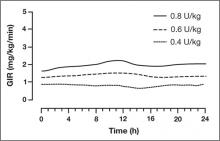
The findings from these investigations demonstrate that insulin degludec has a long half-life, resulting in a prolonged duration of blood glucose lowering with low within-subject pharmacodynamic variability.
FIGURE 1
Mean 24-hour glucose infusion rates (GIR) of insulin degludec at steady state
Copyright 2011 American Diabetes Association. From Diabetes®, Vol. 60,
Suppl. 1; 2011. Reprinted by permission of the American Diabetes Association.
Efficacy, Safety, and Tolerability of Insulin Degludec
Type 2 Diabetes Mellitus
Insulin degludec has been compared with insulin glargine in combination with oral glucose-lowering agents or in combination with a prandial insulin analog; one study investigated insulin degludec and insulin aspart in basal-bolus therapy in T2DM. In the basal-bolus treat-to-target trial, 992 patients with T2DM (mean A1C 8.3%) were randomized to receive insulin degludec or insulin glargine, each in combination with prandial insulin aspart ± metformin ± pioglitazone.15 Basal insulin was titrated to achieve a fasting plasma glucose (FPG) <90 mg/dL. At 1 year, mean A1C values were reduced by 1.1% and 1.2% with insulin degludec and insulin glargine, respectively (estimated treatment difference [ETD], 0.08%; 95% confidence interval (CI), –0.05 to 0.21). FPG was reduced by 41 and 36 mg/dL, respectively (ETD, –5.2 mg/dL; 95% CI, –11.7 to 1.1; P = non significant [NS]). Overall, the rates of confirmed hypoglycemia (plasma glucose <56 mg/dL or severe episodes requiring assistance) were lower in the group treated with insulin degludec than in the group treated with insulin glargine (11.1 vs 13.6 episodes/patient-year; estimated rate ratio [ERR], 0.82; 95% CI, 0.69 to 0.99; P = .0359). Nocturnal confirmed hypoglycemia, defined as episodes occurring between midnight and 6 am, occurred significantly less frequently in the insulin degludec group compared with the insulin glargine group (1.4 vs 1.8 episodes/patient-year, respectively; ERR, 0.75; 95% CI, 0.58 to 0.99; P = .0399) (FIGURE 2). Rates of other adverse events were similar between the 2 groups. At 1 year, the total mean daily insulin doses were 1.46 and 1.42 U/kg in the insulin degludec and insulin glargine groups, respectively, with a ~50:50 basal:bolus ratio for both groups.
Based on these findings, insulin degludec was associated with glycemic control similar to insulin glargine when given as basal-bolus therapy. Overall, confirmed and nocturnal hypoglycemia occurred less frequently with insulin degludec than with insulin glargine.
FIGURE 2
Incidences of nocturnal hypoglycemia with insulin degludec and insulin glargine15,16,18
Type 1 Diabetes Mellitus
Insulin degludec has been investigated in the treatment of patients with T1DM. Two randomized trials involved basal-bolus therapy in combination with insulin aspart. A 1-year treat-to-target trial in 629 adults with T1DM (mean A1C 7.7%) compared insulin degludec with insulin glargine, each given once daily in a basal-bolus regimen with mealtime insulin aspart.16 Both groups were reported to have improved glycemic control, with overall A1C decreased by 0.4%. Similar proportions of patients achieved A1C <7.0% with insulin degludec and insulin glargine (40% vs 43%; P = NS). Mean FPG values were reduced similarly (ETD, 5.9 mg/dL; P = .35). Compared with insulin glargine, rates of confirmed nocturnal hypoglycemia were 25% lower with insulin degludec (4.4 vs 5.9 episodes/patient-year; ERR, 0.75; 95% CI, 0.59 to 0.96; P = .021), whereas rates of overall confirmed hypoglycemia were similar between treatment groups (42.5 vs 40.2 episodes/patient-year; ERR, 1.07; 95% CI, 0.89 to 1.28; P = .48). Overall rates of other adverse events were similar between groups.
Insulin degludec in a fixed-ratio combined formulation with insulin aspart (IDegAsp) was compared with insulin detemir and insulin aspart in basal-bolus therapy in a 26-week, open-label, treat-to-target trial involving 548 patients with T1DM (mean A1C, 8.3%; mean FPG, 189 mg/dL at baseline).17 IDegAsp was given once daily at any meal, with insulin aspart at the remaining meals, whereas insulin detemir was administered according to approved labeling with mealtime insulin aspart at all meals. The mean decrease in A1C was similar for IDegAsp and insulin detemir/insulin aspart (0.73% vs 0.68%, respectively). The decrease in mean FPG was also similar between groups (P = .52). The mean total daily insulin doses were 69 U (0.86 U/kg) for IDegAsp and 79 U (1.00 U/kg) for insulin detemir and insulin aspart. Rates of severe hypoglycemia were 0.33 and 0.42 episodes/patient-year with IDegAsp and insulin detemir, respectively. Rates of overall confirmed hypoglycemia were similar (39 vs 44 episodes/patient-year; P = .27), whereas confirmed nocturnal hypoglycemia was reported significantly less frequently with IDegAsp (3.7 vs 5.7 episodes/patient-year, respectively; P = .0003). Weight increase was significantly greater (by 1.04 kg) with IDegAsp compared with insulin detemir (P = .0021). Overall rates of other adverse events were similar between treatment groups.