To the Editor:
Granulomatous dermatitis (GD) has been described as a rare side effect of MEK and BRAF inhibitor use in the treatment of BRAF V600E mutation–positive metastatic melanoma. As the utilization of BRAF and MEK inhibitors increases for the treatment of a variety of cancers, it is essential that clinicians and pathologists recognize GD as a potential cutaneous manifestation. We present the case of a 52-year-old woman who developed GD while being treated with vemurafenib and cobimetinib for BRAF V600E mutation–positive metastatic cholangiocarcinoma.
A 52-year-old White woman presented with faint patches of nonpalpable violaceous mottling that extended distally to proximally from the ankles to the thighs on the medial aspects of both legs. She was diagnosed with cholangiocarcinoma 10 months prior, with metastases to the lung, liver, and sternum. She underwent treatment with gemcitabine and cisplatin therapy. Computed tomography after several treatment cycles revealed progressive disease with multiple pulmonary nodules as well as metastatic intrathoracic and abdominal adenopathy. Treatment with gemcitabine and cisplatin failed to produce a favorable response and was discontinued after 6 treatment cycles.
Genomic testing performed at the time of diagnosis revealed a positive mutation for BRAF V600E. The patient subsequently enrolled in a clinical trial and started treatment with the BRAF inhibitor vemurafenib and the MEK inhibitor cobimetinib. She developed sun sensitivity and multiple sunburns after starting these therapies. The patient tolerated the next few cycles of therapy well with only moderate concerns of dry sensitive skin.
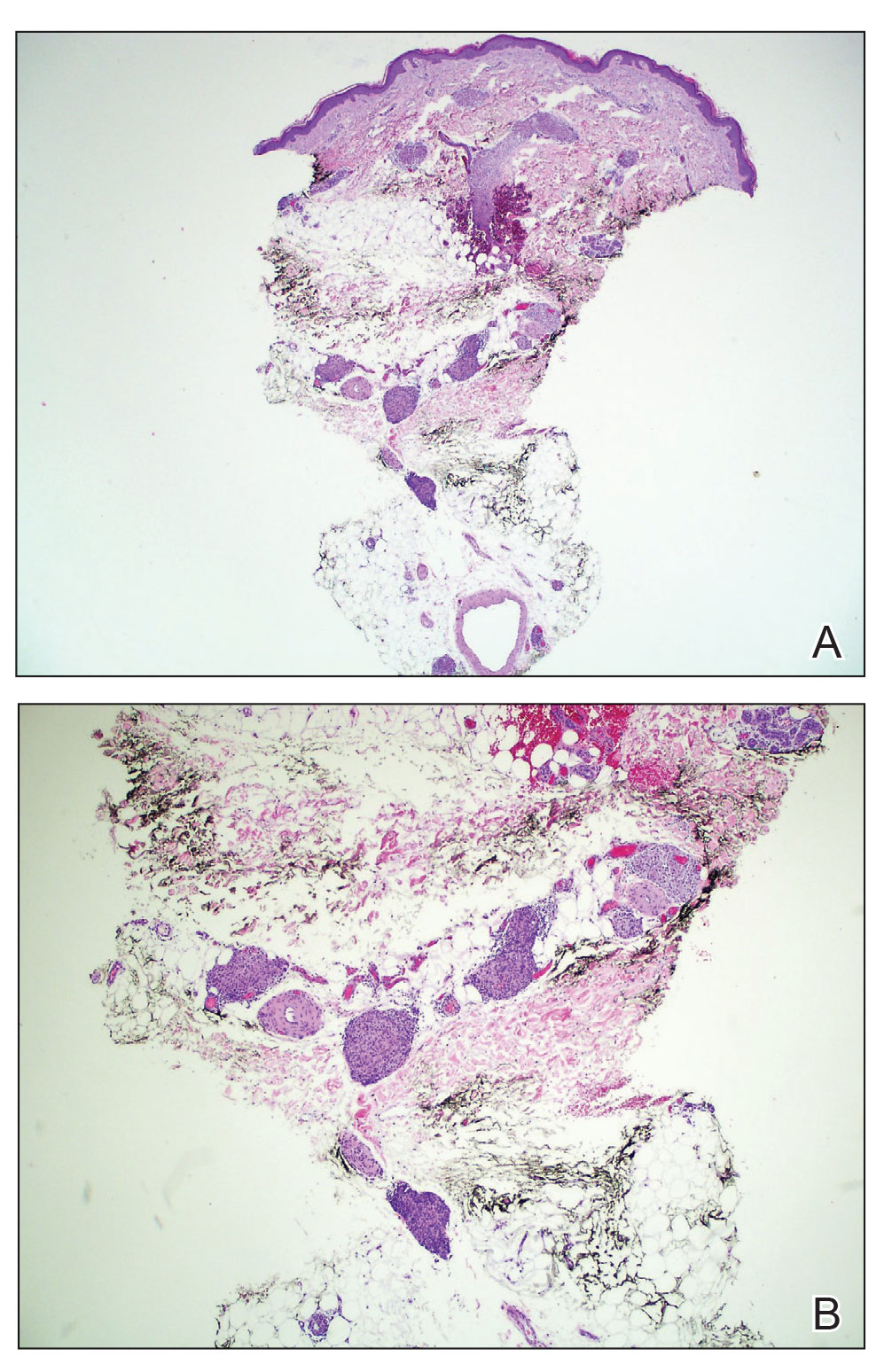
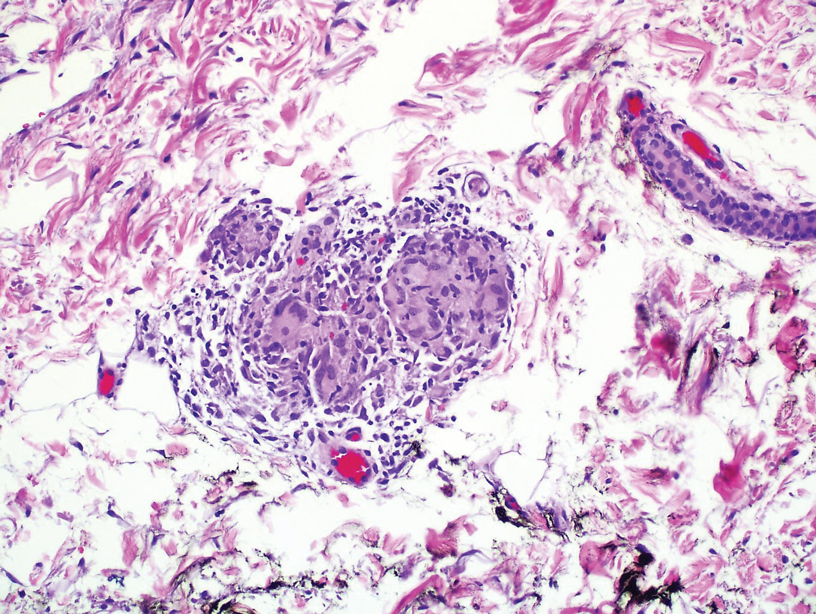
During the sixth cycle of therapy, she presented to dermatology after developing a rash. Over the next 2 weeks, similar lesions appeared on the arms. The patient denied the use of any new lotions, soaps, or other medications. Punch biopsies of the right forearm and right medial thigh revealed nonnecrotizing granulomas in the superficial dermis that extended into the subcutaneous adipose tissue (Figure 1). Surrounding chronic inflammation was scant, and the presence of rare eosinophils was noted (Figure 2). The histiocytes were highlighted by a CD68 immunohistochemical stain. An auramine-O special stain test was negative for acid-fast bacilli, and a Grocott methenamine-silver special stain test for fungal organisms was negative. These findings were consistent with GD. Computed tomography of the chest performed 2 months prior and 1 month after biopsy of the skin lesions revealed no axillary, mediastinal, or hilar lymphadenopathy. The calcium level at the time of skin biopsy was within reference range.
