Acute extremity pain is a common presentation seen daily in EDs. While most etiologies of extremity pain are benign, the complications of acute compartment syndrome are associated with significant morbidity. Moreover, acute compartment syndrome remains a difficult diagnosis that is often missed on initial presentation. Morbidity results from an increased pressure in an anatomically closed space, progressing to decreased perfusion and rapid tissue destruction.
Case
An obese 55-year-old man with a medical history of coronary artery disease, for which he was on aspirin therapy, presented for evaluation of right shin pain. The patient stated that he completed a 5-km race earlier that morning with his son. Immediately following the race, he experienced increasing right shin pain, which he attempted to initially manage with ice compresses and over-the-counter ibuprofen. He noted that neither the ice compresses nor the ibuprofen relieved his pain and that by 5:00 pm, the pain had worsened to the point where he had difficulty walking, prompting his visit to the ED.
Upon arrival at the ED, the patient was ambulatory but had significant pain at both rest and movement. His vital signs and his oxygen saturation on room air were normal. On physical examination, he had normal sensation to the entire right lower extremity and had equal pulses in both feet. The anterolateral aspect of the shin was exquisitely tender to light touch, and the patient was unable to dorsiflex or plantar flex without extreme pain. On passive dorsiflexion and plantar flexion of his right foot, he had exquisite pain. On palpation, the anterior shin was firm compared to the other muscle beds.
Epidemiology
Acute compartment syndrome—elevation of interstitial pressure in closed fascial compartment—affects 10 times as many men as women, at an average age of 32 years old and with an annual incidence of 7.3 per 100,000 men and 0.7 per 100,000 for women.1 McQueen et al1 found that the most common cause of acute compartment syndrome was fracture (69%), followed by soft tissue injury (23%). Younger patients are more likely to develop acute compartment syndrome from trauma because they typically have larger muscle beds with more tissue to become edematous compared to the older, hypotrophic muscles of elderly patients.
Pathophysiology
The fascia surrounds the major muscle groups and neurovascular bundles in the extremities to create distinct compartments. Since the fascia is not a compliant structure, it is typically not able to tolerate increases in volume or pressure in a given compartment. Compartment perfusion pressure is the mean arterial pressure minus the compartment pressure. Normal compartment pressure in adults is between 0 to 8 mm Hg.2 When compartment perfusion pressures are below 70 to 80 mm Hg, there is an increased risk of compartment syndrome.
Although the exact pathophysiology of acute compartment syndrome is still debated,3 the most commonly accepted theory is the arteriovenous pressure gradient theory.4 In this theory, the rise in intracompartment pressure increases venous pressure, which in turn reduces the arteriovenous pressure gradient, reducing local tissue perfusion. The reduction in tissue perfusion, coupled with a reduction in venous drainage, causes significant tissue edema. This change in vascular pressure also causes a reduction in lymphatic drainage, further increasing pressure in the compartment. Finally, the edematous tissue compresses the arterioles leading to end-organ ischemia.5
Initially an absolute threshold compartment pressure was thought to cause irreversible tissue ischemia,6 but this theory has slowly lost favor after it was found that hypertension was actually protective in compartment syndrome.7 Current thinking is that the difference between the diastolic pressure and the compartment pressure leads to tissue ischemia (ie, acute compartment syndrome delta pressure = diastolic blood pressure [BP] – measured compartment pressure).6,8
In 1996, McQueen and Court-Brown6 prospectively admitted all tibial diaphyseal fractures and continuously monitored their anterior compartment pressure. Using a delta pressure value of less than 30 mm Hg, only three patients were diagnosed with acute compartment syndrome and required fasciotomy. The patients’ absolute compartment pressures were 45 mm Hg, 65 mm Hg, and 75 mm Hg, while the delta pressures were 15 mm Hg, 10 mm Hg, and 15 mm Hg, respectively. Conversely, 53 patients had absolute compartment pressures over 30 mm Hg; 30 patients had pressure over 40 mm Hg; four patients had pressure over 50 mm Hg; and none required fasciotomy. This study highlights that the absolute compartment pressure is not helpful in making the diagnosis, and it is the elevated delta pressure that secures the diagnosis.
Etiology
Compartment syndrome is the end result of many different injury patterns. While fracture is the number one cause of compartment syndrome, many types of soft tissue injuries can also lead to compartment syndrome. Nonfracture etiologies of compartment syndrome are relatively uncommon, and as such can lead to a delay in diagnosis.
