User login
HCV Hub
AbbVie
acid
addicted
addiction
adolescent
adult sites
Advocacy
advocacy
agitated states
AJO, postsurgical analgesic, knee, replacement, surgery
alcohol
amphetamine
androgen
antibody
apple cider vinegar
assistance
Assistance
association
at home
attorney
audit
ayurvedic
baby
ban
baricitinib
bed bugs
best
bible
bisexual
black
bleach
blog
bulimia nervosa
buy
cannabis
certificate
certification
certified
cervical cancer, concurrent chemoradiotherapy, intravoxel incoherent motion magnetic resonance imaging, MRI, IVIM, diffusion-weighted MRI, DWI
charlie sheen
cheap
cheapest
child
childhood
childlike
children
chronic fatigue syndrome
Cladribine Tablets
cocaine
cock
combination therapies, synergistic antitumor efficacy, pertuzumab, trastuzumab, ipilimumab, nivolumab, palbociclib, letrozole, lapatinib, docetaxel, trametinib, dabrafenib, carflzomib, lenalidomide
contagious
Cortical Lesions
cream
creams
crime
criminal
cure
dangerous
dangers
dasabuvir
Dasabuvir
dead
deadly
death
dementia
dependence
dependent
depression
dermatillomania
die
diet
direct-acting antivirals
Disability
Discount
discount
dog
drink
drug abuse
drug-induced
dying
eastern medicine
eat
ect
eczema
electroconvulsive therapy
electromagnetic therapy
electrotherapy
epa
epilepsy
erectile dysfunction
explosive disorder
fake
Fake-ovir
fatal
fatalities
fatality
fibromyalgia
financial
Financial
fish oil
food
foods
foundation
free
Gabriel Pardo
gaston
general hospital
genetic
geriatric
Giancarlo Comi
gilead
Gilead
glaucoma
Glenn S. Williams
Glenn Williams
Gloria Dalla Costa
gonorrhea
Greedy
greedy
guns
hallucinations
harvoni
Harvoni
herbal
herbs
heroin
herpes
Hidradenitis Suppurativa,
holistic
home
home remedies
home remedy
homeopathic
homeopathy
hydrocortisone
ice
image
images
job
kid
kids
kill
killer
laser
lawsuit
lawyer
ledipasvir
Ledipasvir
lesbian
lesions
lights
liver
lupus
marijuana
melancholic
memory loss
menopausal
mental retardation
military
milk
moisturizers
monoamine oxidase inhibitor drugs
MRI
MS
murder
national
natural
natural cure
natural cures
natural medications
natural medicine
natural medicines
natural remedies
natural remedy
natural treatment
natural treatments
naturally
Needy
needy
Neurology Reviews
neuropathic
nightclub massacre
nightclub shooting
nude
nudity
nutraceuticals
OASIS
oasis
off label
ombitasvir
Ombitasvir
ombitasvir/paritaprevir/ritonavir with dasabuvir
orlando shooting
overactive thyroid gland
overdose
overdosed
Paolo Preziosa
paritaprevir
Paritaprevir
pediatric
pedophile
photo
photos
picture
post partum
postnatal
pregnancy
pregnant
prenatal
prepartum
prison
program
Program
Protest
protest
psychedelics
pulse nightclub
puppy
purchase
purchasing
rape
recall
recreational drug
Rehabilitation
Retinal Measurements
retrograde ejaculation
risperdal
ritonavir
Ritonavir
ritonavir with dasabuvir
robin williams
sales
sasquatch
schizophrenia
seizure
seizures
sex
sexual
sexy
shock treatment
silver
sleep disorders
smoking
sociopath
sofosbuvir
Sofosbuvir
sovaldi
ssri
store
sue
suicidal
suicide
supplements
support
Support
Support Path
teen
teenage
teenagers
Telerehabilitation
testosterone
Th17
Th17:FoxP3+Treg cell ratio
Th22
toxic
toxin
tragedy
treatment resistant
V Pak
vagina
velpatasvir
Viekira Pa
Viekira Pak
viekira pak
violence
virgin
vitamin
VPak
weight loss
withdrawal
wrinkles
xxx
young adult
young adults
zoloft
financial
sofosbuvir
ritonavir with dasabuvir
discount
support path
program
ritonavir
greedy
ledipasvir
assistance
viekira pak
vpak
advocacy
needy
protest
abbvie
paritaprevir
ombitasvir
direct-acting antivirals
dasabuvir
gilead
fake-ovir
support
v pak
