Patients with cutaneous T-cell lymphoma (CTCL) often are diagnosed with atopic dermatitis (AD) or psoriasis before receiving their CTCL diagnosis. The effects of new biologic therapies for AD such as dupilumab, an IL-4/IL-13 antagonist, on CTCL are unknown. Dupilumab may be beneficial in CTCL given that helper T cell (TH2) cytokines are increased in advanced CTCL.1 We present a patient with definitive CTCL and concomitant AD who was safely treated with dupilumab and experienced improvement in both CTCL and AD.
Case Report
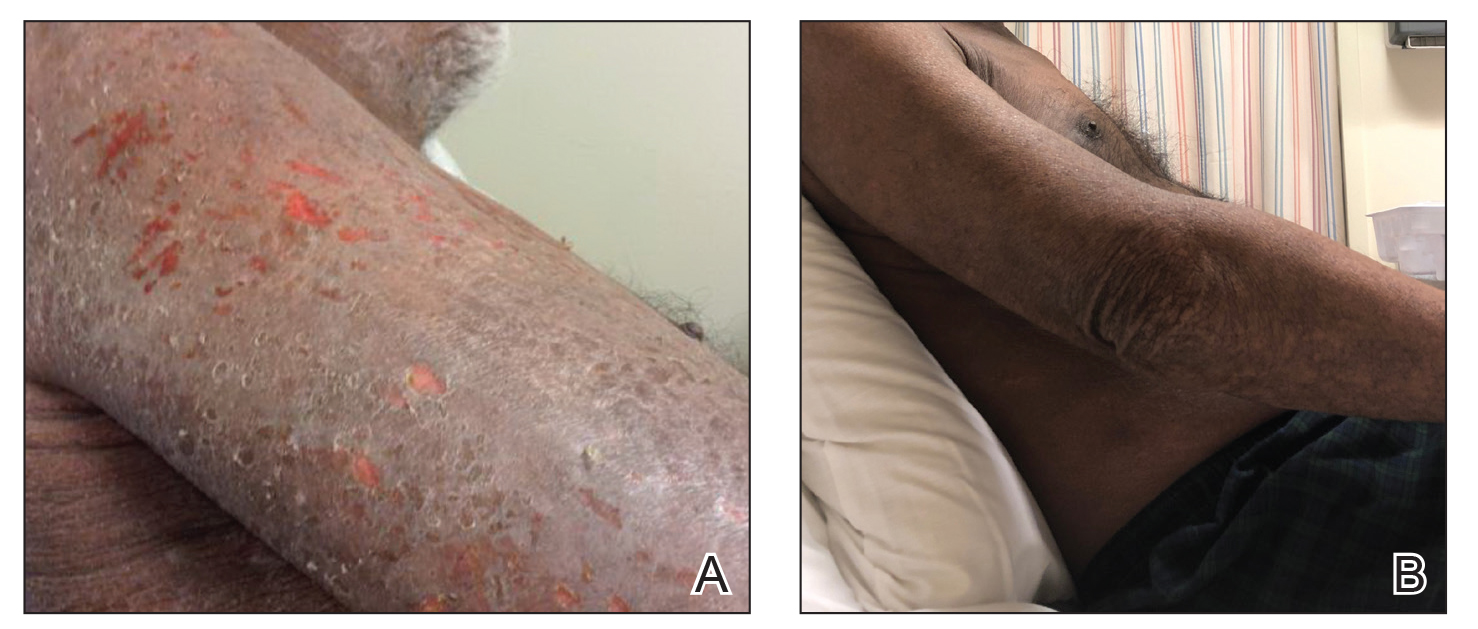
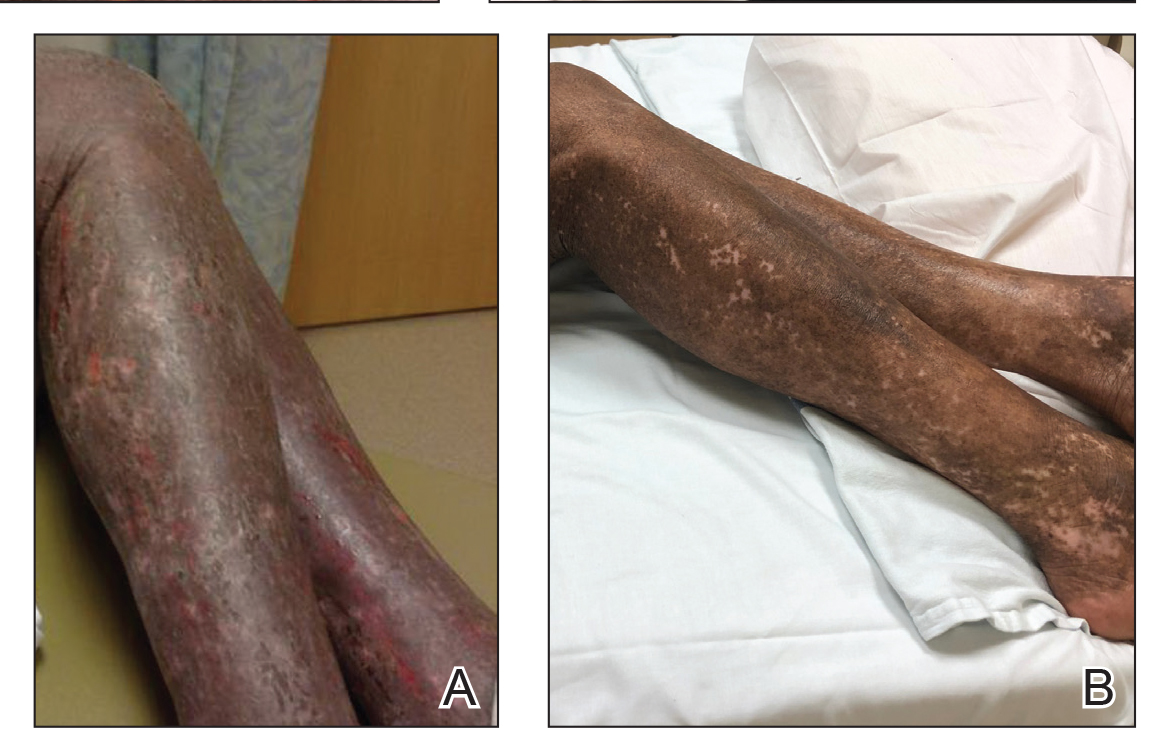
A 68-year-old man presented with increased itching from AD and a new rash on the arms, neck, chest, back, and lower extremities (Figures 1A and 2A). He had a medical history of AD and CTCL diagnosed by biopsy and peripheral blood flow cytometry (stage IVA1 [T4N0M0B2]) that was being treated with comprehensive multimodality therapy consisting of bexarotene 375 mg daily, interferon alfa-2b 3 mIU 3 times weekly, interferon gamma-1b 2 mIU 3 times weekly, total skin electron beam therapy followed by narrowband UVB twice weekly, and extracorporeal photopheresis every 4 weeks, which resulted in a partial clinical response for 6 months. A biopsy performed at the current presentation showed focal spongiosis and features of lichen simplex chronicus with no evidence suggestive of CTCL. Peripheral blood flow cytometry showed stable B1-staged disease burden (CD4/CD8, 2.6:1); CD4+/CD7−, 12% [91/µL]; CD4+/CD26−, 21% [155/µL]). Treatment with potent and superpotent topical steroids was attempted for more than 6 months and was unsuccessful in relieving the symptoms.
Given the recalcitrant nature of the patient’s rash and itching, dupilumab was added to his CTCL regimen. Prior to initiating dupilumab, the patient reported a numeric rating scale itch intensity of 7 out of 10. After 4 weeks of treatment with dupilumab, the patient reported a numeric rating scale itch intensity of 1. Over a 3-month period, the patient’s rash improved dramatically (Figures 1B and 2B), making it possible to decrease CTCL treatments—bexarotene decreased to 300 mg, interferon alfa-2b to 3 mIU