SAN DIEGO – When the Food and Drug Administration gave the nod to a first-in-class tissue removal device in July of 2021, clearance was limited to the treatment of moderate to severe wrinkles in the mid to lower face.
Jill S. Waibel, MD, a dermatologist with the Miami Dermatology and Laser Institute, predicted at the annual conference of the American Society for Laser Medicine and Surgery. “I’m using it in my practice more for laxity and jowls,” she said. Eventually, “I think it’s going to be preventative for 30- to 50-year-olds but that hasn’t been studied. I think it’s going to have a pre- and postrhytidectomy role, so I think the plastic surgeons are going to love this in their practice.”
Developed by Cytrellis, and based on research conducted by William G. Austen Jr., MD, chief of plastic and reconstructive surgery at Massachusetts General Hospital (MGH), Boston, and R. Rox Anderson, MD, director of the Wellman Center for Photomedicine at MGH, the company’s scientific founders, the device uses hollow needles contained in a handpiece to create thousands of microexcisions to physically remove small cores of skin – a process known as microcoring. Dr. Austin and Dr. Anderson were the senior authors, respectively, of seminal trials of the device in swine, published in 2013 and 2015.
This can result in immediate physical hole closures (mechanical closure), which may lead to skin tightening.
“We’re removing dermis and epidermis,” said Dr. Waibel, who noted that the technology has been studied mostly for skin laxity and rhytids. “There are no other devices that are doing this.”
The immediate closure of tiny holes in the skin results in a quantitative and directional reduction in the treated area of skin, she said, which leads to wrinkle improvement, tightening, and smoothing of lax skin. The device contains three 22-guage needles that are less than 500 micrometers in diameter. “Based on optical coherence tomography work we did, these channels of treated skin stay open for about 1.5 minutes,” she added, noting that the tunable depth of the device ranges from 0 to 4 mm. “I tend to treat only with the 4-mm depth,” said Dr. Waibel, who is subsection chief of dermatology at Baptist Hospital of Miami.
The device features a disposable tip that can remove up to 24,000 cores of skin, and the amount of skin removed in a treated area can be 1%, 3%, 5%, 7%, and 8%. “The more cores you do, the more wrinkle improvement we saw in pivotal trials,” she said. “A minimal core count of 12,000 per treatment is recommended for the mid and lower face. Interestingly, higher core counts do not result in more patient downtime.” In her office, she said that the treatment takes about 20 minutes. Recommended postoperative care involves application of petrolatum or Aquaphor over the treated area for 24 hours, or until the holes have closed. “There is very low postoperative downtime,” she noted.
According to pivotal clinical data from Cytrellis on 51 patients treated with Ellacor, patients experienced a mean 1.3-grade improvement on the Lemperle Rating Scale, 86% said that they were satisfied with the procedure, and investigators rated their Global Aesthetic Improvement as 90%.
To date, Dr. Waibel and her colleagues in Miami have treated 102 patients with Ellacor, mostly for wrinkles and skin laxity. In these patients, the minimal downtime experienced was 3.8 days, 75% of patients did not miss any work, and 46% did not miss any social activities. The worst part for patients is the preprocedure numbing, she said. “We do lidocaine injections. Some people do nerve blocks. Once you do the lidocaine injection, the average pain is about 0.36 on a scale of 1-10 during treatment and 0 for all subsequent time points.”
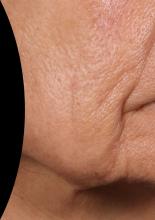
At the meeting, she presented a set of before and after photos that showed improvement of moderate facial wrinkles in a female patient 90 days after one treatment with Ellacor, which removed about 5% of skin in the area of the jowls. “It’s pretty incredible,” Dr. Waibel remarked.
“I don’t have anything in my practice that can help with that kind of laxity other than sending them to a plastic surgeon, and I have about 80 devices.”
At the 2019 meeting of the American Society for Dermatologic Surgery, Dr. Waibel and Roy G. Geronemus, MD, director of the Laser & Skin Surgery Center of New York, presented a small pilot study on the successful use of Ellacor for acne scars and striae. Dr. Waibel said that she and her colleagues in Miami have been using the device to treat skin laxity in several anatomical areas, including the neck, nose, inner thigh, above the knee, elbow, and the axillary region. They have used the device to treat tattoos, rhytidectomy scars, abdominal striae, acne and surgical scars, and idiopathic guttate hypomelanosis, she added.
“We do a lot of combinations with other devices on the same day, and I think this list will increase over the next few years,” she said. “Probably my favorite use in the past 5 months has been doing microcoring and, separated by a month, doing resurfacing.”
Clinical trials of Ellacor were conducted in patients with types I-IV skin, but she has treated several patients with types V-VI skin “with absolutely no safety issues,” which includes treatment of epidermal nevi.
Which variables are the most important for patient selection and procedural success remain unclear, she continued, including patient age, elastic recoil, body mass index, history of a prior procedure (such as radiofrequency or ultrasound), the amount of laxity and rhytids, and overall health, which have not been studied, Dr. Waibel said.
“We have patients that don’t have the same response as others. For the modest improvement seen in some patients, is that their elastic recoil or are we choosing the wrong patients? Do they need more treatments? We are also still learning about the ideal treatment for scars and other indications.”
The device is expected to launch in fourth quarter of 2022.
Dr. Waibel disclosed that she is an advisory board member for Cytrellis. She has conducted clinical trials for and is a consultant to many pharmaceutical and device companies.