oasis
harvoni
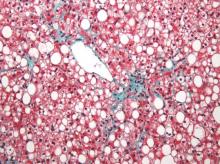
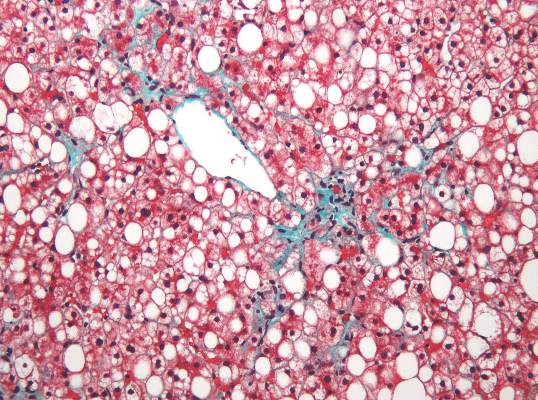
MRI topped transient elastography for staging nonalcoholic fatty liver disease
Two magnetic resonance imaging (MRI) techniques topped transient elastography (TE) for diagnosing hepatic fibrosis and steatosis in patients with nonalcoholic fatty liver disease (NAFLD), according to a first-in-kind study.
Magnetic resonance elastography surpassed all other methods for staging fibrosis, while MRI-based measurement of proton density fat fraction (PDFF) was superior for grading steatosis, with liver biopsy used as the comparative gold standard, said Dr. Kento Imajo at Yokohama (Japan) City University Graduate School of Medicine and his associates. “Magnetic resonance imaging–based noninvasive assessment of liver fibrosis and steatosis is a potential alternative to liver biopsy in clinical practice,” the investigators wrote in the March issue of Gastroenterology.
Assessing liver fibrosis and steatosis is important for staging NAFLD. Although “useful” overall, transient elastography can be unreliable in morbidly obese NAFLD patients or those with ascites because of low-frequency vibrations created by the probe, the researchers noted. To compare TE with MRI-based magnetic resonance elastography and PDFF, they evaluated 142 patients with biopsy-confirmed NAFLD and 10 controls, all of whom they also assessed with five clinical scoring systems for fibrosis – the FIB4 index, the NAFLD fibrosis score, the aspartate aminotransferase (AST) to platelet ratio, the AST-to-alanine transaminase (ALT) ratio, and the BARD score (Gastroenterology. 2015 Dec 8. doi: 10.1053/j.gastro.2015.11.048).
Magnetic resonance elastography detected stage 2 or higher hepatic fibrosis with an area under the receiver operating characteristic (AUROC) curve value of 0.91 (95% confidence interval, 0.86-0.96), compared with 0.82 (0.74-0.89) for transient elastography (P = .001), the investigators reported. The AUROC for MRE also significantly exceeded the AUROCs for all five clinical indexes of fibrosis severity. Furthermore, MRI-based measurement of PDFF identified hepatic steatosis of grade 2 or higher with an AUROC curve value of 0.90 (95% CI, 0.82-0.97), which was significantly greater than the AUROC obtained by using TE to measure the controlled attenuation parameter (0.73; 95% CI, 0.64-0.81; P less than .001).
Adding a measure for serum keratin 18 fragments or ALT did not significantly improve the detection of nonalcoholic steatohepatitis or macrovesicular steatosis affecting at least 5% of hepatocytes by either MRI or TE, the researchers noted. While liver biopsy remains the gold standard for assessing NAFLD, it is associated with sampling errors and intra- and interobserver variability, and these errors could have affected their study results, they acknowledged. The study also did not account for hepatic perfusion, which can elevate liver stiffness measurement independently from liver disease.
Both the magnetic resonance elastography and PDFF techniques require specialized hardware and software that are available from several commercial suppliers, the researchers also noted.
The study was partially supported by the Japanese Ministry of Health, Labor, and Welfare, the Japanese Science and Technology Agency, and Kiban-B, Shingakujuturyouiki. The investigators had no disclosures.
Two magnetic resonance imaging (MRI) techniques topped transient elastography (TE) for diagnosing hepatic fibrosis and steatosis in patients with nonalcoholic fatty liver disease (NAFLD), according to a first-in-kind study.
