The prevalence of allergic disease in the general population is quite high; 8.3% of adults and children have asthma and 11.4% of children have skin allergies.1 Food allergies are present in 8% of children and 5% of adults,2 and up to 10% of anaphylactic reactions in the United States are due to stinging insects.3
Moderate-to-severe food and environmental allergies can negatively affect multiple organ systems and significantly impact morbidity and mortality.4 Quality of life and the financial well-being of patients with allergic diseases, as well as that of their families, can also be significantly impacted by these conditions.4,5 High prevalence and burden of disease mandate that family physicians (FPs) stay up-to-date on the full array of treatment options for allergic diseases. What follows are 6 common questions about allergy immunotherapy (AIT) and the evidence-based answers that will help you to identify and treat appropriate candidates, as well as educate them along the way.
Who is a candidate for AIT?
Patients with moderate-to-severe immunoglobulin (Ig)E-mediated allergies whose symptoms are not adequately controlled by medications and allergen trigger avoidance are candidates for AIT.6-8 Skin prick/puncture testing provides the most reliable and cost-effective confirmation of allergies that are suspected, based on patient history and clinical assessment for allergic symptoms.9 Life-threatening reactions to skin prick/puncture testing are rare.9 While in vitro (laboratory) testing for IgE levels to specific antigens may be more convenient for patients and less invasive than skin prick/puncture testing, it is also less sensitive and less reliable at quantifying the severity of sensitization.9
What constitutes AIT?
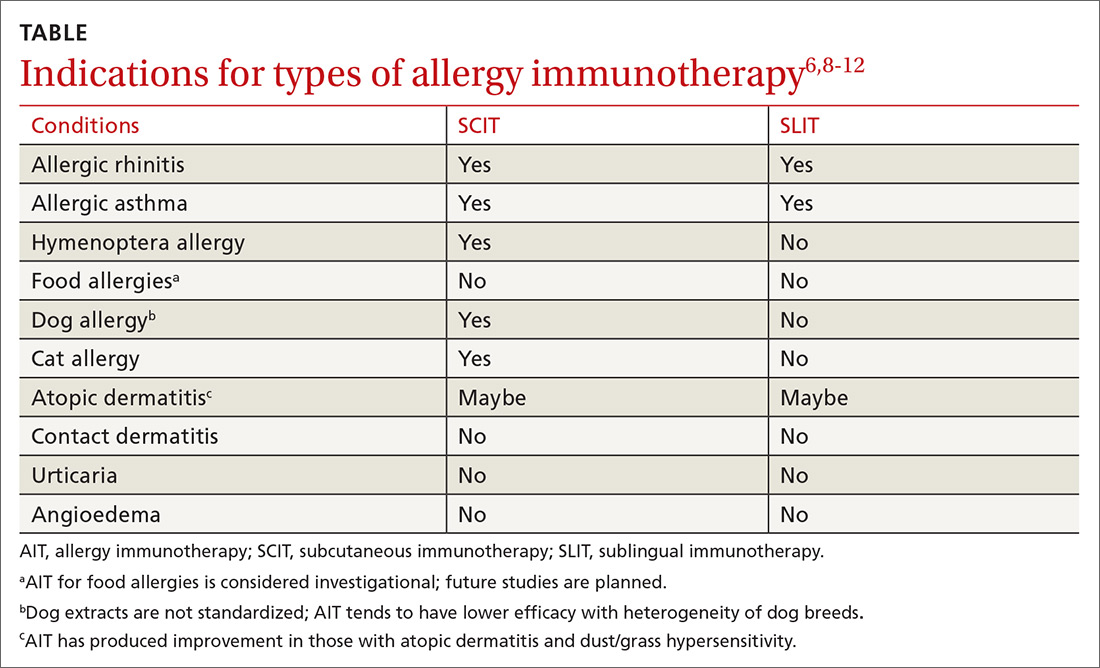
AIT is a disease-modifying treatment that, along with allergen avoidance, can provide long-term remission of allergic disease in certain circumstances.6,7 Consistent gradual exposure to an allergen helps to dampen the inflammatory reaction driven by T cells and B cells, producing clinical tolerance or desensitization that persists after the discontinuation of AIT.8 While subcutaneous immunotherapy (SCIT) is the most widely known type of AIT (ie, allergy shots), there are additional ways that AIT can be administered. These include sublingual immunotherapy (SLIT), venom immunotherapy (VIT), and oral immunotherapy (OIT). The selection of the route of administration depends on the exact nature and symptoms of the allergic condition being treated (TABLE6,8-12).
AIT involves 2 phases
The first phase is the induction or buildup phase during which patients are given gradually increasing amounts of allergen to induce a protective immunologic response.6 After 8 to 28 weeks, the maintenance phase begins, during which continued, consistent allergen exposure is designed to prevent relapse of, and facilitate continued remission of, allergy symptoms.6 The maintenance phase of AIT can last 24 to 48 months.6,10 Certain patients may qualify for an expedited AIT regimen called cluster or rush immunotherapy.6
Conventional schedules for AIT involve increasing the dose of allergen given at each visit (1-3 doses/wk), whereas rush dosing involves multiple, increasing doses given in a single extended visit to reach therapeutic desensitization faster.6 AIT has been shown to produce a 2.7- to 13.7-fold overall improvement in hypersensitivity reactions.10
Length of therapy must be individualized
Experts recommend that the length of treatment with AIT be customized for each patient based on the severity of pretreatment allergy symptoms, the benefit experienced with AIT, the inconvenience of AIT to the patient, and the anticipated impact of symptom relapse.6,10 There are no physiologic symptoms or objective tests that predict which patients will remain in remission after discontinuing AIT; thus, a joint task force of allergy experts suggests that the decision to restart AIT in patients who have a relapse in allergic symptoms should be made based on the same factors used to determine the duration of the maintenance phase.6
Continue to: These allergans are appropriate for AIT