WASHINGTON – Sometimes, the hardest part of solving a problem is figuring out how to work around misaligned resources, and so it has been with sickle cell disease research.
“From my point of view, what I call the geographical disparity in sickle cell disease research can be explained by the fact that the majority of affected individuals are living in the East, and the overwhelming majority of the research takes place in the West,” said Solomon Ofori-Acquah, PhD. He and three physician collaborators from Ghana shared their roadmap to conducting clinical trials in West Africa during an “East Meets West” session of the annual symposium of the Foundation for Sickle Cell Disease Research.
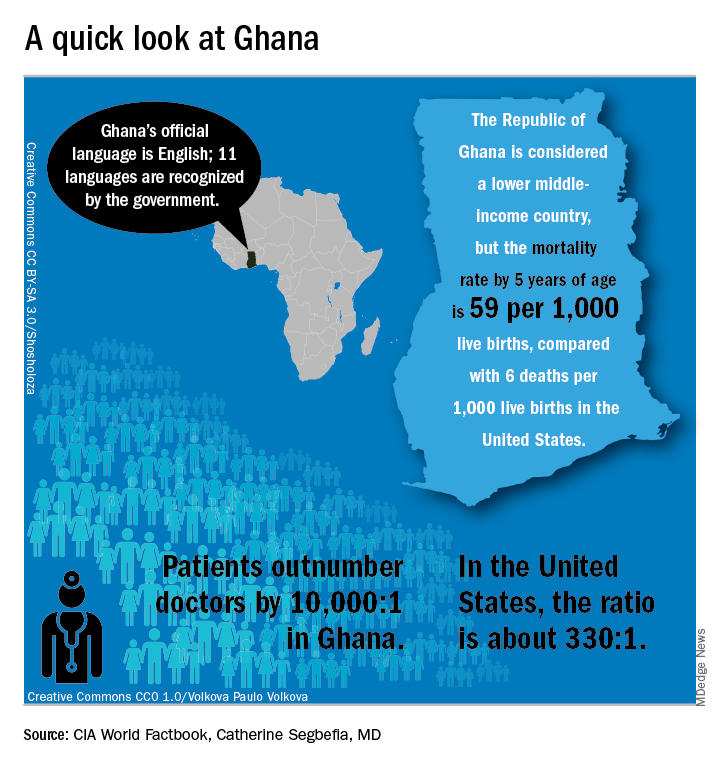
In Ghana, not far from where scientists now believe the hemoglobin sickling mutation originated, fully 2% of newborns have SCD; this translates into 16,000 new cases per year in a population of just 28 million, compared with the 2,000 new SCD cases seen annually in the entire United States. And access even to proven therapies can be limited; historically, little to no clinical drug development work has been conducted in this part of the world.
In the United States, half of the SCD trials that were withdrawn or terminated listed recruitment and retention of study participants as a factor in the study’s discontinuation, said Amma Owusu-Ansah, MD. “I see what we are doing as a very feasible solution to the problem of inadequate accrual to studies in the U.S.,” said Dr. Owusu-Ansah, a hematologist at the University of Pittsburgh’s Center for Translational and International Hematology (CTIH), where she serves as clinical director.
From the African perspective, hosting clinical trials – and building a robust infrastructure to do so – may help alleviate the delay in translation of disease-modifying therapies for SCD to Africa, where most people with the disease live, she said.
An existing example of resource sharing is the Human Heredity & Health in Africa (H3Africa) initiative, said Dr. Ofori-Acquah, who directs the CTIH and also holds an appointment at the University of Ghana. The project, funded by the National Institutes of Health and the Wellcome Trust, “aims to facilitate a contemporary research approach to the study of genomics and environmental determinants of common diseases with the goal of improving the health of African populations,” according to the H3Africa website. Within this framework of 40 research centers conducting genomics research and biobanking, several discrete projects aim to expand knowledge of sickle cell disease.
“All of these networks are going to study thousands of patients,” said Dr. Ofori-Acquah. “I see the H3 as a mechanism to accelerate genomics research in sickle cell disease.”