Magnetic resonance elastography surpassed all other methods for staging fibrosis, while MRI-based measurement of proton density fat fraction (PDFF) was superior for grading steatosis, with liver biopsy used as the comparative gold standard, said Dr. Kento Imajo at Yokohama (Japan) City University Graduate School of Medicine and his associates. “Magnetic resonance imaging–based noninvasive assessment of liver fibrosis and steatosis is a potential alternative to liver biopsy in clinical practice,” the investigators wrote in the March issue of Gastroenterology.
Assessing liver fibrosis and steatosis is important for staging NAFLD. Although “useful” overall, transient elastography can be unreliable in morbidly obese NAFLD patients or those with ascites because of low-frequency vibrations created by the probe, the researchers noted. To compare TE with MRI-based magnetic resonance elastography and PDFF, they evaluated 142 patients with biopsy-confirmed NAFLD and 10 controls, all of whom they also assessed with five clinical scoring systems for fibrosis – the FIB4 index, the NAFLD fibrosis score, the aspartate aminotransferase (AST) to platelet ratio, the AST-to-alanine transaminase (ALT) ratio, and the BARD score (Gastroenterology. 2015 Dec 8. doi: 10.1053/j.gastro.2015.11.048).
Magnetic resonance elastography detected stage 2 or higher hepatic fibrosis with an area under the receiver operating characteristic (AUROC) curve value of 0.91 (95% confidence interval, 0.86-0.96), compared with 0.82 (0.74-0.89) for transient elastography (P = .001), the investigators reported. The AUROC for MRE also significantly exceeded the AUROCs for all five clinical indexes of fibrosis severity. Furthermore, MRI-based measurement of PDFF identified hepatic steatosis of grade 2 or higher with an AUROC curve value of 0.90 (95% CI, 0.82-0.97), which was significantly greater than the AUROC obtained by using TE to measure the controlled attenuation parameter (0.73; 95% CI, 0.64-0.81; P less than .001).
Adding a measure for serum keratin 18 fragments or ALT did not significantly improve the detection of nonalcoholic steatohepatitis or macrovesicular steatosis affecting at least 5% of hepatocytes by either MRI or TE, the researchers noted. While liver biopsy remains the gold standard for assessing NAFLD, it is associated with sampling errors and intra- and interobserver variability, and these errors could have affected their study results, they acknowledged. The study also did not account for hepatic perfusion, which can elevate liver stiffness measurement independently from liver disease.
Both the magnetic resonance elastography and PDFF techniques require specialized hardware and software that are available from several commercial suppliers, the researchers also noted.
The study was partially supported by the Japanese Ministry of Health, Labor, and Welfare, the Japanese Science and Technology Agency, and Kiban-B, Shingakujuturyouiki. The investigators had no disclosures.
Two magnetic resonance imaging (MRI) techniques topped transient elastography (TE) for diagnosing hepatic fibrosis and steatosis in patients with nonalcoholic fatty liver disease (NAFLD), according to a first-in-kind study.
Magnetic resonance elastography surpassed all other methods for staging fibrosis, while MRI-based measurement of proton density fat fraction (PDFF) was superior for grading steatosis, with liver biopsy used as the comparative gold standard, said Dr. Kento Imajo at Yokohama (Japan) City University Graduate School of Medicine and his associates. “Magnetic resonance imaging–based noninvasive assessment of liver fibrosis and steatosis is a potential alternative to liver biopsy in clinical practice,” the investigators wrote in the March issue of Gastroenterology.
Assessing liver fibrosis and steatosis is important for staging NAFLD. Although “useful” overall, transient elastography can be unreliable in morbidly obese NAFLD patients or those with ascites because of low-frequency vibrations created by the probe, the researchers noted. To compare TE with MRI-based magnetic resonance elastography and PDFF, they evaluated 142 patients with biopsy-confirmed NAFLD and 10 controls, all of whom they also assessed with five clinical scoring systems for fibrosis – the FIB4 index, the NAFLD fibrosis score, the aspartate aminotransferase (AST) to platelet ratio, the AST-to-alanine transaminase (ALT) ratio, and the BARD score (Gastroenterology. 2015 Dec 8. doi: 10.1053/j.gastro.2015.11.048).
