Article
Whey Protein Precipitating Moderate to Severe Acne Flares in 5 Teenaged Athletes
Acne vulgaris has been linked to milk ingestion, both whole and skim milk. The milk fraction that promotes acne is unknown.
From the Department of Dermatology, Wayne State University School of Medicine, Detroit, Michigan.
The authors report no conflict of interest.
Correspondence: Jessica Kado, MD, 18100 Oakwood Blvd, Ste 300, Dearborn, MI 48124 (jkado@med.wayne.edu).

Tumor necrosis factor α antagonists are potent biologics used to treat a variety of autoimmune disorders such as rheumatoid arthritis, ankylosing spondylitis, Crohn disease, psoriasis, and psoriatic arthritis. These medications are known to have many side effects (eg, infusion reactions, cytopenia, risk for infection, heart failure); however, only a few cases of acne vulgaris have been associated with the use of these biologics, particularly infliximab and adalimumab. We report a rare case of etanercept-induced cystic acne.
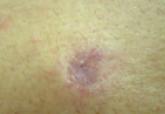
A 35-year-old man with chronic plaque-type psoriasis presented for treatment of a flare-up caused by a combination of hot tub use and 3 missed etanercept injections. The patient had been prescribed subcutaneous etanercept (50 mg once weekly) approximately 2 years prior to presentation for treatment of psoriasis and had used it consistently with 2 brief periods of discontinuation due to upper respiratory infections; treatment was discontinued for 2 weeks until the infections resolved and was restarted at the same dose. Recent hot tub use induced localized folliculitis of the leg and caused koebnerization of his psoriasis. Treatment with etanercept (50 mg twice weekly) was reinitiated, but after 1 month of therapy, the patient developed a nodulocystic eruption on the face. The patient discontinued use of etanercept and subsequently was started on oral minocycline (50 mgonce daily) by his primary care physician. After 6 weeks of minocycline therapy, the patient reported gradual improvement of his cystic acne. At follow-up, physical examination revealed approximately 15 erythematous cystic papules and nodules on the bilateral cheeks. The dose of oral minocycline was increased to 100 mg twice daily and his face subsequently cleared after 12 weeks of treatment.
On rechallenge with etanercept (50 mg weekly) after a separate flare-up of his psoriasis and psoriatic arthritis, the patient again developed nodulocystic acne within 3 weeks of restarting the medication, which confirmed the association between etanercept and the eruption. Etanercept was subsequently discontinued and the patient was started on ustekinumab for treatment of psoriasis and psoriatic arthritis as well as minocycline (100 mg twice daily) for treatment of nodulocystic acne. His acne subsequently cleared after 12 weeks of therapy. The patient’s psoriasis currently is well controlled on a regimen of methotrexate 20 mg weekly, as continued use of ustekinumab was not covered by his health insurance policy.
Etanercept, along with infliximab and adalimumab, is a tumor necrosis factor α (TNF-α) antagonist. Tumor necrosis factor α is a major cytokine of the immune system that is deregulated in autoimmune disorders, causing inflammation and a variety of systemic effects. Inhibition of TNF-α results in a decrease in inflammatory markers such as IL-1 and IL-6, leading to a reduction in endothelial permeability and leukocyte migration.1 Etanercept is unique in that it is a fully humanized soluble fusion protein consisting of a TNF-α receptor linked to the Fc region of IgG1, whereas infliximab and adalimumab are monoclonal antibodies that target TNF-α. Etanercept works by acting as a decoy receptor for TNF-α, thereby preventing it from binding cell surface receptors and subsequently decreasing inflammation. It is an approved treatment of rheumatoid arthritis, polyarticular juvenile rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, and plaque psoriasis.2
Many drugs have been implicated in acneform eruptions mimicking acne vulgaris, such as corticosteroids, cyclosporine, antipsychotics, anticonvulsants, epidermal growth factor receptor inhibitors, antidepressants, danazol, antituberculosis drugs, quinidine, azathioprine, testosterone, and TNF-α antagonists.1 The TNF-α antagonists that have been associated with acne are infliximab and adalimumab, especially when used to treat Crohn disease or rheumatoid arthritis, though there also are reports of acneform eruptions when using these drugs to treat psoriasis.1,3 There have been reports of the use of etanercept in treating severe acne and acne conglobata4,5; our case documents etanercept-induced acne associated with psoriasis treatment. Theoretically, anti–TNF-α agents should suppress acne rather than induce it due to their inhibition of the inflammatory markers TNF-α, IL-1a, and IFN-γ, which are thought to play a role in hypercornification of the infundibulum.6 Thus the mechanism of TNF-α antagonists and associated acneform eruptions remains unknown. This observation should prompt further research to investigate the correlation between the use of TNF-α inhibitors, specifically etanercept, and cystic acne.
Acne vulgaris has been linked to milk ingestion, both whole and skim milk. The milk fraction that promotes acne is unknown.
Etanercept is a biologic agent prescribed by dermatologists for the treatment of moderate to severe plaque psoriasis and psoriatic arthritis.
This case report describes the successful treatment of severe plaque psoriasis with etanercept in a patient who underwent a liver transplant. It...