twice weekly, interferon gamma-1b to 2 mIU twice weekly, extracorporeal photopheresis every 5 weeks, and narrowband UVB was discontinued completely. A comparison of the patient’s flow cytometry analysis from before treatment to 3 months after dupilumab showed an overall slight reduction in CTCL B1 blood involvement and normalization of the patient’s absolute eosinophil count and serum lactate dehydrogenase level. The patient tolerated the treatment well without any adverse events and has maintained clinical response for 6 months.
Comment
Cutaneous T-cell lymphomas represent a heterogeneous group of T-cell lymphoproliferative disorders involving the skin.2 The definitive diagnosis of CTCL is challenging, as the clinical and pathologic features often are nonspecific in early disease. Frequently, undiagnosed patients are treated empirically with immunosuppressive agents. Tumor necrosis factor inhibitors and cyclosporine are both associated with progression or worsening of undiagnosed CTCL.3,4 Dupilumab was the first US Food and Drug Administration–approved biologic for the treatment of moderate to severe AD. Cutaneous T-cell lymphoma has immunologic features, such as TH2 skewing, that overlap with AD; however, the effects of dupilumab in CTCL are not yet known.5,6 Our group has seen patients initially thought to have AD who received dupilumab without improvement and were subsequently diagnosed with CTCL, suggesting dupilumab did not affect CTCL tumor cells. Given these findings, there was concern that dupilumab might exacerbate undiagnosed CTCL. Our patient with definitive, severe, refractory CTCL noted marked improvement in both AD and underlying CTCL with the addition of dupilumab. No other treatments were added. The response was so dramatic that we were able to wean the doses and frequencies of several CTCL treatments. Our findings suggest that dupilumab may be beneficial in a certain subset of CTCL patients with a history of AD or known concomitant AD. Prospective studies are needed to fully investigate dupilumab safety and efficacy in CTCL and whether it has any primary effects on tumor burden in addition to benefit for itch and skin symptom relief.