Magnetic resonance elastography detected stage 2 or higher hepatic fibrosis with an area under the receiver operating characteristic (AUROC) curve value of 0.91 (95% confidence interval, 0.86-0.96), compared with 0.82 (0.74-0.89) for transient elastography (P = .001), the investigators reported. The AUROC for MRE also significantly exceeded the AUROCs for all five clinical indexes of fibrosis severity. Furthermore, MRI-based measurement of PDFF identified hepatic steatosis of grade 2 or higher with an AUROC curve value of 0.90 (95% CI, 0.82-0.97), which was significantly greater than the AUROC obtained by using TE to measure the controlled attenuation parameter (0.73; 95% CI, 0.64-0.81; P less than .001).
Adding a measure for serum keratin 18 fragments or ALT did not significantly improve the detection of nonalcoholic steatohepatitis or macrovesicular steatosis affecting at least 5% of hepatocytes by either MRI or TE, the researchers noted. While liver biopsy remains the gold standard for assessing NAFLD, it is associated with sampling errors and intra- and interobserver variability, and these errors could have affected their study results, they acknowledged. The study also did not account for hepatic perfusion, which can elevate liver stiffness measurement independently from liver disease.
Both the magnetic resonance elastography and PDFF techniques require specialized hardware and software that are available from several commercial suppliers, the researchers also noted.
The study was partially supported by the Japanese Ministry of Health, Labor, and Welfare, the Japanese Science and Technology Agency, and Kiban-B, Shingakujuturyouiki. The investigators had no disclosures.
FROM GASTROENTEROLOGY
Key clinical point: Two specialized MRI techniques surpassed transient elastography for staging fibrosis and steatosis in nonalcoholic fatty liver disease.
Major finding: The areas under the curve for magnetic resonance elastography and the proton density fat fraction measure were significantly greater than those for transient elastography and the TE-based controlled attenuation parameter (P is less than .001 for both comparisons).
Data source: A cross-sectional study of 142 patients with nonalcoholic fatty liver disease and 10 controls.
Disclosures: The study was partially supported by the Japanese Ministry of Health, Labor, and Welfare, the Japanese Science and Technology Agency, and Kiban-B, Shingakujuturyouiki. The investigators had no disclosures.
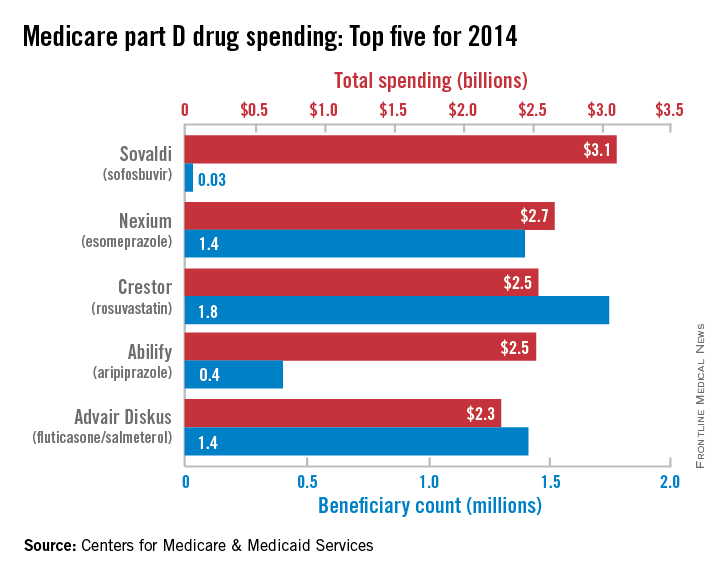
Sovaldi topped Medicare part D spending in 2014
In its first year on the market, the hepatitis C virus drug Sovaldi soared to the top of the Medicare part D spending list, the Centers for Medicare & Medicaid Services reported.
With innovative oral HCV drugs getting at least partial credit for a big jump in U.S. health expenditures in 2014, total part D spending for Sovaldi (sofosbuvir) was more than $3.1 billion, close to half a billion more than second-place Nexium (esomeprazole), which had almost $2.7 billion in spending for the year. Next on the list was Crestor (rosuvastatin) at $2.54 billion, followed by Abilify (aripiprazole) at $2.53 billion and Advair Diskus (fluticasone/salmeterol) at $2.3 billion, according to the Medicare drug spending dashboard.