Fracture
Almost 70% of all cases of compartment syndrome are due to fracture.1 Fractures of the tibia, distal radius, and ulna are the most common injuries that lead to acute compartment syndrome. Interestingly, acute compartment syndrome is caused by an equal distribution of high-energy and low-energy mechanisms of injuries.1 Because the increase in compartment pressure is highest at the fracture site,9 it is imperative to measure pressures at the site of the fracture. Contrary to traditional teaching, an open fracture does not reduce the risk of compartment syndrome. McQueen and Court-Brown6 found there was no difference in the intracompartment pressure between open and closed fractures.
Fracture reduction and manipulation can actually increase the risk of compartment syndrome. In one case series, fracture manipulation increased compartment pressure by reducing the total volume in a stretched compartment.10 Dresing et al10 found the average pressure increased by 21 mm Hg during wrist reduction, warranting close observation after fracture reduction and close observation of the patient’s pain and neurovascular status.
McQueen et al11 evaluated the risk factors for the development of acute compartment syndrome from tibial diaphyseal fractures and found that younger patients were at the highest risk. Patients between ages 10 to 19 years old had an odds ratio (OR) of 12.09; 20 to 29 years old had an OR of 9.84; and patients older than age 40 years had an OR of 1.11 As previously stated, younger patients have larger muscle volumes compared to their older counterparts and therefore have less space for edema after the primary muscle injury.
Soft Tissue Injury
Direct soft tissue injury can lead to a rise in compartment pressures due to trauma, infections, and burns even in the absence of fractures. Unfortunately, under these circumstances, patients with direct soft tissue injury are at high risk for a delay in diagnosis.12 The primary injury can be worsened by underlying coagulopathies.1 A circumferential constrictive eschar from burns can also cause external compression to a compartment13 as well as edema, which decreases the compliance of the fascia, leading to a rise in compartment pressure.
Vascular Injuries and Unusual Causes
Arterial Vessel Damage. Injuries to single arterial vessels can also lend to the development of acute compartment syndrome. Arterial damage from high-energy trauma causes acute compartment syndromes due to the rapid development of a hematoma and pressure in affected compartments. Loss of the arterial blood flow from the traumatized artery also causes cell necrosis and edema to the muscle bed, further increasing the compartment pressure. The result of these injuries is the development of acute compartment syndrome in uncommon locations such as the thigh14 and foot.15
Arterial damage from relatively low-energy ankle-inversion injuries have also been implicated in development of acute compartment syndrome of the foot.15 Conversely, damage to branches of an artery may cause symptoms in the compartments of the proximal extremity, but spare the blood flow and pulsations to the distal portion.13 This atypical mechanism of injury requires the physician to maintain a high index of suspicion and consider arteriography and direct pressure management in diagnosis and treatment of this condition.
Deep Vein Thrombosis. Deep vein thrombosis (DVT) can also be associated with acute compartment syndrome. A large clot burden, such as that observed in phlegmasia cerulea dolens, can lead to reduced venous flow and increased pressure, resulting in decreased arteriovenous gradient and tissue perfusion. Acute compartment syndrome caused by extensive DVT is often treated with anticoagulation therapy, thrombolysis or thrombectomy, but fasciotomy also has a role as an adjunct treatment to reduce compartment pressure sufficiently to return blood flow.16
Medication-Induced Compartment Syndrome
Injections of medications or illicit drugs can lead to increased compartment pressure through several independent mechanisms (Table).17 Local tissue vasotoxicity from direct injection of a caustic agent can cause direct muscle necrosis and edema. In addition, prolonged external compression while lying in a coma-like state induced by alcohol intoxication or central nervous system suppressant drugs, or a state of unconsciousness from any cause, can produce direct injury to the compartment (Table).
Diagnosis
Signs and Symptoms
Diagnosis of acute compartment syndrome is primarily clinical, using compartment pressure measurement as an adjunct in evaluation. Because the features of early acute compartment syndrome are nonspecific, a high clinical suspicion must be maintained for all at-risk populations.
The classic features such as pain, pallor, paresthesias, paralysis, and pulselessness are all late findings of acute compartment syndrome and are associated with irreversible damage. However, pain out of proportion to injury and pain with passive stretch of muscles are early symptoms that require further attention and monitoring.8
The earliest objective finding on physical examination is compartment firmness.8 Unfortunately, the sensitivity of physical examination by orthopedic physicians is low (22%-26%) on cadaver models with elevated compartment pressures.18 Peripheral nerve tissue is very sensitive to ischemia and will stop functioning after 75 minutes.9 A review of clinical findings in acute compartment syndrome showed that the positive predictive values of these individual symptoms are low, but there is a high likelihood of compartment syndrome when at least three clinical findings are present simultaneously.19 In patients who are at high risk for developing acute compartment syndrome, but who may not be able to describe or who do not show clear symptoms (eg, patients who are obtunded, intubated, or very young/old), compartment pressure measurement can be a valuable aid in the diagnosis.
