There are several things you should know about necessary vaccinations, and sometimes potential supply problems, if your families will be traveling internationally.
Yellow fever and vaccine supply
Yellow fever is caused by a Flavivirus transmitted by the bite of an infected mosquito. It occurs in sub-Saharan Africa and in tropical areas in South America. Multiple factors determine a traveler’s risk for acquisition, including destination, season, duration of potential exposure, activities, and the local transmission rate. The majority of those infected are asymptomatic or have minimal clinical symptoms. The incubation period is 3-6 days, which is then followed by an influenza-like illness. Approximately 15% of infected individuals develop more serious symptoms including jaundice, hemorrhagic symptoms, shock, and, ultimately, multiorgan system failure with a fatality rate of 90%. There is no specific treatment.
Prevention is avoidance of mosquito bites and receipt of yellow fever vaccine (YF-Vax). It is a relatively safe vaccine and indicated for use in persons at least 9 months of age. There are a few situations in which it can be administered to patients as young as 6 months. The vaccine becomes valid 10 days after administration, and it must be documented on an International Certificate of Vaccination or Prophylaxis card (Yellow Card).Previously, vaccine boosters were required every 10 years. However, the duration of immunity was extensively reviewed by the World Health Organization and effective July 11, 2016, boosters are no longer required. A single dose of vaccine is now valid for the lifetime of the individual. This includes those persons vaccinated prior to July 11, 2016. Since it is a live vaccine, administration is contraindicated in certain individuals. Exemption letters are provided for those who have a medical contraindication.
Caution is advised in persons receiving their initial dose of YF-VAX who are older than 60 years of age because they have an increased risk of serious side effects. This is not a concern for the pediatrician. The vaccine can only be administered at state approved facilities. It is one vaccine that is not only recommended, but may be required for entry into certain countries. Go to www.cdc.gov/yellowfever for a complete list.
Sanofi Pasteur is the only U.S. manufacturer of YF-VAX. Production has ceased until mid-2018, when a new manufacturing facility will open. Current supplies are anticipated to be depleted by mid-2017, and orders have been limited to 5 doses per month. Sanofi Pasteur, in conjunction with the Food and Drug Administration, will make Stamaril – a yellow fever vaccine manufactured by the company in France and licensed in over 70 countries – available to U.S. travelers through an Expanded Access Investigational New Drug Application. Details on how and when this program will be operational are forthcoming. What is known is that, nationwide, there will be a limited number of sites administering Stamaril. Once finalized, a list of locations will be posted on the CDC Yellow Fever site.
How does this affect your patients? If travel to a yellow fever risk area is anticipated, they should not delay in seeking pretravel advice and immunizations until the last minute. Individual clinic inventories will not be stable. Postponing a trip or changing destinations is preferred if the vaccine is not available. Yellow fever exemption letters are only provided for those persons who have a medical contraindication to receive YF-VAX.
Zika, dengue, and chikungunya
These three Flaviviruses all are transmitted by mosquitoes and can present with fever, rash, and headache. Their distribution is overlapping in several parts of the world. Most infected people are asymptomatic. If symptoms develop, they usually are self-limited. Disease prevention is by mosquito avoidance. There are no preventive vaccines.
Zika virus is the only one associated with a congenital syndrome. It is characterized by brain abnormalities with or without microcephaly, neural tube defects, and ocular abnormalities.
Guidelines for the evaluation and management of Zika virus–exposed infants were initially published in January, 2016, with the most recent update published in August 2016 (MMWR Morb Mortal Wkly Rep. 2016 Aug 26;65[33]:870-8).
Preliminary data from the U.S. Zika pregnancy registry of 442 completed pregnancies between Jan. 15 to Sept. 22, 2016, identified birth defects in 26 fetuses/ infants (6%). There were 21 infants with birth defects among 395 live births and 5 fetuses with birth defects among 47 pregnancy losses. Birth defects were reported for 16 of 271 (6%) asymptomatic and 10 of 167 (6%) symptomatic women. There were no birth defects in infants when exposure occurred after the first trimester. Of the 26 affected infants, 4 had microcephaly and no neuroimaging and 3 (12%) had no fetal or infant testing. Approximately 41% (82/442) of infants did not have Zika virus testing (JAMA. 2017 Jan 3;317[1]:59-68).
It is unclear why testing was not performed. One concern is that the pediatrician may not have been aware of the maternal Zika virus exposure or test results. It may behoove us to begin asking questions about parental international travel to provide optimal management for our patients. We also should be familiar with the current guidelines for evaluating any potentially exposed infants, which include postnatal neuroimaging, Zika virus testing, a comprehensive newborn examination including neurologic exam, and a standard newborn hearing screen prior to hospital discharge.
Regardless of maternal Zika virus test results, infants with any clinical findings suggestive of congenital Zika virus syndrome and possible maternal exposure based on epidemiologic link also should be tested. Zika virus travel alerts and the most up to date information can be found on the Centers for Disease Control and Prevention website (www. cdc.gov/Zika).
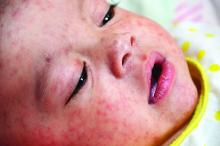
Measles
Although endemic measles was eliminated in the United States in 2000, it is still common in many countries in Europe, Africa, and the Pacific. Most cases in the United States occur in unvaccinated individuals, with 78 cases reported in 2016. As of March 25, 2017, 28 cases have been reported. At least 10 countries – including Belgium, France, Italy, Germany, Portugal, and Thailand – have reported outbreaks of measles since April 2017. As reminder, all children aged 6-11 months should receive one dose of MMR and those 12 months or older should receive two doses of MMR at least 28 days apart if international travel is planned. Adults born after 1956 also should have received two doses of MMR prior to international travel.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She reported having no relevant financial disclosures.