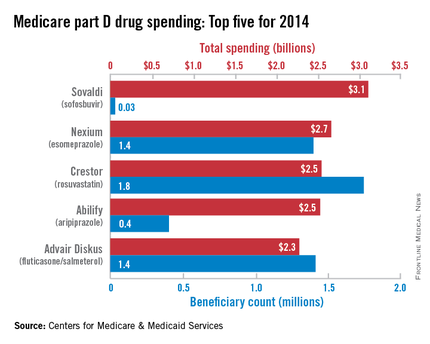
Sovaldi’s spot at the top, however, was not a result of its popularity. Compared with the other top five drugs, it was used by the fewest people (33,000) and had the highest average per-unit cost ($1,017). Crestor was most commonly prescribed among the five, with 1.7 million users in 2014. Advair Diskus was used by 1.42 million part D beneficiaries, putting it just ahead of Nexium, which had 1.4 million users. Abilify was well behind those three with 405,000 users, but it did have the second-highest per-unit cost, $28.65. The per-unit costs were similar for Crestor ($6.07), Advair Diskus ($4.94), and Nexium ($7.82), the CMS data show.
In 2014 overall, 3,761 different prescription drug products were covered by Medicare part D, with total program spending of $121.5 billion. There were 115 drugs with spending over $250 million for the year, and combined spending for those 115 drugs was $76.7 billion, or 63% of the part D total, the CMS said.
In its first year on the market, the hepatitis C virus drug Sovaldi soared to the top of the Medicare part D spending list, the Centers for Medicare & Medicaid Services reported.
With innovative oral HCV drugs getting at least partial credit for a big jump in U.S. health expenditures in 2014, total part D spending for Sovaldi (sofosbuvir) was more than $3.1 billion, close to half a billion more than second-place Nexium (esomeprazole), which had almost $2.7 billion in spending for the year. Next on the list was Crestor (rosuvastatin) at $2.54 billion, followed by Abilify (aripiprazole) at $2.53 billion and Advair Diskus (fluticasone/salmeterol) at $2.3 billion, according to the Medicare drug spending dashboard.

Sovaldi’s spot at the top, however, was not a result of its popularity. Compared with the other top five drugs, it was used by the fewest people (33,000) and had the highest average per-unit cost ($1,017). Crestor was most commonly prescribed among the five, with 1.7 million users in 2014. Advair Diskus was used by 1.42 million part D beneficiaries, putting it just ahead of Nexium, which had 1.4 million users. Abilify was well behind those three with 405,000 users, but it did have the second-highest per-unit cost, $28.65. The per-unit costs were similar for Crestor ($6.07), Advair Diskus ($4.94), and Nexium ($7.82), the CMS data show.
In 2014 overall, 3,761 different prescription drug products were covered by Medicare part D, with total program spending of $121.5 billion. There were 115 drugs with spending over $250 million for the year, and combined spending for those 115 drugs was $76.7 billion, or 63% of the part D total, the CMS said.
In its first year on the market, the hepatitis C virus drug Sovaldi soared to the top of the Medicare part D spending list, the Centers for Medicare & Medicaid Services reported.
With innovative oral HCV drugs getting at least partial credit for a big jump in U.S. health expenditures in 2014, total part D spending for Sovaldi (sofosbuvir) was more than $3.1 billion, close to half a billion more than second-place Nexium (esomeprazole), which had almost $2.7 billion in spending for the year. Next on the list was Crestor (rosuvastatin) at $2.54 billion, followed by Abilify (aripiprazole) at $2.53 billion and Advair Diskus (fluticasone/salmeterol) at $2.3 billion, according to the Medicare drug spending dashboard.

Sovaldi’s spot at the top, however, was not a result of its popularity. Compared with the other top five drugs, it was used by the fewest people (33,000) and had the highest average per-unit cost ($1,017). Crestor was most commonly prescribed among the five, with 1.7 million users in 2014. Advair Diskus was used by 1.42 million part D beneficiaries, putting it just ahead of Nexium, which had 1.4 million users. Abilify was well behind those three with 405,000 users, but it did have the second-highest per-unit cost, $28.65. The per-unit costs were similar for Crestor ($6.07), Advair Diskus ($4.94), and Nexium ($7.82), the CMS data show.
In 2014 overall, 3,761 different prescription drug products were covered by Medicare part D, with total program spending of $121.5 billion. There were 115 drugs with spending over $250 million for the year, and combined spending for those 115 drugs was $76.7 billion, or 63% of the part D total, the CMS said.
