To the Editor:
Immune checkpoint inhibitors have revolutionized the treatment of advanced-stage melanoma, with remarkably improved progression-free survival.1 Anti–programmed death receptor 1 (anti–PD-1) therapies, such as nivolumab and pembrolizumab, are a class of checkpoint inhibitors that have been approved by the US Food and Drug Administration for unresectable metastatic melanoma. Anti–PD-1 agents block the interaction of programmed death-ligand 1 (PD-L1) found on tumor cells with the PD-1 receptor on T cells, facilitating a positive immune response.2
Although these therapies have demonstrated notable antitumor efficacy, they also give rise to numerous immune-related adverse events (irAEs). As many as 70% of patients treated with PD-1/PD-L1 inhibitors experience some type of organ system irAE, of which 30% to 40% are cutaneous.3-6 Dermatologic adverse events are the most common irAEs, specifically spongiotic dermatitis, lichenoid dermatitis, pruritus, and vitiligo.7 Bullous pemphigoid (BP), an autoimmune bullous skin disorder caused by autoantibodies to basement membrane zone antigens, is a rare but potentially serious cutaneous irAE.8 Systemic corticosteroids commonly are used to treat immune checkpoint inhibitor–induced BP; other options include tetracyclines for maintenance therapy and rituximab for corticosteroid-refractory BP associated with anti-PD-1.9 We present a case of recalcitrant BP secondary to nivolumab therapy in a patient with metastatic melanoma who had near-complete resolution of BP following 2 months of cyclosporine.
A 41-year-old man presented with a generalized papular skin eruption of 1 month’s duration. He had a history of stage IIIC malignant melanoma of the lower right leg with positive sentinel lymph node biopsy. The largest lymph node deposit was 0.03 mm without extracapsular extension. Whole-body positron emission tomography–computed tomography showed no evidence of distant disease. The patient was treated with wide local excision with clear surgical margins plus 12 cycles of nivolumab, which was discontinued due to colitis. Four months after the final cycle of nivolumab, the patient developed widespread erythematous papules with hemorrhagic yellow crusting and no mucosal involvement. He was referred to dermatology by his primary oncologist for further evaluation.
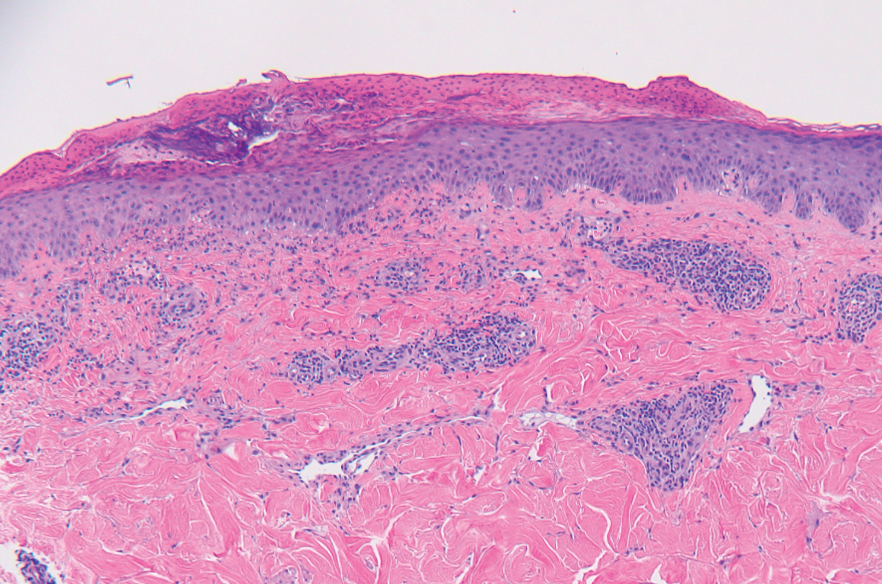
A punch biopsy from the abdomen showed parakeratosis with leukocytoclasis and a superficial dermal infiltrate of neutrophils and eosinophils (Figure 1). Direct immunofluorescence revealed linear basement membrane deposits of IgG and C3, consistent with subepidermal blistering disease. Indirect immunofluorescence demonstrated trace IgG and IgG4 antibodies localized to the epidermal roof of salt-split skin and was negative for IgA antibodies. An enzyme-linked immunoassay was positive for BP antigen 2 (BP180) antibodies (98.4 U/mL [positive, ≥9 U/mL]) and negative for BP antigen 1 (BP230) antibodies (4.3 U/mL [positive, ≥9 U/mL]). Overall, these findings were consistent with a diagnosis of BP.
The patient was treated with prednisone 60 mg daily with initial response; however, there was disease recurrence with tapering. Doxycycline 100 mg twice daily and nicotinamide 500 mg twice daily were added as steroid-sparing agents, as prednisone was discontinued due to mood changes. Three months after the prednisone taper, the patient continued to develop new blisters. He completed treatment with doxycycline and nicotinamide. Rituximab 375 mg weekly was then initiated for 4 weeks.
