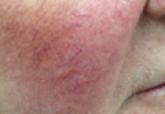
A recent study in the Journal of Drugs in Dermatology (2014;13:56-61) featured long-term data regarding use of brimonidine gel, which was approved by the US Food and Drug Administration last year as the only topical agent on the market to treat erythema associated with rosacea. Compared to the initial safety and efficacy in phase 2 and 3 trials with 4-week use, this study spanned 12 months and assessed the erythema and adverse events associated with the drug. The decrease in erythema with once-daily application was observed after first use and was durable until the end of the study with no tachyphylaxis. The side-effect profile was mild and included flushing and irritancy events that typically improved over time.
What’s the issue?
The manufacturers of this new product tout that “Red is Wrong” (http://www.rediswrong.com). Although this strong statement virtually forces people to sprint to the Web site to see how they could improve their facial erythema and my patients have reported quite favorably on its efficacy and tolerability, obtaining the medication has been problematic. Similar to many of the coupon cards and assistance provided by pharmaceutical companies, the true segment of the population for which they serve is more limited than it appears, as health insurance plans with prescription deductibles, Medicare, Medicaid (and any spin-offs of the sort in the last few months involving US health care cost-management gymnastics) typically are excluded from discounts, and the out-of-pocket cost can be well over $200 per month. In an era when we have heroically developed a new and effective drug for a common and problematic indication, how will we practically provide it to the masses?
We want to know your views! Tell us what you think.