Common adverse effects of MTX, such as nausea, vomiting, stomatitis, and fatigue, may be minimized by IM or SC administration, splitting the dose, or providing folate supplementation.21-24 Given in doses of 1 to 5 mg/d, folate may reduce adverse hematologic, gastrointestinal, and hepatic effects without decreasing efficacy.22
The major severe toxicities are myelosuppression, hepatotoxicity, and pulmonary fibrosis.24 MTX-induced hepatotoxicity is similar to nonalcoholic fatty liver disease (NAFLD) and is thought to exacerbate preexisting NAFLD, which is common in patients with metabolic syndrome. A liver biopsy or serum assays for liver fibrosis (amino-terminal peptide of pro-collagen III) may be warranted during therapy.24
MTX is an abortifacient and teratogen, so contraception during treatment and for up to 3 months thereafter is mandatory for women of childbearing age.26 Men should be advised that MTX decreases sperm count. (For more on methotrexate, see: “When a fetus survives methotrexate exposure,” at http://www.jfponline.com/Pages.asp?AID=10299).
Cyclosporine (CSA), an oral calcineurin inhibitor, is a potent immunosuppressant that rapidly clears psoriasis.27 Because duration of use correlates with permanent nephrotoxicity, hypertension, and potential increased risk of SCC and lymphoma, intermittent 12-week courses are recommended. Calcium channel blockers are the preferred treatment for CSA-induced hypertension because of their effect on smooth muscle vasodilation.21
Oral retinoids. Acitretin modulates epidermal proliferation and is anti-inflammatory. Because it lacks immunosuppression, acitretin is generally considered the treatment of choice in HIV patients with severe psoriasis.28 Acitretin is teratogenic and contraindicated in women who plan to become pregnant or who are unwilling to use adequate contraception for 3 years after discontinuing the drug.21
CASE Given the significant percentage of body surface area involved and symptoms consistent with psoriatic arthritis, Tom required an aggressive therapeutic regimen. His history of nonalcoholic fatty liver and social drinking precluded the use of methotrexate. A biologic therapy was the next therapeutic choice that could relieve both his cutaneous and joint symptoms.
FIGURE
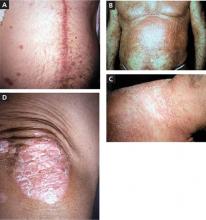
4 psoriasis patients, 4 different presentations
A. The linear erythematous, scaly plaque along this patient’s cardiac bypass scar demonstrates koebnerization of plaque psoriasis.
B. Diffuse erythematous scaly papules coalesce into plaques on this patient’s anterior chest, arms, abdomen, and periumbilicus.
C. The pinpoint pustules on an erythematous base on this patient’s lateral neck, shoulders, and upper back are characteristic of pustular psoriasis.
D. This patient has erythematous plaques with overlying silvery scale on the elbow—a classic
TABLE 2
Traditional systemic therapy21-24
| Methotrexate (MTX) | Cyclosporine (CSA)* | Acitretin | |
|---|---|---|---|
| Dosing | ≤30 mg in one weekly dose (PO, IM, or SC) | 2.5-5.0 mg/kg/d in 2 divided doses for 12 wk, then 12-wk nontreatment period Dose decreased (by 0.5-1.0 mg/kg) with disease clearance or when hypertension or nephrotoxicity are detected | 10-50 mg/d given as a single dose Lower doses (25 mg/d) used to minimize adverse effects and in combination regimens When added to UV, light dose should be reduced 30%-50% |
| Contraindications |
|
|
|
| Baseline monitoring† |
|
|
|
| BP, blood pressure; BUN, blood urea nitrogen; CBC, complete blood cell count; CR, creatinine; H&P, history and physical; IM, intramuscular; LFTs, liver function tests; PO, by mouth; PPD, purified protein derivative; PUVA, psoralen and ultraviolet A; SC, subcutaneous; UV, ultraviolet; UVB, ultraviolet B. *Avoid live vaccinations; caution required with major infection and poorly controlled diabetes. †Ongoing monitoring for MTX: BUN, CBC, CR, LFTs; possible liver biopsy (for high-risk patients or cumulative dose >3.5-4 g); CSA: BP, BUN, CBC, CR, LFTs; lipd profile; magnesium, uric acid, potassium tests; pregnancy testing; Acitretin: CBC, LFTs, lipid profile, renal function test, pregnancy testing. | |||
Biologics require lab work and a detailed medication list
Before beginning biologic therapy for a patient, the National Psoriasis Foundation29 recommends obtaining a complete history, physical, medication list, future plans (ie, pregnancy or travel to locations requiring vaccinations), and baseline labs to identify possible risk factors and/or contraindications. Periodic evaluation to monitor development of new symptoms, including infection and malignancy ( TABLE 3 ),24,30-33 is needed, as well.