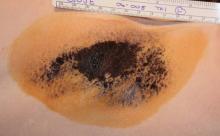
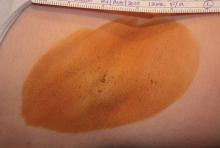
GRAPEVINE, TEX. – A novel microwave device significantly reduced underarm sweating in two studies involving a total of 151 patients with axillary hyperhidrosis.
The condition affects millions of people, yet current treatments are limited by either duration of effect or efficacy. The microwave device has the potential for a longer-term and possibly even permanent effect via eradication of eccrine sweat glands, Dr. Suzanne L. Kilmer and Dr. Mark Lupin reported in separate presentations at the annual meeting of the American Society for Laser Medicine and Surgery.
Miramar Labs' miraDry system focuses microwave energy to selectively heat the interface between the skin and underlying fat, where the sweat glands reside. The system comprises a console, handpiece, and disposable tip. The in-office procedure takes approximately 40 minutes. The system was cleared for licensure in the United States by the Food and Drug Administration in January 2011, and will be marketed later this year, according to a company spokesperson.
Dr. Kilmer, of the Laser and Skin Surgery Center of Northern California, Sacramento, presented data on a second-generation version of the device that was FDA approved but never marketed. In a multicenter, sham-controlled study, 120 adults with hyperhidrosis were randomized to miraDry (81) or sham treatment (39) for one to three sessions. The patients had to have a Hyperhidrosis Disease Severity Scale (HDSS) score of 3 or 4 ("my underarm sweating is barely tolerable and frequently/always interferes with my daily activities") at baseline, and gravimetric readings greater than 50 mg at each axilla (in 5 minutes).
Follow-up was 6 months for the sham group and 12 months for the treatment group. Responders were defined as subjects reporting a reduction to an HDSS score of 1 ("My underarm sweating is never noticeable and never interferes with daily activities") or 2 ("My underarm sweating is tolerable but sometimes interferes with daily activities"). The patients had a mean age of 33 years, 58% were female, and 84% were white.
Efficacy for the treatment group was 89% at 30 days, 74% at 3 months, 67% at 6 months, and 69% at the 9- and 12-month visits. In the sham group, efficacy was 54% at 30 days and 44% for the 3- and 6-month visits. At all time points, the differences between the treatment and sham groups were significant, Dr. Kilmer reported.
Treatment-related adverse events were generally mild, and all but one resolved. The most common adverse events in the treatment group were transient patches of altered sensation in the treatment limb, occurring in 8 patients (10%), and axillary pain requiring prescription medication in 5 patients (6%). Most subjects experienced transient post-treatment local sequelae in the axilla such as edema, tenderness, and bruising.
Follow-up of the treated subjects showed stable efficacy through 12 months, she said.
The second study, involving 31 patients, investigated the third-generation, optimized version of the device. As in the earlier study, all patients had to have primary axillary hyperhidrosis with an HDSS score of 3 or 4. The patients also were required to have a gravimetric sweat assessment of at least 50 mg in each axilla (in 5 minutes). None of the patients had surgery for axillary hyperhidrosis or botulinum toxin injections in the axillae in the prior 12 months, said Dr. Lupin of Cosmedica Laser Centre, Victoria, B.C.
The patients had a mean age of 33 years (range 18-65 years), and three-quarters were female. They had a mean BMI of 24.8 kg/m2. Of 28 patients seen at 30 days, all but one (96%) had HDSS scores of 1 or 2. Of 25 seen at 3 months, all had HDSS scores of 1 or 2 (100% efficacy). Gravimetric assessments 3 months after treatment showed that 94% of patients had at least a 50% reduction in axillary sweat compared with baseline, with an average sweat reduction of 82%.
Nineteen of the 31 initially enrolled patients (61%) experienced at least one treatment-related adverse event, of which most (88%) were rated as mild. The most common were numbness or tingling in an area of the treated limb (12 patients, 39%), and edema in the chest or treatment limb lasting about a week (9 patients, 29%). Most of the patients also experienced acute post-treatment transient effects in the treatment area such as localized edema, tenderness, or erythema. Follow-up of safety is ongoing, Dr. Lupin noted.