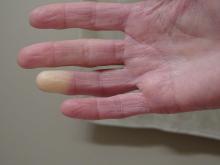
Researchers have identified two genes that may contribute to Raynaud’s phenomenon, a condition where blood vessels in the extremities constrict and limit blood flow.
Raynaud’s is a relatively common condition, affecting 2%-5% of the general population. Though Raynaud’s can be an annoyance for some, it can also cause severe pain and can require medication.
These newly identified genes will hopefully lead to new therapeutic options, said Maik Pietzner, PhD, chair in health data modeling at Queen Mary University of London’s Precision Healthcare University Research Institute (PHURI) and group leader in the Computational Medicine Group at the Berlin Institute of Health at Charité – Universitätsmedizin Berlin, Germany.
Dr. Pietzner led the research along with Claudia Langenberg, MD, PhD, director of PHURI.
The study was published in Nature Communications.
Largest genomic study of Raynaud’s to date
The researchers looked through electronic medical records from the UK Biobank, a large-scale database that contains genetic and health information on half a million participants. They identified more than 5,100 individuals with Raynaud’s, of which 68% had primary Raynaud’s. These participants were compared with more than 439,000 controls who did not have Raynaud’s.
In a secondary analysis, the team also used health records from the Queen Mary University of London Genes & Health Study, which contains health information on individuals of South Asian ancestry.
The researchers identified two genes that are likely involved with Raynaud’s. The first, ADRA2A, encodes for the alpha-2A adrenergic receptor that can cause vasoconstriction of small blood vessels in response to stress hormones. Researchers have long suspected that this type of receptor could be involved with Raynaud’s, but there was debate over which receptor subtype was responsible.
“Our finding of alpha-2A receptors is quite interesting because the focus has always been on alpha-2C receptors,” said Dr. Pietzner. “It’s only a letter, but it’s a massive difference in terms of biology and physiology,” he said, and could be why therapies targeting 2C receptors have been ineffective.
The second strongest association was for the transcription factor IRX1. Less is known about this gene, but the data we do have suggest that it is involved with regulating the dilation of blood vessels, Dr. Pietzner noted.
“There might be balance between the ADRA2A finding being responsible for constriction and the IRX1 finding indirectly linked to the dilation of those vessels following constrictions. Having both may explain why these prolonged episodes of vasoconstriction lead to a loss of oxygen to the tissues,” so they turn white and then blue, he said.
Because the Biobank cohort was European-centric, Dr. Pietzner and colleagues also identified 400 cases of Raynaud’s in British individuals of Bangladeshi and Pakistani ancestry and were able to replicate the association between IRX1 and Raynaud’s. Data on ADRA2A were unavailable.
The genes identified are associated with primary Raynaud’s. Secondary Raynaud’s is a rarer type of the condition that occurs along with autoimmune disorders, such as scleroderma, and is generally more severe.
It’s long been suspected that Raynaud’s had some genetic component, because half of patients with Raynaud’s have another family member with the same condition, said Laura Hummers, MD, who codirects the John Hopkins Scleroderma Center in Baltimore. She was not involved with the study.
This is “the largest study of this kind that’s been done,” she said, and the first to show a potential mechanism behind this genetic association.
The main gene finding, ADRA2A, “points to a receptor on the cells that regulate the tone of these blood vessels,” she continued. “It suggests maybe there’s too many of these receptors or they’re overly sensitive; something about them is different that makes patients more susceptible to these cold triggers. Knowing that is potentially really important, because it could give you a direct way to intervene, if true.”