For use in children. Asthma controller medications approved for use in children younger than 5 years include the fluticasone dry-powder inhalers (Flovent, Rotadisk, and Flovent Diskus), which are approved for children as young as 4 years (Flovent Diskus is not yet commercially available), and nebulized budesonide inhalation suspension (Pulmicort Respules), which is approved for children as young as 12 months.
The LABAs formoterol (Foradil) and salmeterol (Serevent Diskus) are approved for children as young as 5 and 4 years, respectively. Cromolyn sodium nebulizer solution is approved for children as young as 2 years, and theophylline is available for use at any age.
Based on safety and extrapolation of efficacy data in older patients, the oral granule formulation of the leukotriene receptor antagonist (LTRA) montelukast (Singulair) is approved for children as young as 1 year, and the chewable tablets are approved for children 2 to 5 years of age. Zafirlukast (Accolate) is approved for use in children 5 years and older.
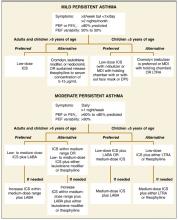
New recommendations for mild persistent asthma. Recommendations for the treatment of mild and moderate persistent asthma have changed considerably from the 1997 guidelines. ICSs are now the preferred controller medications, based on greater efficacy. The updated guidelines no longer recommend an initial trial of cromolyn or nedocromil for the treatment of mild persistent asthma; these agents, along with the leukotriene modifiers and slow-release theophylline, are now considered alternatives to low-dose ICSs for adults and children older than 5 years with mild persistent disease (Figure).
According to the NAEPP update, daily low-dose ICS treatment also is preferred for the control of mild persistent asthma in preschool children. As in older children, cromolyn and nedocromil are no longer considered appropriate initial treatments for infants and children 5 years and younger. Cromolyn is considered an alternative controller, whereas nedocromil is no longer recommended for use.
New recommendations for moderate persistent asthma. For adults and children older than 5 years with moderate persistent asthma, revision to the guidelines involved recommendation of a low- to medium-dose ICS plus a LABA as the preferred controller treatment (Figure). Comparative low, medium, and high daily doses for ICSs are shown in Table 3 .1
For preschool children, preferred controller treatments for moderate persistent asthma include low-dose ICSs plus a LABA, or increasing ICSs within the medium-dose range (Figure). Recommendations for the use of LABAs as add-on therapy in this age group are based on extrapolation of data from older patients, since therapy with an ICS/LABA combination has not been adequately studied in children younger than 5 years. Four studies included in the NAEPP evaluation showed clear benefit of medium-dose ICSs in this age group, supporting the use of medium-dose ICSs as a preferred option.6-9 LABAs are not recommended for use without an ICS, and the only ICS/LABA combination product currently available has been FDA approved only for patients aged 12 years and older.
TABLE 2
Levels of evidence for NAEPP assessments*
| Medication | NAEPP assessment | SOR* |
|---|---|---|
| ICS | Preferred treatment for children of all ages with persistent asthma | A (A) |
| SABA | ICSs improve asthma control compared with as-needed SABAs | A (A) |
| Cromolyn/nedocromil | For use as alternative, not preferred, treatment of mild persistent asthma in children of all ages (cromolyn) or children >5 years of age (nedocromil) | A (A) |
| LABA | For use with ICSs as the preferred combination treatment for moderate and severe persistent asthma in children >5 years of age | A (A) |
| For use as a preferred option for combination treatment in children 5 years of age | B (B) | |
| Leukotriene modifier | For use as alternative, not preferred, treatment of mild persistent asthma and as ICS adjunct in moderate persistent asthma | B (B) |
| Theophylline | For use as an alternative ICS add-on in moderate or severe persistent asthma if serum concentrations are monitored | D (D) |
| Not considered an alternative controller for young children with mild persistent asthma due to potential adverse effects in infants with frequent febrile illnesses | ||
| *Highest level of evidence available is reported. Strengths of recommendation are based on the method of Jadad et al.3 Strength of evidence based on the Oxford Center for Evidence-Based Medicine5 is in parentheses. SOR, strength of recommendation; NAEPP, National Asthma Education and Prevention Program; ICS, inhaled corticosteroid; SABA, short-acting β2-adrenergic agonist; LABA, long-acting β2-adrenergic agonist. | ||
TABLE 3
Estimated comparative daily doses for inhaled corticosteroids*
| Drug | Low daily dose | Medium daily dose | High daily dose | |||
|---|---|---|---|---|---|---|
| Adult | Child† | Adult | Child† | Adult | Child† | |
| Beclomethasone CFC 42 or 84 μg/puff | 168–504 μg | 84–336 μg | 504–840 μg | 336–672 μg | >840 μg | >672 μg |
| Beclomethasone HFA 40 or 80 μg/puff | 80–240 μg | 80–160 μg | 240–480 μg | 160–320 μg | >480 μg | >320 μg |
| Budesonide DPI 200 μg/inhalation | 200–600 μg | 200–400 μg | 600–1200 μg | 400–800 μg | >1200 μg | >800 μg |
| Budesonide inhalation suspension for nebulization (child dose) | 0.5mg | 1.0 mg | 2.0 mg | |||
| Fluticasone MDI 44, 110, or 220 μg/puff | 88–264 μg | 88–176 μg | 264–660 μg | 176–440 μg | >660 μg | >440 μg |
| Fluticasone DPI 50, 100, or 250 μg/inhalation | 100–300 μg | 100–200 μg | 300–600 μg | 200–400 μg | >600 μg | >400 μg |
| Triamcinolone acetonide 100 μg/puff | 400–1000 μg | 400–800 μg | 1000–2000 μg | 800–1200 μg | >2000 μg | >1200 μg |
| *The most important determinant of appropriate dosing is the clinician’s judgment of the patient’s response to therapy. This updated comparative dose chart is based on review of recently published clinical trials involving more than 5000 patients and published reviews. Some doses may be outside package labeling, especially in the high-dose range. | ||||||
| †Children 12 years of age. | ||||||
| CFC, chlorofluorocarbon; HFA, hydrofluoroalkane; DPI, dry-powder inhaler; MDI, metered-dose inhaler. | ||||||
FIGURE
Updated National Asthma Education and Prevention Program recommendations for long-term controller treatment in mild and moderate persistent asthma