fusion of fingers and toes, and aggressive squamous-cell carcinomas. Median survival is only 30 years.
EB is caused by loss-of-function mutations in the gene that encodes for collagen type VII (C7). The mutations cause a severe decrease in the expression of C7, "a collagen localized at the dermal-epidermal junction" that contributes to the formation of "anchoring fibrils that tether the epidermal basement membrane to the dermal matrix," noted the investigators. When C7 is not expressed properly, these fibrils fail to form properly, "and epidermal-dermal adherence is lost beneath the lamina densa of the basement membrane," Dr. Wagner and his colleagues explained.
"To date, the care of patients with recessive dystrophic EB has been palliative and restricted to the treatment of individual wounds," they added.
The investigators explored whether bone marrow or cord blood transplantation might correct the collagen mutations systemically in a murine model. When that proved successful, they undertook the clinical trial in the seven pediatric patients (aged 15 months to 14.5 years). All had extensive cutaneous disease and four had severe mucosal disease requiring esophageal dilation and placement of a gastrostomy tube for nutritional support. Five patients had severe mitten deformities, four required wheelchairs, two had renal impairment, and four had severe iron-deficiency anemia.
One patient died before transplantation from hemorrhagic cardiomyopathy "that was probably due to cyclophosphamide cardiotoxicity," and a second had to delay transplantation until different complications from the conditioning chemotherapy resolved. At transplantation, five patients received unfiltered marrow stem cells from a human leukocyte antigen – identical but healthy sibling, and one of these five also received umbilical cord blood from the same donor. A sixth patient received umbilical cord blood from an unrelated donor.
All six patients showed improved wound healing and decreased mucocutaneous blistering within the first 100 days, with three showing marked improvement within 30 days. The percentage of affected body surface area was reduced significantly in three patients, as assessed by clinician and parent reports and by documented reductions in the need for bandages.
Four patients were able to discontinue all immunosuppressive therapy and the fifth has tolerated tapering of cyclosporine, according to the investigators.
Skin biopsy specimens showed increases in C7 immunoreactivity at the dermal-epidermal junction after transplantation in all six patients. Testing with an anti-C7 antibody showed an increase in C7 expression over time in five of the six.
At baseline, electron micrographs had shown "a complete absence of mature anchoring fibrils." After transplantation, five of the six patients showed "scanty, wispy structures under the lamina densa," which could represent rudimentary anchoring fibrils or fragmented elastic fibrils. More study is needed to further characterize these structures. None of the micrographs showed the morphologic hallmarks of normal anchoring fibrils, the investigators reported (N. Engl. J. Med. 2010;363:629-39).
"Unexpectedly, we detected substantial proportions of donor cells in the skin and mucosa after treatment; these proportions varied over time and with the location of the biopsy site," they wrote.
Although the precise identity and function of these donor cells has yet to be determined, "we favor the possibility that these healthy donor cells residing in the skin secrete C7 and that the secreted C7 is subsequently incorporated into the lamina densa at the dermal-epidermal junction," Dr. Wagner and his colleagues noted,