GAITHERSBURG, MD. – A portable, battery-powered device that delivers electrical fields to the brain has a favorable risk-benefit profile in patients who have used up other treatment options for recurrent glioblastoma multiforme, according to a Food and Drug Administration advisory committee.
The FDA’s Neurological Devices Advisory Panel voted 7-3 with two abstentions on March 17 that the benefits of the NovoTTF-100A system exceed its risks when used as monotherapy in adults who have exhausted surgical and radiation treatment options for histologically or radiologically confirmed recurrent glioblastoma multiforme (GBM).
The device’s manufacturer, Israel-based Novocure Ltd., is seeking an indication in this population based on the results of a company-sponsored prospective randomized trial that compared treatment with the device to best standard of care (BSC) chemotherapy in 237 patients with recurrent GBM.
Panelists emphasized that the indication should reflect that patients have also failed chemotherapy options, and that while the treatment appears to be safe short term, the potential for chronic adverse effects should be monitored closely.
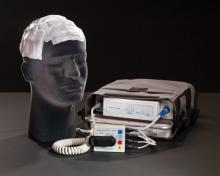
The device weighs about 6 pounds and is intended to be worn about 20 hours per day. It delivers "tumor treating fields" (TTF) via four electrodes placed on the scalp. This creates "a low intensity, alternating electric field within the tumor that exerts physical forces on electrically charged cellular components, preventing the normal mitotic process and causing cancer cell death prior to division," according to the company. Normal cells are not affected, it said.
In the pivotal study, median overall survival, the primary end point, was 6.3 months among those treated with the device and 6.4 months among those treated with the best available chemotherapy. Investigators have presented subgroup analyses showing better survival in some populations.
Both the FDA and the panelists had concerns about methodologic problems in the study, which was designed to show that TTF therapy is superior to the best standard of care chemotherapy. But those voting positively cited the safety of the device, and evidence that it benefited some patients with few treatment options. Median survival of patients diagnosed with GBM is 15 months, and the 5-year overall survival rate is less than 10%, according to data cited by the company and the FDA.
NovoCure is conducting a phase III study of the device in patients with newly diagnosed GBM, in combination with temozolomide. The device has been approved in the European Union as a treatment for newly diagnosed and recurrent GBM and for the treatment of non–small cell lung cancer.
The FDA usually follows the recommendations of its advisory committees. Panel members have been cleared of potential conflicts of interest by the FDA before meetings.