The Centers for Disease Control and Prevention (CDC) has released recommendations made by the Advisory Committee for Immunization Practices (ACIP) for using influenza vaccine for the upcoming influenza season.1 The recommendations, which are easier to follow than in past years, continue to advise that patients older than 6 months of age (without a contraindication) be vaccinated annually. But there are some newer recommendations, as well, and they are reviewed here.
2011-2012 vaccine choices include a new product
Although the virus strains in the 2011-2012 vaccines are the same as in 2010-20112—A/California/7/2009 (H1N1)-like, A/Perth/16/ 2009 (H3N2)-like, and B/Brisbane/60/2008-like antigens—individuals vaccinated last year should receive the vaccine again this year. Over the course of a year, antibodies that developed in response to an influenza vaccine decline, and it is believed that even if the vaccine strains have not changed, annual vaccination confers optimal protection.
Although all influenza vaccine products available in the United States contain the same virus strains, the products contain either killed virus (trivalent influenza vaccine [TIV]) or live virus (live attenuated influenza vaccine [LAIV]). The only LAIV vaccine available is the intranasally administered FluMist (MedImmune). It is licensed for use in those between the ages of 2 and 49 years who are healthy, nonpregnant, and without high-risk medical conditions. The CDC does not state a preference for LAIV or TIV in this age group.
A new intradermally administered TIV, Fluzone Intradermal (Sanofi Pasteur),3 was licensed in May 2011 for use in individuals ages 18 through 64 years. It contains less antigen than intramuscular TIV options and is administered in a smaller volume (0.1 rather than 0.5 mL). The preferred site of administration is over the deltoid muscle. Injection-site erythema, induration, swelling, and pruritus occur more frequently than with intramuscular vaccine, but usually these reactions are self-limited, resolving within 3 to 7 days.
Again this coming season, a higher antigen product, Fluzone High-Dose (Sanofi Pasteur), will be available for adults ages 65 and older.4 Fluzone High-Dose contains 4 times the amount of influenza antigen as other TIV options. Ongoing studies are comparing this product with others for effectiveness and rates of adverse reactions. At this time, however, ACIP has not identified a preferred TIV product for this age group.
Easier decision making with young children
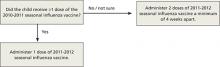
How to determine the number of doses needed by a child younger than 9 years has been simplified. If the child received 1 or more doses of vaccine last season, only 1 dose is needed this year. If no vaccine was received last year or if that status is unknown, 2 doses are recommended (FIGURE).1
FIGURE
How many doses of flu vaccine for children 6 months through 8 years of age?1
Egg allergy does not necessarily prohibit vaccination
The last recommendation change this season is that a history of egg allergy is no longer an automatic contraindication to influenza vaccine.1 The only contraindication to receiving the vaccine is a prior severe allergic reaction to influenza vaccine. ACIP now states that individuals who have experienced only hives after exposure to egg should receive the vaccine, but only a TIV product and only from a health care provider who is familiar with the potential manifestations of egg allergy. Additionally, those who receive the vaccine should be observed for at least 30 minutes for signs of a reaction.
In the past, some providers have used a 2-step approach (giving a small proportion as a dose first; then, if no reaction occurs, administering the remaining portion). Others have recommended skin testing with vaccine before administration. Neither of these approaches is necessary, according to ACIP, which cites studies that showed skin prick testing with vaccine is poorly predictive of allergic reactions and administration of both full doses and 2-step doses have been well tolerated.1
Many people reporting egg allergy will not have a reaction to influenza vaccine.1 In addition, current influenza vaccine products contain very low levels of egg protein. Individuals more likely to have a serious reaction are those who have had severe reactions to egg—eg, angioedema, respiratory distress, light-headedness, or recurrent emesis, or who required epinephrine or other emergency medical interventions. Such people should be referred to a physician with expertise in the management of allergic conditions for further risk assessment.1 As another precaution, the ACIP recommendations state that all vaccines should be administered in settings where equipment is on hand for treatment of anaphylaxis and where providers are trained in the recognition and treatment of allergic reactions.