To the Editor:
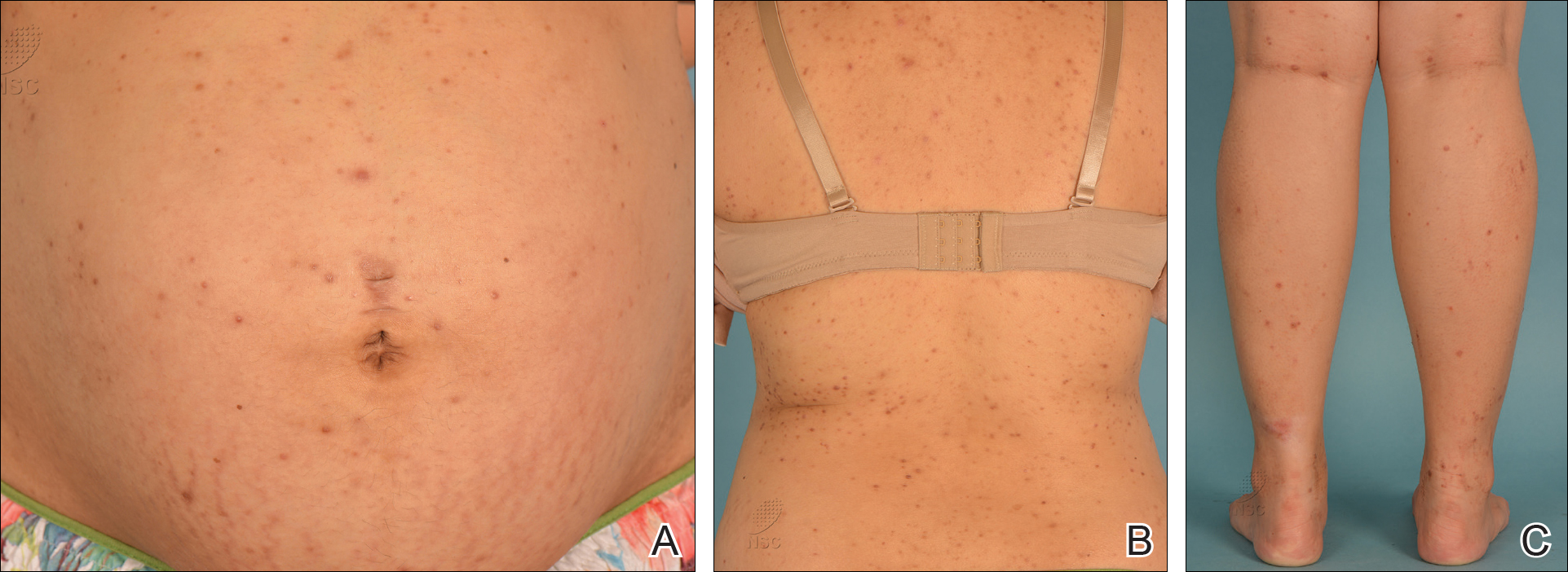
A 28-year-old primigravid woman at 32 weeks’ gestation presented to an outpatient dermatology clinic with a generalized rash and itch of 3 months’ duration. She was distressed with the itch and had tried antihistamines (eg, chlorpheniramine, cetirizine) without relief. She had no notable medical history. Physical examination revealed generalized erythematous papules and nodules on the chest, back, periumbilical region, arms, and legs (Figure). A few pustules were noted on the upper back. No wheals, plaques, vesicles, or bullae were seen.
Laboratory investigations revealed elevated alkaline phosphatase (187 U/L [reference range, 30–120 U/L]), aspartate aminotransferase (45 U/L [reference range, 10–30 U/L]), alanine aminotransferase (120 U/L [reference range, 10–40 U/L]), and γ-glutamyltransferase (48 U/L [reference range, 9–40 U/L]) levels. A fungal scrape of the papules on the upper back demonstrated spores. Subsequent tests included ultrasonography of the liver, which showed fatty changes, as well as rising levels of alkaline phosphatase. Fasting glucose and 2-hour oral glucose tolerance tests showed poorly controlled gestational diabetes mellitus (DM) as well as raised triglycerides.
