Continuous use regimen. A randomized controlled trial evaluating the frequency and management of breakthrough bleeding with continuous use of the transvaginal contraceptive ring reported a reduction in bleeding, flow, and pelvic pain, and a high continuation rate. Most patients considered the bleeding profile with the continuous vaginal ring acceptable compared with the baseline 21/7 use.22 Each ring contains up to a 28-day supply of hormones.
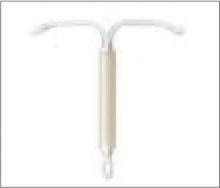
LNG-IUS (Mirena)
Fewer interactions. Transvaginal absorption of hormones with the vaginal ring avoids a first pass through the liver, thus decreasing many medication interactions. Irregular bleeding experienced with OCs or the patch may be effectively reduced with the steadily released, rapidly acting hormones in the ring.23
Intrauterine contraception
Intrauterine contraception is increasingly accepted by women who want long-term and effective contraception without having to comply with a particular regimen.
Copper IUD: Many contraindications are lifted
The nonhormonal IUD ParaGard T 380A is indicated for contraception for up to 10 years in women who are 16 years of age and older.24 This IUD’s active ingredient is the spermicidal copper wire wound around the short arms of the device. A recent meta-analysis reported an association between the use of a copper IUD and a decrease in the risk of endometrial cancer (OR=0.39; 95% CI, 0.29-0.51), though the mechanism for this association is unclear.25
In late 2005, the FDA broadened the use of copper IUDs to include women who are nulliparous; have a history of pelvic inflammatory disease (PID), sexually transmitted disease, or ectopic pregnancy; are in nonmonogamous relationships; or have a history of premenopausal breast cancer. This method also may be used by women with asymptomatic human immunodeficiency virus infection, Actinomyces infection, abnormal Papanicolaou (Pap) test results, or vaginitis. Furthermore, data support its use in adolescents who are at particularly high risk for unintended pregnancy.26
The copper IUD remains contra-indicated for patients with acute PID or current high-risk behavior for sexually transmitted infections, as well as for those who have mucopurulent cervicitis or have had postpartum endometritis within the past 3 months.24 Wilson’s disease is also a contraindication.
Insertion tip. Misoprostol may be used to soften the nulliparous cervix for insertion.27
Levonorgestrel intrauterine system: An alternative to the copper IUD
The levonorgestrel intrauterine system (LNG IUS), sold under the brand name Mirena, is gaining tremendous popularity in the United States. Multiple mechanisms of action, including endometrial thinning, cervical mucus thickening, inhibition of sperm function, and intermittent ovulation suppression are responsible for the >99% efficacy of this 5-year contraceptive.
Irregular menses can be expected initially, and 20% of patients reported amenorrhea at 1 year of use. In the unlikely event that a woman becomes pregnant while using Mirena, evaluate for ectopic pregnancy, which occurs in about half of pregnancies in women using this system.
Noncontraceptive benefits. The system’s primary noncontraceptive benefit is the dramatic reduction of menstrual blood loss, reported to be up to 90%.28 This contraceptive has been used as a cost-effective alternative to hysterectomy and endometrial ablation.29,30 Mirena imparts a protective effect against PID, likely secondary to progestin-mediated cervical mucus thickening.31
Subdermal implant (Implanon)
It appears safe and expeditious to provide both counseling and intrauterine contraception insertion in one visit, provided pregnancy is excluded.32 Confirm normalcy of cervical cytology and screen for sexually transmitted disease, if indicated. Prophylactic antibiotics are unnecessary, as the risk of PID within 20 days of insertion is only 9.7 per 1000 woman- years.33 After 20 days, the risk declines to 1.4 per 1000 woman-years, the same as that of the general population.34
Subdermal implant: Easily reversible
In July 2006, the FDA approved Implanon, a subdermal contraceptive implant.35 It has been available worldwide since 1998. The 40 × 2 mm single-rod implant containing etonogestrel (ENG) 68 mg diffuses the hormone at a rate of 60 mcg/d immediately after insertion and then steadily at 30 mcg/d for up to 3 years.
Its primary mechanism of action is ovulation suppression, with no ovulation detected for 30 months in a study group of more than 17,000 women.35 Increased cervical mucus viscosity also contributes to its effectiveness.35 In a large clinical trial, no pregnancies were reported in more than 6100 cycles.36 However, this trial excluded women weighing more than 130% of their ideal body weight, so no data are available to support the effectiveness of Implanon in obese women.36