A topical steroid was prescribed; however, it was not utilized by the patient. Within 2 months of onset, the GD lesions resolved with no treatment. The GD lesions did not affect the patient’s enrollment in the clinical trial, and no dose reductions were made. Due to progressive disease with metastases to the brain, the patient eventually discontinued the clinical trial.
BRAF inhibitors are US Food and Drug Administration approved for the treatment of metastatic melanoma to deactivate the serine-threonine kinase BRAF gene mutation, which leads to decreased generation and survival of melanoma cells.1,2 Vemurafenib, dabrafenib, and encorafenib are the only BRAF inhibitors approved in the United States.3 The most common side effects of vemurafenib include arthralgia, fatigue, rash, and photosensitivity.1,4 There are 4 MEK inhibitors currently available in the United States: cobimetinib, trametinib, selumetinib and binimetinib. The addition of a MEK inhibitor to BRAF inhibitor therapy has shown increased patient response rates and prolonged survival in 3 phase 3 studies.5-10
Response rates remain low in the treatment of advanced cholangiocarcinoma with standard chemotherapy. Recent research has explored if targeted therapies at the molecular level would be of benefit.11 Our patient was enrolled in the American Society of Clinical Oncology Targeted Agent and Profiling Utilization Registry (TAPUR) trial, a phase 2, prospective, nonrandomized trial that matches eligible participants to US Food and Drug Administration–approved study medications based on specific data from their molecular testing results.12 Some of the most common mutations in intrahepatic cholangiocarcinoma include HER2, KRAS, MET, and BRAF.13-17 Our patient’s molecular test results were positive for a BRAF V600E–positive mutation, and she subsequently started therapy with vemurafenib and cobimetinib. The use of personalized genomic treatment approaches for BRAF V600E mutation–positive cholangiocarcinoma has produced a dramatic patient response to BRAF and MEK inhibitor combination therapies.11,18-20