Compartment Pressure Measurement
There are several readily available methods to directly measure the compartment pressure. It is imperative to measure the compartment pressure closest to the fracture location (within 5 cm) because the pressure dissipates as distance increases from the fracture site.20
Solid-State Transducer Intracompartmental Catheter. The Stryker Intra-Compartmental Pressure Monitor System (Stryker Surgical) is a commonly used solid-state transducer intracompartmental catheter (STIC) that allows measurement of compartment pressure.
The STIC system consists of a side-port needle, syringe of saline flush, and a digital read-out manometer. It has been validated against commonly used alternatives and found to be accurate21,22 with a confidence interval between ± 5 to 6.23. This device uses a side port needle to allow for testing multiple compartments with the same needle as it is less likely to be occluded by tissue when compared to a standard needle.
The following technique should be employed to properly measure compartment pressure using the Stryker STIC device23:
1. Place the side port needle on the tapered end of the diaphragm chamber.
2. Connect the prefilled syringe of normal saline to the diaphragm chamber.
3. Place the diaphragm chamber in the pressure monitor with the black side down and push until it is seated in the device.
4. Close the cover until it snaps.
5. Place the needle up and fill the system with normal saline from the syringe until there are no air bubbles in the system.
6. Turn the pressure monitor on.
7. Select an intended angle and press the “Zero” button and wait until it reads “00.”
8. Under sterile technique and appropriately anesthetized skin, insert the device into the compartment. Once in the compartment, slowly inject a small amount of saline into the compartment and record the provided number.
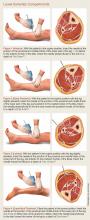
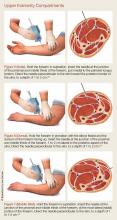
For details on needle-placement techniques, including depths, see Figures 1 to 4 for lower extremity compartments and Figures 5 to 7 for upper extremity compartments.24
Arterial Line Transducer System. An arterial pressure monitoring system can be adapted to measure compartment pressures. This technique has been validated against commercially available products.1,7,8
The following technique should be followed to properly measure compartment pressure using an arterial monitoring system25,26:
1. Connect 1 L of normal saline to the pressure-monitoring tubing.
2. Place the normal saline into a pressure bag.
3. Flush the line and all stopcocks in the pressure monitoring tubing.
4. Inflate the pressure bag to 300 mm Hg.
5. “Zero” the system that is level with the compartment you are testing.
6. Connect an 18-gauge spinal needle to the arterial line tubing.
7. Flush fluid through the needle.
8. Under sterile technique and appropriately anesthetized skin, insert the needle into the compartment approximately 2 to 3 cm deep.
9. To confirm the needle is in the correct location, squeeze the compartment to note a transient rise on the monitor.
Laboratory Evaluation
Although the diagnosis of compartment syndrome is made by clinical findings and direct pressure measurement, laboratory tests can support the diagnosis.
Serum creatinine phosphokinase (CPK) is elevated with muscle necrosis. Both CPK and myoglobin proteins are glomerulotoxic, and acute kidney injury is a common complication of acute compartment syndrome. A CPK of greater than 1,000 IU/L has a sensitivity of 0.91 for acute compartment syndrome, but a specificity of only 0.52.2
In a multivariate model for predicting acute compartment syndrome, CPK greater than 4,000 IU/L, chloride greater than 104 mEq/L, and a blood urea nitrogen less than 10 mmol/L were found to be predictive of compartment syndrome during a patient’s hospital admission. No patient had compartment syndrome when all three variables were negative, and all patients with all three positive variables had acute compartment syndrome.22 This model was conducted on admitted patients during their inpatient hospital stay; therefore its application in the ED may not be valid, and the model has yet to be validated prospectively.
Treatment
Prompt surgical consultation for decompressive fasciotomy is paramount to the management of acute compartment syndrome in the ED. When acute compartment syndrome is suspected, elevation of the affected extremity is suggested in an attempt to decrease swelling.27 The optimum height of elevation remains controversial; to prevent a decrease in arterial blood flow, it has been suggested not to raise the affected extremity above the level of the heart.8
A low systemic BP should be corrected to hopefully increase the compartment perfusion, and any applied external compressive forces (eg, casts, splints, dressings, eschars) should be removed.8 Removal of a cast can reduce the intracompartment pressure by 85%.5 Finally, applying cool compresses to the affected region can help reduce edema as a temporizing measure. Direct application of ice to the skin should be avoided to prevent cold-induced injury to the skin.
