Effects on guidelines
The intravenous iron recommendations in the European HF guidelines refer only to ferric carboxymaltose without mentioning other forms, such as ferric derisomaltose, “but this is now a class effect given the similarities between AFFIRM-AHF and IRONMAN,” said Gregory D. Lewis, MD, Mass General Brigham, Boston, invited discussant for Dr. Kalra’s presentation at the AHA session.
“In the United States, we relegate IV iron to improvement in functional capacity as a comorbidity of heart failure. Perhaps this role will expand,” added Dr. Lewis, who is medical director of his center’s heart transplant program.
He also wondered aloud whether the purported clinical benefits of intravenous iron in HF patients with iron deficiency, not as yet supported by a significant primary-endpoint showing in one of the major trials, currently justify expansion of its use in practice.
“With the benefits of IV iron on exercise capacity and quality of life, and the safety of administering high doses of IV iron,” potentially reducing HF polypharmacy, he noted, “should we be considering IV iron more commonly for utilization in our patients even if we find that heart failure hospitalizations and mortality are only modestly improved?”
IRONMAN “asked whether there’s benefit to IV iron in the longer term,” Kiran Musunuru, MD, PhD, MPH, University of Pennsylvania,Philadelphia, observed at the media briefing. As the trial was reported, “that does in fact, seem to be the case,” said Dr. Musunuru, who was not involved in IRONMAN.
Therefore, he said, “this study reinforces the message that we should be routinely monitoring our heart failure patients for iron deficiency and supplementing them as needed.”
A commentary linked to the IRONMAN publication agreed. The trial “increases the evidence base for the treatment of iron deficiency with intravenous iron supplementation,” wrote the editorialists, led by Theresa A. McDonagh, MD, King’s College Hospital and School of Cardiovascular Sciences, London.
Patients with acute or chronic HF, iron deficiency, and reduced or mildly reduced ejection fractions “should be offered treatment with intravenous iron to reduce their risk of hospital admission for heart failure,” they concluded.
Mostly reduced-EF outpatients
The open-label, blinded-endpoint IRONMAN trial, conducted at 70 centers in the United Kingdom, entered adults with HF, ejection fractions 45% or lower within the previous 2 years, and iron deficiency defined as transferrin saturation less than 20% or serum ferritin levels below 100 mcg/L, the report states. They were either hospitalized for HF, had such a hospitalization within the past 6 months, or were outpatients with elevated natriuretic peptide levels; the third category accounted for two thirds of the trial population.
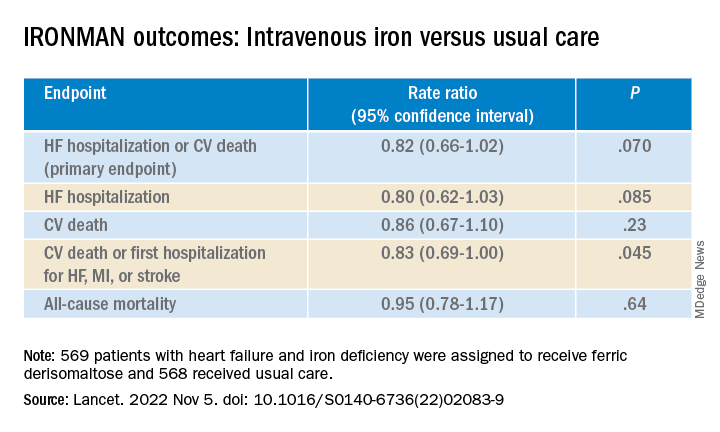
Of the 1,137 randomized patients, 569 were assigned to receive intravenous ferric derisomaltose at weight- and hemoglobin-adjusted dosages; 568 went to the usual-care group.
Those receiving intravenous iron visited the trial clinic 4 weeks later and then every 4 months. At those visits, they received a round of ferric derisomaltose if their ferritin levels were below 100 mcg/L, or 400 mcg/L or lower if transferrin saturation was below 25%, the published report states.
Mean scores on the Minnesota Living with Heart Failure Questionnaire improved by a marginally significant 3.33 points (P = .050) at 4 months in the intravenous iron group. The gain receded to a nonsignificant 2.57 points by 20 months (P = .23).
In COVID-related sensitivity analysis, the intravenous iron group showed a significant benefit for the primary endpoint and a trend for improved HF hospitalizations.
- HF hospitalization or CV death: RR, 0.76 (95% confidence interval, 0.58-1.00; P = .047)
- HF hospitalization: RR 0.76 (95% CI, 0.56-1.03; P = .077)
Fewer patients in the intravenous iron group experienced serious cardiac adverse events, 36% compared with 43% in for those on usual care, P = .016.
The recently updated European Society of Cardiology guidelines for HF made it a class 1 recommendation to assess iron status in every patient, Kalra observed. “It doesn›t specify how frequently, but I think we should be thinking about every 4-6 months.”
Dr. Kalra disclosed receiving research grants from Pharmacosmos; and consulting or lecturing for Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Novartis, Pfizer, Pharmacosmos, Servier, and Vifor Pharma. Dr. Musunuru disclosed significant ownership interest in Verve Therapeutics and Variant Bio. Dr. Lewis disclosed relationships with NXT, American Regent, and RIVUS; and receiving research grants from Cytokinetics and Amgen.
A version of this article first appeared on Medscape.com.