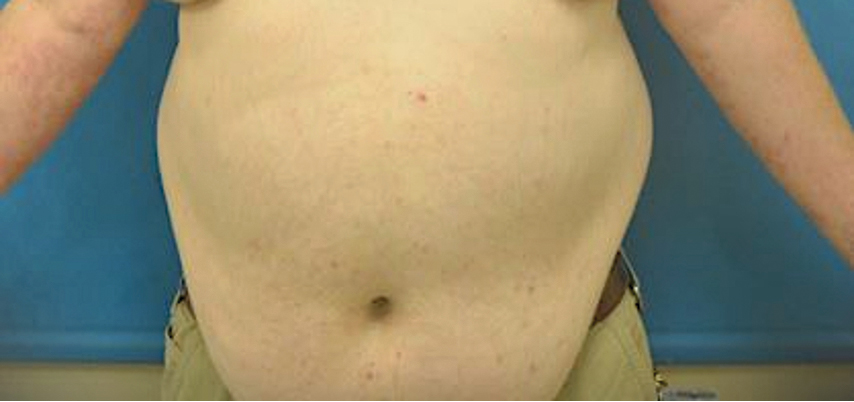
At 2-week follow-up after completing the rituximab course, the patient reported worsening symptoms and presented with new bullae on the abdomen and upper extremities (Figure 2). Because of the recent history of mood changes while taking prednisone, a trial of cyclosporine 100 mg twice daily (1.37 mg/kg/d) was initiated, with notable improvement within 2 weeks of treatment. After 2 months of cyclosporine, approximately 90% of the rash had resolved with a few tense bullae remaining on the left frontal scalp but no new flares (Figure 3). One month after treatment ended, the patient remained clear of lesions without relapse.
Programmed death receptor 1 inhibitors have shown dramatic efficacy for a growing number of solid and hematologic malignancies, especially malignant melanoma. However, their use is accompanied by nonspecific activation of the immune system, resulting in a variety of adverse events, many manifesting on the skin. Several cases of BP in patients treated with PD-1/PD-L1 inhibitors have been reported.9 Cutaneous irAEs usually manifest within 3 weeks of initiation of PD-1 inhibitor therapy; however, the onset of BP typically occurs later at approximately 21 weeks.4,9 Our patient developed cutaneous manifestations 4 months after cessation of nivolumab.
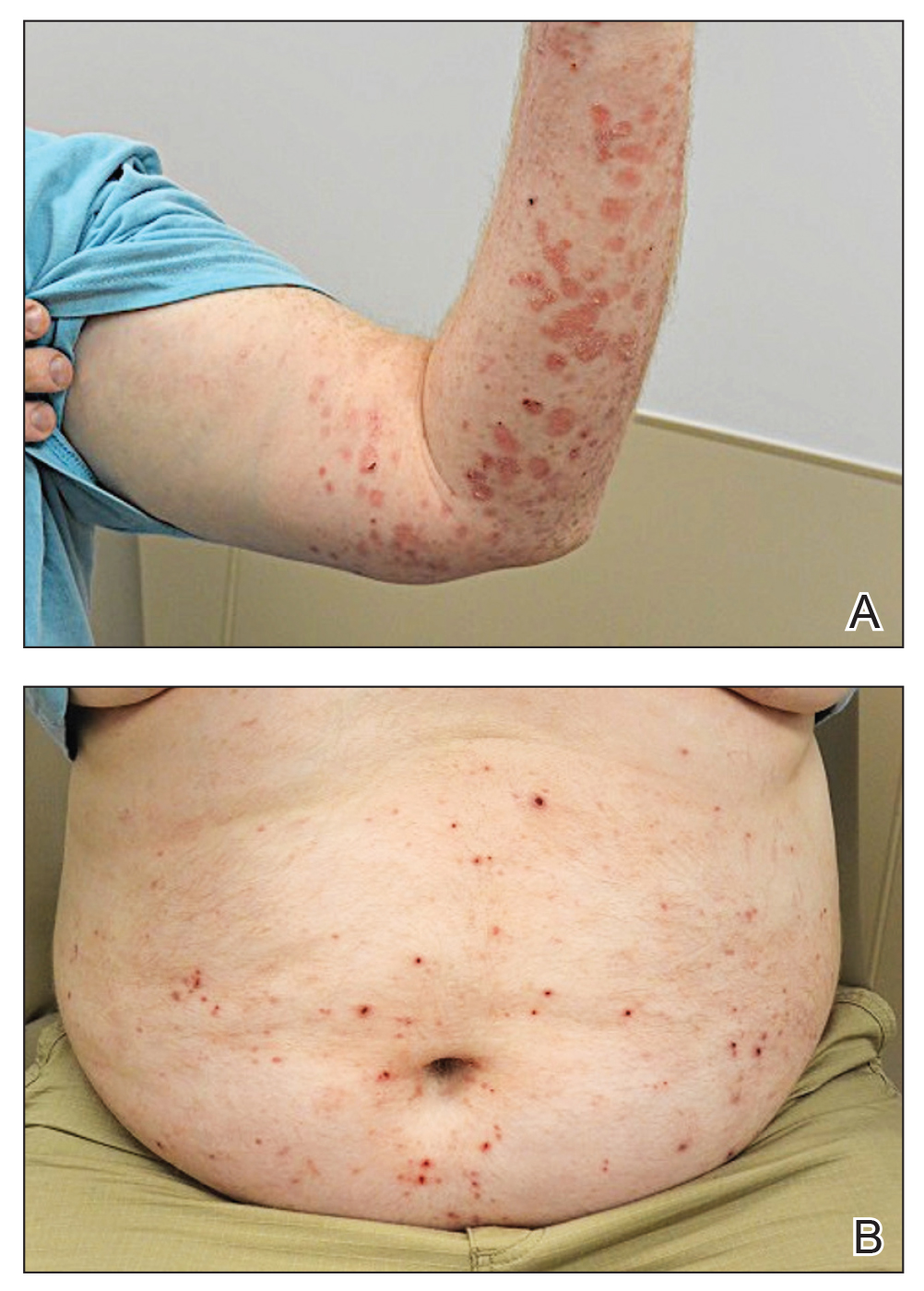
FIGURE 2. Recalcitrant bullous pemphigoid secondary to nivolumab therapy for malignant melanoma persisted despite therapy with prednisone, doxycycline, nicotinamide, and rituximab. A, Diffuse erythematous papules, plaques, and bullae of varying duration with overlying hemorrhagic crusting and erosions were evident on the left arm. B, Scattered erythematous papules and bullae with overlying hemorrhagic crusting on the abdomen.
Bullous pemphigoid classically manifests with pruritus and tense bullae. Notably, our patient’s clinical presentation included a widespread eruption of papules without bullae, which was similar to a review by Tsiogka et al,9 which reported that one-third of patients first present with a nonspecific cutaneous eruption. Bullous pemphigoid induced by anti–PD-1 may manifest differently than traditional BP, illuminating the importance of a thorough diagnostic workup.