Appropriate medical resuscitation is imperative to good outcomes. Identifying and intervening when hypotension is present is necessary to improve tissue perfusion. Cellular derangement and death that can lead to hypocalcaemia, hyperkalemia, metabolic acidosis, and renal failure, require prompt recognition and correction.
At-Risk Populations
Pediatric Patients
Diagnosis of acute compartment syndrome in the general pediatric population is very difficult and therefore unfortunately associated with delays in diagnosis. The average time from injury to diagnosis can vary from 18.2to 31.1 hours.28,29 In children younger than age 3 years, 60% of acute compartment syndrome cases are due to trauma; 27% are due to invasive infections; and 13% develop from intravenous (IV) infiltration.30 Supracondylar humerus fractures are associated with increased risk of compartment syndrome. The volar compartment of the forearm is at risk after reduction of the fracture and when the elbow is flexed beyond 90°.31
Intubated and Obtunded Patients
Intubated and obtunded patients require special attention to prevent and/or identify the presence of acute compartment syndrome. Since clinical examination for compartment syndrome in these patients is unreliable, serial or continuous compartment pressure measurements are required to monitor for acute compartment syndrome.
Laboratory analysis, including monitoring of CPK levels, can also help identify developing compartment syndrome in intubated, sedated, or neurologically compromised patients.32 Onset of unexplained myoglobinuria or acute renal failure in an intubated patient should lead to consideration of compartment syndrome. In addition to laboratory studies, examination of atypical locations, such as the back or gluteal compartments, can also assist in identifying compartment syndrome in impaired patients.
Complications
The complications of compartment syndrome can be severe, and are typically organized as early and late stages of the disease.
Early Clinical Complications
Even with prompt diagnosis, acute compartment syndrome can lead to significant metabolic derangements. Patients with compartment syndrome are at significant risk for rhabdomyolysis and resultant renal failure from acute tubal necrosis. Likewise, both myocyte damage and death can cause extracellular electrolyte shifts, and hyperkalemia, metabolic acidosis, and hypocalcemia are frequently encountered under these circumstances.
Late Clinical Complications
Necrotic muscle is a significant risk factor for bacterial superinfection.33 Necrotic muscle may quickly be seeded by bacteria, and lead to sepsis. Necrotic muscle may therefore require repeated debridement and even possible extremity amputation for infection control. Likewise, muscle necrosis can lead to muscle contractures and loss of function of the affected extremity.3
Medicolegal Complications
Delay in the diagnosis of acute compartment syndrome has become an increasing source of medicolegal liability. In a 2004 review by Bhattacharyya and Vrahas34 of 23 years of claims from a medical malpractice insurer, only 19 claims were made for compartment syndrome. In this series, the following four risk factors were associated with an unsuccessful defense: (1) a linear association between the number of documented cardinal signs of compartment syndrome and an indemnity payment; (2) delays in fasciotomy; (3) poor communication with the patient and nursing staff; (4) and failure to intervene after documentation of an abnormal physical finding. All of the above were associated with a negative legal outcome.
Case Conclusion
The patient had a firm anterior compartment, CPK of 9,100 IU/L, normal renal function, compartment pressure of 60 mm Hg, and diastolic pressure of 80 mm Hg at the time of the procedure. Because the patient had a delta pressure of 20 mm Hg, orthopedic services were consulted, and the patient was taken to the operating room, where he underwent a bicompartment fasciotomy of the right lateral calf. The compartments were tense when opened and there was no evidence of myonecrosis. The patient was given continuous IV fluids and observed in the hospital for 2 days as his CPKs trended downward without subsequent renal injury.
Conclusion
Compartment syndrome requires high clinical suspicion for early diagnosis and treatment to prevent major disability. Early recognition of this condition is paramount, as the classical presentation of the five “Ps”—pain, pallor, pulselessness, paresthesias, and paralysis—are all late findings associated with irreversible consequences.
Given the difficulty in establishing the diagnosis by physical examination findings, the emergency physician (EP) should check and monitor compartment pressures when considering the diagnosis of acute compartment syndrome. In patients with acute compartment syndrome, delayed fasciotomies lead to poor outcomes and a 10-fold increase in surgical complications, such as infection and renal failure.35
Although traumatic fractures are the most common cause of acute compartment syndrome, EPs must also recognize that obtundation, intubation, coagulopathies, and seemingly minor traumas all can potentially cause or lead to acute compartment syndrome.