RSV vaccine in pregnancy
In August, the FDA approved Pfizer’s RSVpreF vaccine for use during pregnancy—as a single dose given at 32 to 36 weeks’ gestation—for the prevention of RSV LRTD in infants in the first 6 months of life. In the clinical trials, the vaccine was given at 24 to 36 weeks’ gestation. However, there was a statistically nonsignificant increase in preterm births in the RSVpreF group compared to the placebo group.6 While there were insufficient data to prove or rule out a causal relationship, the FDA advisory committee was more comfortable approving the vaccine for use only later in pregnancy, to avoid the possibility of very early preterm births after vaccination. The ACIP agreed.
From time of maternal vaccination, at least 14 days are needed to develop and transfer maternal antibodies across the placenta to protect the infant. Therefore, infants born less than 14 days after maternal vaccination should be considered unprotected.
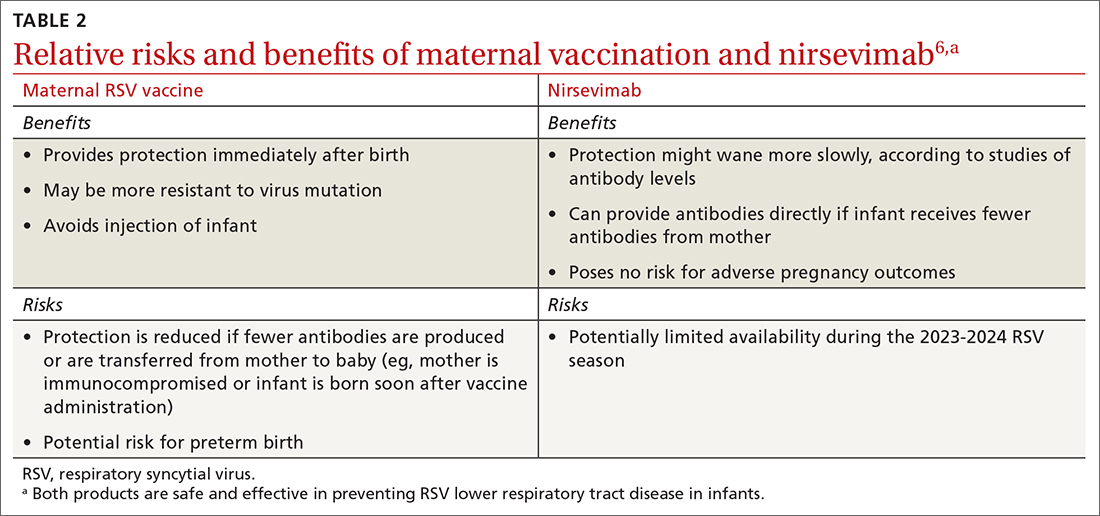
Both maternal vaccination with RSVpreF and infant injection with nirsevimab are now options to protect newborns and infants from RSV. However, use of both products is not needed, since combined they do not offer significant added protection compared to either product alone (exceptions to be discussed shortly).6 When the estimated due date will occur in the RSV season, maternity clinicians should provide information on both products and assist the mother in deciding whether to be vaccinated or rely on administration of nirsevimab to the infant after birth. The benefits and risks of these 2 options are listed in TABLE 2.6
There are some rare situations in which use of both products is recommended, and they include6:
- When the baby is born less than 14 days from the time of maternal vaccination
- When the mother has a condition that could produce an inadequate response to the vaccine
- When the infant has had cardiopulmonary bypass, which would lead to loss of maternal antibodies
- When the infant has severe disease placing them at increased risk for severe RSV.
Conclusion
All of these new RSV preventive products should soon be widely available and covered with no out-of-pocket expense by commercial and government payers. The exception might be nirsevimab—because of the time needed to produce it, it might not be universally available in the 2023-2024 season.