Isotretinoin is the most effective treatment of recalcitrant acne, but because of its teratogenicity and potential association with psychiatric adverse effects, it has been heavily regulated by the US Food and Drug Administration (FDA) through the iPLEDGE program since 2006.1,2 To manage the risk of teratogenicity associated with isotretinoin, various pregnancy prevention programs have been developed, but none of these programs have demonstrated a zero fetal exposure rate. The FDA reported 122 isotretinoin-exposed pregnancies during the first year iPLEDGE was implemented, which was a slight increase from the 120 pregnancies reported the year after the implementation of the System to Manage Accutane-Related Teratogenicity program, iPLEDGE’s predecessor.3 The iPLEDGE program requires registration of all wholesalers distributing isotretinoin, all health care providers prescribing isotretinoin, all pharmacies dispensing isotretinoin, and all female and male patients prescribed isotretinoin to create a verifiable link that only enables patients who have met all criteria to pick up their prescriptions. For patients of reproductive potential, there are additional qualification criteria and monthly requirements; before receiving their prescription every month, patients of reproductive potential must undergo a urine or serum pregnancy test with negative results, and patients must be counseled by prescribers regarding the risks of the drug and verify they are using 2 methods of contraception (or practicing abstinence) each month before completing online questions that test their understanding of the drug’s side effects and their chosen methods of contraception.4 These requirements place burdens on both patients and prescribers. Studies have shown that in the 2 years after the implementation of iPLEDGE, there was a 29% decrease in isotretinoin prescriptions.1-3
We conducted a survey study to see if clinicians chose not to prescribe isotretinoin to appropriate candidates specifically because of the administrative burden of iPLEDGE. Secondarily, we investigated the medications these clinicians would prescribe instead of isotretinoin.
Methods
In March 2020, we administered an anonymous online survey consisting of 12 multiple-choice questions to verified board-certified dermatologists in the United States using a social media group. The University of Rochester’s (Rochester, New York) institutional review board determined that our protocol met criteria for exemption (IRB STUDY00004693).
Statistical Analysis—Primary analyses used Pearson χ2 tests to identify significant differences among respondent groups, practice settings, age of respondents, and time spent registering patients for iPLEDGE.
Results
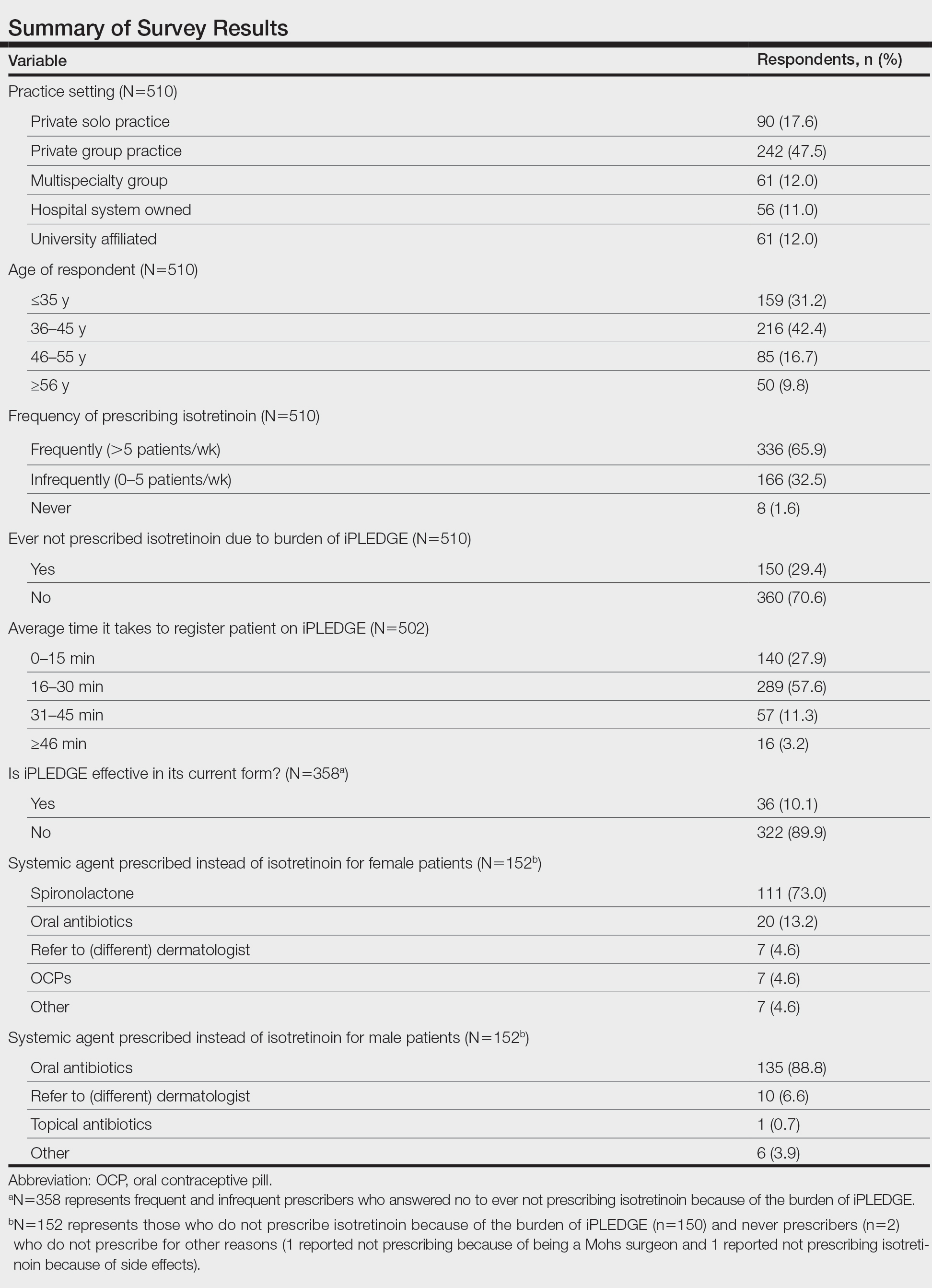
Survey results from 510 respondents are summarized in the Table.
Burden of iPLEDGE—Of the respondents, 336 (65.9%) were frequent prescribers of isotretinoin, 166 (32.5%) were infrequent prescribers, and 8 (1.6%) were never prescribers. Significantly more isotretinoin prescribers estimated that their offices spend 16 to 30 minutes registering a new isotretinoin patient with the iPLEDGE program (289 [57.6%]) compared with 0 to 15 minutes (140 [27.9%]), 31 to 45 minutes (57 [11.3%]), and morethan 45 minutes (16 [3.2%])(χ23=22.09, P<.0001). Furthermore, 150 dermatologists reported sometimes not prescribing, and 2 reported never prescribing isotretinoin because of the burden of iPLEDGE.
Systemic Agents Prescribed Instead of Isotretinoin—Of the respondents, 73.0% (n=111) prescribed spironolactone to female patients and 88.8% (n=135) prescribed oral antibiotics to male patients instead of isotretinoin. Spironolactone typically is not prescribed to male patients with acne because of its feminizing side effects, such as gynecomastia.5 According to the American Academy of Dermatology guidelines on acne, systemic antibiotic usage should be limited to the shortest possible duration (ie, less than 3–4 months) because of potential bacterial resistance and reported associations with inflammatory bowel disease, Clostridium difficile infection, and candidiasis.6,7