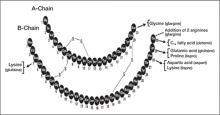
FIGURE 1
Modifications of human insulin to make insulin analogs
Arrows denote substitution; dashed line denotes addition
TABLE 1
Insulins commonly used in the United States18-23
| Generic | Brand | Form | Time of action (h) | ||
|---|---|---|---|---|---|
| Onset | Peak | Duration | |||
| Bolus or prandial insulin | |||||
| Rapid-acting | |||||
| Aspart | Novolog | Analog | < 0.25 | 1-3 | 3-5 |
| Glulisine | Apidra | Analog | < 0.25 | 1-2 | 3-4 |
| Lispro | Humalog | Analog | < 0.25 | 1-2 | 3-4 |
| Short-acting | |||||
| Regular | Humulin R; Novolin R | Human | 0.5-1 | 2-3 | 3-6 |
| Basal insulin | |||||
| Intermediate-acting | |||||
| NPH | Humulin N; Novolin N | Human | 2-4 | 4-10 | 10-16 |
| Long-acting | |||||
| Detemir | Levemir | Analog | 1-2 | Relatively flat | ≤ 24 |
| Glargine | Lantus | Analog | 1-2 | Relatively flat | ≤ 24 |
| NPH, neutral protamine Hagedorn. | |||||
Rapid-Acting Insulin Analogs
The pharmacokinetic and pharmacodynamic profiles of the rapid-acting insulin analogs have been compared with those of short-acting regular human insulin. Many of those investigations have used the euglycemic clamp technique, which allows for the assessment of insulin absorption and insulin activity through simultaneous intravenous infusion of insulin and glucose to maintain a consistent glucose level, with close monitoring of blood glucose levels. Investigations have generally not measured the onset of biologic activity directly but have measured surrogate markers, such as the time to maximum plasma concentration (tmax). One comparison reported a tmax of 70 minutes for insulin aspart compared with 129 minutes for regular human insulin, and 42 minutes for insulin lispro compared with 101 minutes for regular human insulin.24,25
Onset of activity, duration of activity, and glucose-lowering effect are dependent on absorption of the insulin molecules from the injection site. Variability in absorption has been a limitation of some insulins, but variability is lower with the rapid-acting insulin analogs. The variability of tmax between injections in the same patient with insulin aspart and regular human insulin has been reported to be 15% and 24% (P < .05), respectively. The respective variability of tmax between individuals was 20% and 37% (P < .001).24 Greater variability in tmax may contribute to greater variability in blood glucose levels as well as risk of hypoglycemia.
The shorter onset of action of the rapid-acting insulin analogs more closely mimics the postprandial physiologic profile of endogenous insulin secretion and activity relative to regular human insulin. Thus it would be expected that the rapid-acting insulin analogs may be administered within 15 minutes of a meal compared with the necessary 30 minutes with regular human insulin. The shorter preprandial administration time with the rapid-acting insulin analogs may improve patient-perceived convenience. Treatment outcomes may also be improved due to less potential for insulin administration to be followed by a missed or incompletely eaten meal.
Because the rapid-acting insulin analogs are more physiologically similar to endogenous insulin and provide a more rapid onset and time to peak activity relative to regular human insulin, the frequency of severe hypoglycemia observed with the rapid-acting insulin analogs after meals may be reduced.26 A Cochrane review of 49 randomized controlled studies reported that the incidence of severe hypoglycemia with rapid-acting insulin analogs was approximately half that of regular human insulin in patients with T1DM (median, 21.8 vs 46.1 episodes/100 patient-years, respectively) and one fifth that in patients with T2DM (median, 0.3 vs 1.4 episodes/100 patient-years, respectively). However, the review also reported that the incidence of all hypoglycemic episodes with the rapid-acting insulin analogs was similar to that with regular human insulin, with similar glycemic control.27 This finding contradicts our clinical experience which suggests that the incidence of hypoglycemia is lower with the rapid-acting insulin analogs compared with regular human insulin.
Basal Insulin Analogs
Approved in 2000, insulin glargine was the first basal insulin analog to become available in the United States. Insulin detemir was subsequently approved in 2005. Insulin glargine is formulated in an acidic solvent with pH 4.0 that forms stable hexamers following subcutaneous injection. For insulin detemir, modification of the insulin structure to include a long-chain fatty acid facilitates self-association and binding to serum albumin.28 Through these different mechanisms, both insulin detemir and insulin glargine are slowly absorbed following subcutaneous administration, such that they have a longer duration of action than does NPH insulin and a relatively flat time-concentration profile.
Also using the euglycemic clamp technique, the pharmacokinetic and pharmacodynamic properties of insulin detemir and insulin glargine were compared with those of NPH insulin in patients with T1DM or T2DM.29-32 One study was a head-to-head comparison of insulin detemir, insulin glargine, and NPH insulin in 54 patients with T1DM.32 Over the 24-hour period following the administration of 4 single subcutaneous doses of 0.4 U/kg, the time-action profiles (ie, the glucose infusion rates over time) of insulin detemir and insulin glargine were reported to be relatively flat, whereas that of NPH insulin had a more pronounced peak (FIGURE 2).32
FIGURE 2
Individual time-action profiles (glucose infusion rates over time) of patients randomized to (A) insulin detemir, (B) NPH insulin, or (C) insulin glargine. The 4 euglycemic clamps in one subject are summarized in one plot32