Minor residual staining found adequate for colonoscopy
A Boston Bowel Preparation Scale (BBPS) score of 2 – indicating mild residual staining and small stool fragments – was as good as the optimal preparation score of 3 for visualizing polyps and adenomas larger than 5 mm and advanced adenomas during colonoscopy, researchers said.
A score of 2 might increase the chances of missing smaller polyps, but is adequate for detecting clinically significant masses, Dr. Brian Clark of Yale University, New Haven, Conn., and his associates reported in the February issue of Gastroenterology. But a score of 1 – meaning that there is enough staining or stool to obscure the mucosa – significantly increased the chances of missing adenomas larger than 5 mm, they said. Patients should undergo early repeat colonoscopy if their BBPS score is 1 or 0 in any colon segment, they emphasized.
Source: American Gastroenterological Association
Bowel preparation for colonoscopy is considered adequate if endoscopists can detect polyps larger than 5 mm, but no prior study had quantified the amount of preparation needed. This prospective observational study assessed adequate preparation in terms of the BBPS, which scores each of three colon segments on a scale of 0 (solid stool covering the mucosa) to 3 points (entire mucosa seen well, with no residual staining). Study participants included 438 men aged 50-75 years who underwent screening or surveillance colonoscopy at a single Veterans Affairs center, followed by repeat colonoscopies within 60 days performed by different blinded endoscopists. The investigators excluded patients who scored 0 in all colon segments or had familial polyposis syndrome, inflammatory bowel disease, polyps so large that they could not be completely removed, or a history of colonic or rectal resection. In all, they analyzed 1,161 colon segments (Gastroenterology. 2015 Dec 7. doi: 10.1053/j.gastro.2015.09.041).
Endoscopists missed about 5% of adenomas greater than 5 mm, regardless of whether BBPS scores were 2 or 3 in a model that accounted for age, reason for colonoscopy, colon segment, number of polyps removed in the first examination, and endoscopist performing the procedure, the researchers said. But when BBPS scores were 1, endoscopists missed 16% of adenomas larger than 5 mm, a difference of about 10%. Furthermore, 43% of screening and surveillance intervals would have been incorrect had they been based solely on an initial examination for which scores were 1 in at least one segment. In contrast, only about 15% of intervals would have been incorrect for patients who scored 2 or 3 in all segments.
In all, 80% of patients were sufficiently prepared, having scored at least 2 in all segments on the first examination. “Determining whether a patient’s preparation quality is adequate is one of the most common and important decisions made by gastroenterologists each day,” the researchers said. Between 25% and 30% of screening and surveillance colonoscopies occur at “inappropriately shortened intervals,” often because of uncertainty about what constitutes adequate visualization, they added. Defining adequate visualization based on bowel preparation could save billions of dollars in health care costs every year, minimize complications from unnecessary procedures, and pinpoint those patients who truly need an early repeat colonoscopy to help prevent interval colorectal cancer, they emphasized.
The National Institutes of Health funded the study. The investigators had no disclosures.
We have seen a dramatic increase in attention to improving the adenoma detection rate (ADR) during colonoscopy because patients of endoscopists with a higher ADR have a lower risk of colorectal cancer after colonoscopy. One major contributor to missed adenomas is inadequate bowel preparation, though little was known about how best to define adequacy.
 |
| Dr. Jason Domonitz |
Clark and colleagues’ elegant tandem colonoscopy study helps address this knowledge gap using the Boston Bowel Preparation Scale (BBPS), a validated instrument that is easy to implement. They hypothesized that a BBPS colon-segment score of 2 was noninferior to a score of 3 for identifying adenomas greater than 5 mm, but that a BBPS colon-segment score of 1 would be inferior to scores of 2 or 3. Their findings support this hypothesis and give us long overdue data that we can now use to define an adequate bowel preparation. Given that the adenoma miss rate was 16% when the segment score was 1, but only about 5% with higher scores, it is reasonable to recommend repeat colonoscopy within 12 months if any segment score is less than 2. Otherwise, standard surveillance intervals should be recommended. Finally, unless and until other scoring systems are similarly validated, these findings should encourage the widespread adoption of the BBPS.