Based on the patient’s reports of itch, signs of erythematous papules and nodules, and laboratory results of cholestasis, a diagnosis of intrahepatic cholestasis of pregnancy (ICP) was made. The finding of Pityrosporum folliculitis also prompted screening for gestational DM, which was positive.
Treatment with ursodeoxycholic acid (UDCA) 250 mg twice daily was prescribed, which led to some relief of the skin symptoms. Her cutaneous symptoms were discussed with her obstetrician, and a decision was made for emergency cesarean delivery at 37 weeks’ gestation in light of nonreassuring fetal status during her follow-up antenatal ultrasonograph. Her pruritus and poor liver function resolved within 2 weeks after delivery.
Intrahepatic cholestasis of pregnancy is a rare form of reversible cholestasis occurring in the second half of pregnancy. The incidence varies with geographical location and ethnicity.1 It is one of the specific dermatoses of pregnancy and usually presents in the third trimester. It is characterized by pruritus, elevation of serum total bile acids and mild elevations of other liver function tests, and increased rates of adverse fetal outcomes. A positive diagnosis is made by the elevation of the serum total bile acid levels (>11.0 μmol/L [reference range, 0.73–5.63 μmol/L]). It is important for clinicians to recognize ICP because it is associated with fetal prematurity, intrapartal fetal distress, and stillbirths.2
The pathogenesis of ICP is not fully understood. During pregnancy, estrogens interfere with bile acid secretion, and progestins inhibit hepatic glucuronyltransferase. Increased IFN-γ, natural killer cells, and natural killer T cells, as well as decreased T cells in decidua parietalis, also have been reported.3
Mutations in the ATP binding cassette subfamily B member 4 gene, ABCB4, which encodes the multidrug resistance protein 3, a canalicular phosphatidylcholine translocase, have been found in several women with ICP.4 Clinically, patients usually present with pruritus that may precede or follow laboratory abnormalities. The pruritus worsens as the pregnancy advances and can resolve within 48 hours of delivery. Pruritus usually affects the palms and soles but may extend to the legs and abdomen or become generalized.4
Generally, there are no cutaneous signs other than excoriation marks, contrary to primary skin lesions found in other specific dermatoses of pregnancy. Mild jaundice can develop 2 to 4 weeks after the onset of pruritus, which may be associated with subclinical steatorrhea and increased risk of intrapartum and postpartum hemorrhage.5 Of note, ICP may be associated with increased risk for gestational DM, as illustrated in our case.6
Ursodeoxycholic acid currently is the most effective pharmacologic treatment of ICP. It reduces bile acids in cord blood, colostrum, and amniotic fluid.7 A meta-analysis of randomized controlled trials demonstrated that UDCA (450–1200 mg daily) is highly effective in alleviating pruritus and normalizing laboratory abnormalities associated with ICP.8 No severe adverse events have been reported related to UDCA.9,10
Intrahepatic cholestasis of pregnancy has been associated with increased risk for preterm delivery (19%–60%), meconium staining of amniotic fluid (≤27%), fetal bradycardia (≤14%), fetal distress (22%–41%), and fetal loss (0.4%–4.1%).11 The risk for serious fetal complications in ICP makes intensive fetal surveillance mandatory, including weekly nonstress cardiotocography or biophysical assessment from 34 weeks’ gestation. Delivery at 36 weeks or earlier (if lung maturity is achieved and cervix favorable) should be considered for severe cases with jaundice, progressive elevations in serum total bile acids, and suspected fetal distress. At more than 36 weeks’ gestation, amniocentesis and delivery should be considered if cervix is favorable and fetal lung maturity satisfactory.12-14
Our case highlights the importance of diagnosing ICP when a pregnant patient presents with generalized itch associated with elevated liver function tests. Interdisciplinary management involving dermatologists, obstetricians, pediatricians, and gastroenterologists is mandatory to acquire a better outcome for the mother and the fetus.