To the Editor:
Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome—a severe cutaneous adverse drug reaction—is characterized by a cutaneous rash and systemic upset in the form of various internal organ and hematologic disturbances. This delayed and idiosyncratic syndrome went by several names, including anticonvulsant hypersensitivity syndrome, before Bocquet et al1 proposed the term DRESS syndrome.
Phenytoin, a hydantoin derivative used in neurology, was implicated in 41% of cases of DRESS syndrome in a study of 100 patients conducted in southern India.2,3 While DRESS syndrome is a newer name, the clinical picture of DRESS secondary to phenytoin use remains similar in that it manifests with a morbilliform rash and systemic upset. We sought to describe the clinical and laboratory characteristics of phenytoin-induced DRESS syndrome in this case series.
The analysis included 23 patients with DRESS syndrome secondary to phenytoin use who presented to a tertiary care institution in North India between July 2021 and December 2022, satisfied the European Registry of Severe Cutaneous Adverse Reaction (RegiSCAR) criteria,4 and achieved a DRESS diagnostic score of more than 1. The mean age of the patients was 44 years (range, 14–74 years). There was a slight female predominance with a male to female ratio of 0.9:1. More than half of the patients (52.2% [12/23]) presented directly to the dermatology outpatient department; the remaining patients were referred from other departments (47.8% [11/23]). Patients primarily were receiving phenytoin for neurologic indications. Specific reasons included antiseizure prophylaxis following a traffic accident (34.8% [8/23]); epilepsy (26.1% [6/23]); and neoplastic (17.4% [4/23]), vascular (17.4% [4/23]), and infectious (4.3% [1/23]) causes. The mean latency period from drug intake to symptom onset was 29 days (range, 6–62 days), and the mean illness duration was 9 days (range, 1–45 days).
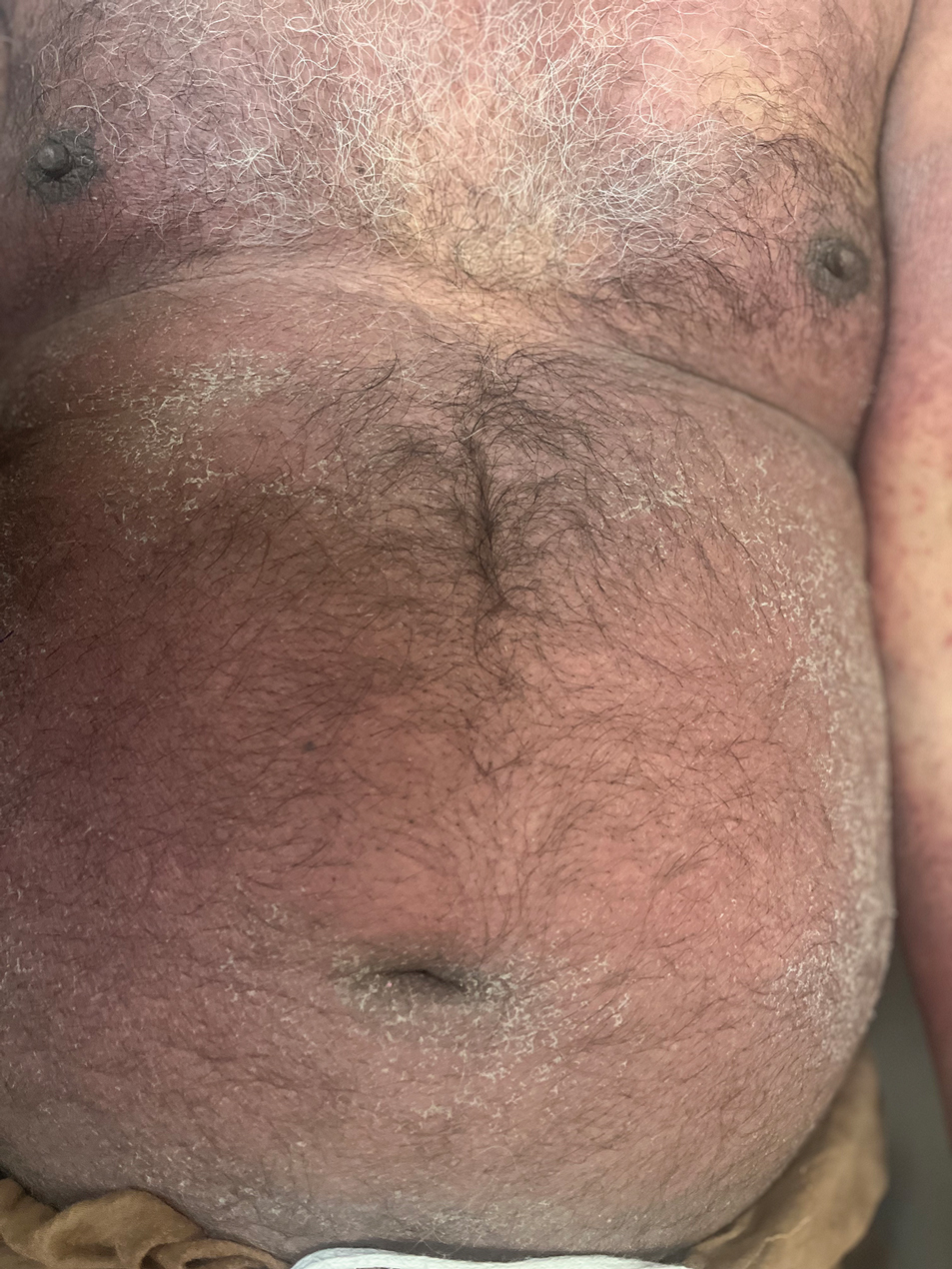
The majority of patients experienced pruritus (91.3% [21/23]) and fever (74.0% [17/23]), and all initially had a rash. Maculopapular morphology was seen in all patients. Erythema multiforme–like (17.4% [4/23]), erythrodermic (17.4% [4/23]), and vesicular (13.0% [3/23]) rashes also were documented (Figure 1). The trunk (100% [23/23]) and extremities (95.7% [22/23]) were involved most often, followed by the palms and soles (56.5% [13/23]). The mean total body surface area affected was 73.65%. Only 7 patients (30.4%) had mucosal involvement; nonhemorrhagic cheilitis was the most common manifestation.