Dr. Jason A. Dominitz, AGAF, is the national program director for gastroenterology for the Veterans Health Administration and is professor of medicine in the division of gastroenterology at the University of Washington, Seattle. He has no conflicts of interest.
We have seen a dramatic increase in attention to improving the adenoma detection rate (ADR) during colonoscopy because patients of endoscopists with a higher ADR have a lower risk of colorectal cancer after colonoscopy. One major contributor to missed adenomas is inadequate bowel preparation, though little was known about how best to define adequacy.
 |
| Dr. Jason Domonitz |
Clark and colleagues’ elegant tandem colonoscopy study helps address this knowledge gap using the Boston Bowel Preparation Scale (BBPS), a validated instrument that is easy to implement. They hypothesized that a BBPS colon-segment score of 2 was noninferior to a score of 3 for identifying adenomas greater than 5 mm, but that a BBPS colon-segment score of 1 would be inferior to scores of 2 or 3. Their findings support this hypothesis and give us long overdue data that we can now use to define an adequate bowel preparation. Given that the adenoma miss rate was 16% when the segment score was 1, but only about 5% with higher scores, it is reasonable to recommend repeat colonoscopy within 12 months if any segment score is less than 2. Otherwise, standard surveillance intervals should be recommended. Finally, unless and until other scoring systems are similarly validated, these findings should encourage the widespread adoption of the BBPS.
Dr. Jason A. Dominitz, AGAF, is the national program director for gastroenterology for the Veterans Health Administration and is professor of medicine in the division of gastroenterology at the University of Washington, Seattle. He has no conflicts of interest.
We have seen a dramatic increase in attention to improving the adenoma detection rate (ADR) during colonoscopy because patients of endoscopists with a higher ADR have a lower risk of colorectal cancer after colonoscopy. One major contributor to missed adenomas is inadequate bowel preparation, though little was known about how best to define adequacy.
 |
| Dr. Jason Domonitz |
Clark and colleagues’ elegant tandem colonoscopy study helps address this knowledge gap using the Boston Bowel Preparation Scale (BBPS), a validated instrument that is easy to implement. They hypothesized that a BBPS colon-segment score of 2 was noninferior to a score of 3 for identifying adenomas greater than 5 mm, but that a BBPS colon-segment score of 1 would be inferior to scores of 2 or 3. Their findings support this hypothesis and give us long overdue data that we can now use to define an adequate bowel preparation. Given that the adenoma miss rate was 16% when the segment score was 1, but only about 5% with higher scores, it is reasonable to recommend repeat colonoscopy within 12 months if any segment score is less than 2. Otherwise, standard surveillance intervals should be recommended. Finally, unless and until other scoring systems are similarly validated, these findings should encourage the widespread adoption of the BBPS.
Dr. Jason A. Dominitz, AGAF, is the national program director for gastroenterology for the Veterans Health Administration and is professor of medicine in the division of gastroenterology at the University of Washington, Seattle. He has no conflicts of interest.
A Boston Bowel Preparation Scale (BBPS) score of 2 – indicating mild residual staining and small stool fragments – was as good as the optimal preparation score of 3 for visualizing polyps and adenomas larger than 5 mm and advanced adenomas during colonoscopy, researchers said.
A score of 2 might increase the chances of missing smaller polyps, but is adequate for detecting clinically significant masses, Dr. Brian Clark of Yale University, New Haven, Conn., and his associates reported in the February issue of Gastroenterology. But a score of 1 – meaning that there is enough staining or stool to obscure the mucosa – significantly increased the chances of missing adenomas larger than 5 mm, they said. Patients should undergo early repeat colonoscopy if their BBPS score is 1 or 0 in any colon segment, they emphasized.
Source: American Gastroenterological Association
Bowel preparation for colonoscopy is considered adequate if endoscopists can detect polyps larger than 5 mm, but no prior study had quantified the amount of preparation needed. This prospective observational study assessed adequate preparation in terms of the BBPS, which scores each of three colon segments on a scale of 0 (solid stool covering the mucosa) to 3 points (entire mucosa seen well, with no residual staining). Study participants included 438 men aged 50-75 years who underwent screening or surveillance colonoscopy at a single Veterans Affairs center, followed by repeat colonoscopies within 60 days performed by different blinded endoscopists. The investigators excluded patients who scored 0 in all colon segments or had familial polyposis syndrome, inflammatory bowel disease, polyps so large that they could not be completely removed, or a history of colonic or rectal resection. In all, they analyzed 1,161 colon segments (Gastroenterology. 2015 Dec 7. doi: 10.1053/j.gastro.2015.09.041).
