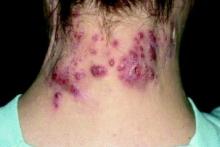
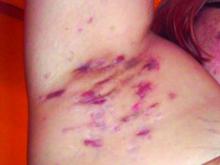
at the completion of treatment, a retrospective study showed.
“These findings suggest a course of 12 to 16 weeks of ertapenem may be appropriate as a new standard length of therapy in HS patients, which is at least twice the current recommendation of the North American treatment guidelines,” wrote corresponding author Steven R. Cohen, MD, MPH, of the departments of dermatology at Weill Cornell Medicine and Albert Einstein College of Medicine, New York, and his coauthors. The results were published online February 14, 2024, in JAMA Dermatology.
In an earlier study , some of the same researchers evaluated the efficacy of daily IV ertapenem for 6 weeks in seven patients with HS. The patients experienced “notable remediation of disease that was rapidly lost within 1 month of withdrawal.”
Treatment guidelines published in 2019 recommend ertapenem as a highly effective third-line therapy limited to one 6-week course “as rescue therapy or during surgical planning, given the practical barriers to home infusions and concerns about antibiotic resistance” .
For the current analysis, Dr. Cohen and colleagues explored the effects of a longer duration of treatment with ertapenem in this patient population. They retrospectively reviewed the medical records of 98 patients with HS who received care at Albert Einstein College of Medicine’s Montefiore HS Center between 2018 and 2022. Each patient used an elastomeric pump to self-administer 1 g IV ertapenem daily for 12-16 weeks.
Key outcome measures of interest were the HS Physician Global Assessment (PGA) score (a 6-point scale ranging from clear to very severe) and a numerical rating scale (NRS) for pain (an 11-point scale in which a score of 0 indicates no pain and a score of 10 indicates the worst possible pain) and markers of inflammation such as leukocytes, erythrocyte sedimentation rate, C-reactive protein (CRP), and interleukin (IL)-6. The researchers measured these outcomes at baseline, the midcourse of IV ertapenem treatment, at the end of the course, and post therapy.
The mean age of the patients was 35.8 years, 62.2% were female, and 60.2% were Black. The mean treatment duration was 13.1 weeks and the mean posttherapy follow-up occurred after a mean of 7.8 weeks.
Between baseline and posttherapy follow-up, the HS PGA scores dropped from a mean of 3.9 to 2.7 and the NRS for pain dropped from 4.2 to 1.8 (P < .001 for both associations). Markers of inflammation also dropped between baseline and post therapy.
Specifically, values for CRP dropped from 5.4 to 2.4 mg/dL; IL-6 dropped from 25.2 to 13.7, and leukocytes dropped from 11.3 to 10.0 (P < .001 for all associations). Among the 76 patients who participated in a follow-up telephone survey, 63 (80.3%) reported medium to high satisfaction with their course of ertapenem, and 69 (90.8%) said they would recommend the treatment to other patients with HS.
The authors noted certain limitations of their study, including its retrospective, single-center design, the lack of a control group, and the fact that the HS-PGA scores at each visit did not meet the threshold of a 2-point decrease that is considered a clinically meaningful in the medical literature.
The definitive mechanism of ertapenem efficacy remains elusive, the authors pointed out. “Although oral antibiotics are generally accepted as a core therapeutic approach to HS, much less is known about the efficacy of IV antibiotics, especially ertapenem, a parenteral carbapenem possessing activity against many gram-positive bacteria, gram-negative bacteria, and anaerobic organisms,” they wrote.
In an accompanying editorial, Haley B. Naik, MD, MHSc, a dermatologist at the University of California, San Francisco, said that adopting prolonged courses of ertapenem treatment “comes with substantial individual and public health considerations”.
“Even though HS is a noninfectious disease, microbes might play a role in inciting HS immune dysregulation, prompting the inclusion of antimicrobial therapy in treatment regimens. However, broad-spectrum antibiotics for HS are associated with high levels of antibiotic resistance,” she wrote. Prolonged use of ertapenem and other carbapenems in HS treatment “will likely increase antimicrobial resistance, thereby limiting management of both HS and comorbid infections.”
Jennifer L. Hsiao, MD, a dermatologist who directs the HS clinic at the University of Southern California, Los Angeles, who was asked to comment on the study, said that, despite significant advances in the management of HS over the past decade, there are still patients who do not respond adequately to standard treatments.
For these patients, IV ertapenem can serve as a valuable bridge to a longer-term therapeutic option, “be it surgery or escalated immunomodulation,” such as dual biologic therapy, she said. “In my personal experience, IV ertapenem, which like the authors I also typically use for a 12-week course, delivers impressive and fast results even in the worst disease cases.
“It can be difficult to maintain the therapeutic benefit of ertapenem after it is discontinued, which is why patients should be on concomitant medications as they were in this study and have a post-ertapenem treatment plan in place,” said Dr. Hsiao, who was not involved with the study. “Hopefully, we will be able to one day understand why ertapenem is so effective for HS and be able to harness that benefit for patients without concern for antimicrobial resistance.”
Dr. Cohen reported receiving personal fees from Verrica Pharmaceuticals and belonging to the Board of Trustees of the American Skin Association outside the submitted work. No other disclosures were reported. Dr. Naik reported having received grants from AbbVie and the National Institutes of Health; personal fees from Novartis, UCB, Boehringer Ingelheim, 23andMe, Aristea Therapeutics, Medscape, Sonoma Biotherapeutics, DAVA Oncology, and Pfizer; and shares from Radera during the conduct of the study. She is a board member of the Hidradenitis Suppurativa Foundation. Dr. Hsiao disclosed that she is a member of the board of directors for the Hidradenitis Suppurativa Foundation. She has served as a consultant for AbbVie, Aclaris, Boehringer Ingelheim, Incyte, Novartis, UCB, as a speaker for AbbVie, Novartis, and UCB, and as an investigator for Amgen, Boehringer Ingelheim, and Incyte.