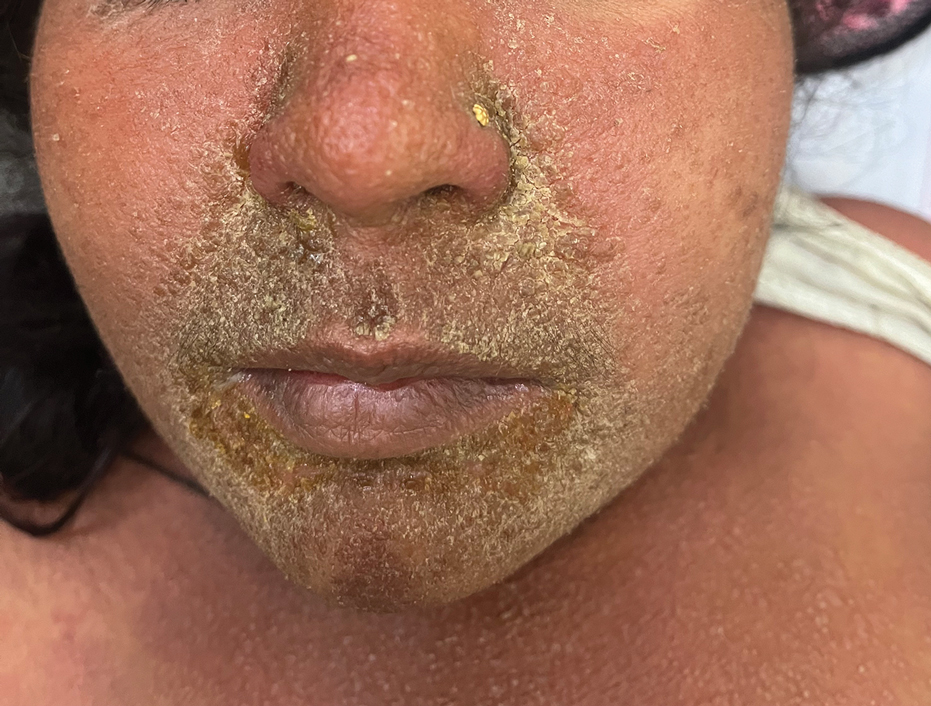
Facial edema, a hallmark feature of DRESS syndrome, was noted in 69.6% (16/23) of patients (Figure 2). Lymphadenopathy was present in 43.5% (10/23) of patients; of those cases, the inguinal (40.0%; n=4) and cervical (30%; n=3) nodes most commonly were involved. Although DRESS syndrome can affect internal organs, this was an issue for only 2 (8.7%) patients who experienced mild hepatomegaly.
Laboratory investigations revealed a mean differential eosinophil percentage of 10.3% (reference range, 1%–4%), while the mean absolute eosinophil count was 1.0634×109/L (reference range, 0.02–0.5×109/L). Other hematologic findings included the mean percentages of neutrophils (60%; reference range, 50%–60%), lymphocytes (19.95%; reference range, 20%–50%), and monocytes (8.70%; reference range, 2%–8%).
Liver function tests revealed transaminitis5 as the most common finding, with mean aspartate aminotransferase levels of 109 U/L (reference range, 8–33 U/L), mean alanine aminotransferase of 97.9 U/L (reference range, 7–56 U/L), and mean alkaline phosphatase levels of 211.35 U/L (reference range, 44–147 U/L). Half of the patients had notable (>2 times the upper limit of normal) transaminitis.
Renal blood workup revealed slightly elevated blood urea nitrogen levels with a mean value of 28.4 mg/dL (reference range, 6–24 mg/dL), and mean serum creatinine was 0.78 mg/dL (reference range for men, 0.7–1.3 mg/dL; for women, 0.6–1.1 mg/dL).
All patients were treated with oral steroids (prednisolone 1 mg/kg/d) before tapering slowly over the following 6 to 8 weeks. The culprit drug (phenytoin) was stopped on the day of presentation. Resolution of rash and itching was seen in all patients by 3 weeks after presentation without any relapse by follow-up at 6 weeks from presentation to the hospital.
Our case series seeks to discuss the clinical and laboratory features of phenytoin-induced DRESS syndrome. Our patients had more erythrodermic and erythema multiforme–like morphologies, less mucosal involvement, more hepatic involvement, and earlier resolution.