Patients were sent home with a postcard that served as a 2-week follow-up. Two weeks after treatment patients rated their current pain, maximum pain over the 2-week period, and typical pain over the 2-week period, using the previously described VAS.
Data analysis
Previous research on the effect of magnets on pain has shown reduction in pain on a 10-point VAS ranging from 1.1 to 4.4 points with standard deviations of 1.6 and 3.1, respectively.1 Corresponding sample sizes to detect these differences would range from 34 per group to 8 per group. Standard sample size formulas for power equal to 0.80, ( equal to 0.05, and a standard deviation of 2.5 estimated that a sample size of 15 per group could detect a difference of 2.6 points between groups.
Data were analyzed using chi-square analysis for categorical data, paired t tests for within group comparisons, and independent t tests for between group comparisons on age and pain. Confirmation of normal distributions for the VAS variables was made using the Kolmogorov-Smirnov goodness-of-fit test.
Results
Of the 160 patients contacted by mail, 45 replied, 38 qualified for participation, and 30 patients completed the 45-minute treatment protocol: 15 with a magnetic device and 15 with a placebo. Descriptive statistics for the 2 groups are provided in Table 1. Groups did not differ significantly in age or any of the presenting symptoms including numbness, tingling, burning, and pain. There were no men in the magnet group and 4 in the placebo group ( P =.01).
Table 2 contains the mean pain scores for both groups at different points in time. There were no significant differences for any of the pain variables. Twenty of the participants in this study completed a 2-week follow-up questionnaire, 10 in each group. There were no significant differences between groups in the pain at 2 weeks post-treatment, the greatest pain experienced during the 2 weeks, and the typical pain experienced during the 2 weeks. The mean pain score at 2 weeks post-treatment and their typical pain across the 2 weeks had not returned to their baseline pain levels measured before device application.
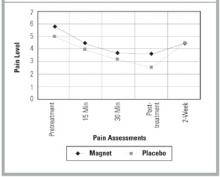
The Figure shows the pain trend across the 45-minute treatment for both groups. The steep decline across each pain measurement period was almost identical for each group but illustrates the significant pain relief provided by both the magnet and the placebo devices. Paired t test analysis revealed that the mean change between pre- and post-treatment was -2.4 ( P =.004) for the magnet group and -2.4 ( P =.003) for the placebo group.
TABLE 1
BASELINE CHARACTERISTICS OF THE STUDY GROUPS
| Characteristic | Magnet N (%) | Placebo N (%) | P |
| Mean age, years, N (SD) | 50.7 (15.5) | 48.5 (11.7) | .67* |
| Women | 15 (100) | 11 (73) | .01† |
| Repetitive work | 11 (73) | 13 (87) | .36† |
| Numbness | |||
| None | 5 (33) | 7 (49) | .13† |
| Some | 2 (13) | 5 (33) | |
| A great deal | 8 (53) | 3 (20) | |
| Tingling | |||
| None | 8 (47) | 9 (60) | .68† |
| Some | 2 (13) | 3 (20) | |
| A great deal | 5 (33) | 3 (20) | |
| Burning | |||
| None | 12 (80) | 11 (73) | .22† |
| Some | 0 (0) | 2 (13) | |
| A great deal | 3 (20) | 2 (13) | |
| Pain | |||
| None | 5 (33) | 6 (40) | .25† |
| Some | 3 (20) | 6 (40) | |
| A great deal | 7 (47) | 3 (20) | |
| *t test analysis | |||
| † Chi-square analysis | |||
| SD denotes standard deviation. | |||
TABLE 2
COMPARISON OF GROUP VISUAL ANALOG SCALE MEANS BEFORE, DURING, AND AFTER DEVICE APPLICATION
| Pain Score | Magnet Mean (SD) | Placebo Mean (SD) | Difference (95% CI)* |
|---|---|---|---|
| Pretreatment pain† | 5.9 (2.6) | 5.0 (2.4) | 0.9 (-.90 to 2.84) |
| Pain at 15 minutes† | 4.5 (2.6) | 3.9 (2.8) | 0.6 (-1.49 to 2.47) |
| Pain at 30 minutes† | 3.7 (2.6) | 3.2 (2.6) | 0.5 (-1.47 to 2.36) |
| Post-treatment pain† | 3.6 (3.1) | 2.6 (2.7) | 1.0 (-1.21 to 3.15) |
| Total pain decrease† | -2.4 (2.7) | -2.4 (2.6) | 0.0 (-2.02 to 1.97) |
| Pain at 2 week follow-up‡ | 4.3 (2.9) | 4.3 (3.5) | 0.0 (-3.0 to 3.03) |
| Greatest pain during 2 weeks‡ | 5.5 (2.7) | 4.9 (2.8) | 0.6 (-2.07 to 3.15) |
| Typical pain during 2 weeks‡ | 4.1 (2.7) | 3.7 (2.4) | 0.4 (-1.99 to 2.83) |
| *95% confidence interval for the difference between the mean pain scores. None of the differences were statistically significant. | |||
| SD denotes standard deviation; CI, confidence interval. | |||
| † N=150 | |||
| ‡ N=100 | |||
FIGURE
PAIN TREND BY GROUP
Discussion
The delivery of a unipolar static magnetic field through a magnetized device directly applied to the point of greatest wrist pain resulted in no significant difference in relief of pain when compared with an identical placebo device. However, both magnet and placebo produced a significant decrease in pain during the 45-minute application that was still detectable at the 2-week follow-up. The decrease in pain observed in both experimental and control groups could be attributed to a variety of causes. Most likely, this is a placebo effect due to the patients’ belief in the efficacy of the device. Also, it is possible that pressure over the area of pain, due to application of the bracelet, somehow reduces the amount of pain experienced.
A limitation of this study is the small sample size. It is possible that a larger study would detect small improvements in outcomes, but it is questionable whether these would be clinically significant.