Although the pathogenesis of immune checkpoint inhibitor–induced BP has not been fully elucidated, it is hypothesized to be caused by increased T cell cytotoxic activity leading to tumor lysis and release of numerous autoantigens. These autoantigens cause priming of abnormal T cells that can lead to further tissue damage in peripheral tissue and to generation of aberrant B cells and subsequent autoantibodies such as BP180 in germinal centers.4,10,11
Cyclosporine is a calcineurin inhibitor that reduces synthesis of IL-2, resulting in reduced cell activation.12 Therefore, cyclosporine may alleviate BP in patients who are being treated, or were previously treated, with an immune checkpoint inhibitor by suppressing T cell–mediated immune reaction and may be a rapid alternative for patients who cannot tolerate systemic steroids.
Treatment options for mild to moderate cases of BP include topical corticosteroids and antihistamines, while severe cases may require high-dose systemic corticosteroids. In recalcitrant cases, rituximab infusion with or without intravenous immunoglobulin often is utilized.8,13 The use of cyclosporine for various bullous disorders, including pemphigus vulgaris and epidermolysis bullosa acquisita, has been described.14 In recent years there has been a shift away from the use of cyclosporine for these conditions following the introduction of rituximab, a monoclonal antibody directed against the CD20 antigen on B lymphocytes. We utilized cyclosporine in our patient after he experienced worsening symptoms 1 month after completing treatment with rituximab.
Improvement from rituximab therapy may be delayed because it can take months to deplete CD20+ B lymphocytes from circulation, which may necessitate additional immunosuppressants or re-treatment with rituximab.15,16 In these instances, cyclosporine may be beneficial as a low-cost alternative in patients who are unable to tolerate systemic steroids, with a relatively good safety profile. The dosage of cyclosporine prescribed to the patient was chosen based on Joint American Academy of Dermatology–National Psoriasis Foundation management guidelines for psoriasis with systemic nonbiologic therapies, which recommends an initial dosage of 1 to 3 mg/kg/d in 2 divided doses.17
As immunotherapy for treating various cancers gains popularity, the frequency of dermatologic irAEs will increase. Therefore, dermatologists must be aware of the array of cutaneous manifestations, such as BP, and potential treatment options. When first-line and second-line therapies are contraindicated or do not provide notable improvement, cyclosporine may be an effective alternative for immune checkpoint inhibitor–induced BP.