Endoscopists missed about 5% of adenomas greater than 5 mm, regardless of whether BBPS scores were 2 or 3 in a model that accounted for age, reason for colonoscopy, colon segment, number of polyps removed in the first examination, and endoscopist performing the procedure, the researchers said. But when BBPS scores were 1, endoscopists missed 16% of adenomas larger than 5 mm, a difference of about 10%. Furthermore, 43% of screening and surveillance intervals would have been incorrect had they been based solely on an initial examination for which scores were 1 in at least one segment. In contrast, only about 15% of intervals would have been incorrect for patients who scored 2 or 3 in all segments.
In all, 80% of patients were sufficiently prepared, having scored at least 2 in all segments on the first examination. “Determining whether a patient’s preparation quality is adequate is one of the most common and important decisions made by gastroenterologists each day,” the researchers said. Between 25% and 30% of screening and surveillance colonoscopies occur at “inappropriately shortened intervals,” often because of uncertainty about what constitutes adequate visualization, they added. Defining adequate visualization based on bowel preparation could save billions of dollars in health care costs every year, minimize complications from unnecessary procedures, and pinpoint those patients who truly need an early repeat colonoscopy to help prevent interval colorectal cancer, they emphasized.
The National Institutes of Health funded the study. The investigators had no disclosures.
A Boston Bowel Preparation Scale (BBPS) score of 2 – indicating mild residual staining and small stool fragments – was as good as the optimal preparation score of 3 for visualizing polyps and adenomas larger than 5 mm and advanced adenomas during colonoscopy, researchers said.
A score of 2 might increase the chances of missing smaller polyps, but is adequate for detecting clinically significant masses, Dr. Brian Clark of Yale University, New Haven, Conn., and his associates reported in the February issue of Gastroenterology. But a score of 1 – meaning that there is enough staining or stool to obscure the mucosa – significantly increased the chances of missing adenomas larger than 5 mm, they said. Patients should undergo early repeat colonoscopy if their BBPS score is 1 or 0 in any colon segment, they emphasized.
Source: American Gastroenterological Association
Bowel preparation for colonoscopy is considered adequate if endoscopists can detect polyps larger than 5 mm, but no prior study had quantified the amount of preparation needed. This prospective observational study assessed adequate preparation in terms of the BBPS, which scores each of three colon segments on a scale of 0 (solid stool covering the mucosa) to 3 points (entire mucosa seen well, with no residual staining). Study participants included 438 men aged 50-75 years who underwent screening or surveillance colonoscopy at a single Veterans Affairs center, followed by repeat colonoscopies within 60 days performed by different blinded endoscopists. The investigators excluded patients who scored 0 in all colon segments or had familial polyposis syndrome, inflammatory bowel disease, polyps so large that they could not be completely removed, or a history of colonic or rectal resection. In all, they analyzed 1,161 colon segments (Gastroenterology. 2015 Dec 7. doi: 10.1053/j.gastro.2015.09.041).
Endoscopists missed about 5% of adenomas greater than 5 mm, regardless of whether BBPS scores were 2 or 3 in a model that accounted for age, reason for colonoscopy, colon segment, number of polyps removed in the first examination, and endoscopist performing the procedure, the researchers said. But when BBPS scores were 1, endoscopists missed 16% of adenomas larger than 5 mm, a difference of about 10%. Furthermore, 43% of screening and surveillance intervals would have been incorrect had they been based solely on an initial examination for which scores were 1 in at least one segment. In contrast, only about 15% of intervals would have been incorrect for patients who scored 2 or 3 in all segments.
In all, 80% of patients were sufficiently prepared, having scored at least 2 in all segments on the first examination. “Determining whether a patient’s preparation quality is adequate is one of the most common and important decisions made by gastroenterologists each day,” the researchers said. Between 25% and 30% of screening and surveillance colonoscopies occur at “inappropriately shortened intervals,” often because of uncertainty about what constitutes adequate visualization, they added. Defining adequate visualization based on bowel preparation could save billions of dollars in health care costs every year, minimize complications from unnecessary procedures, and pinpoint those patients who truly need an early repeat colonoscopy to help prevent interval colorectal cancer, they emphasized.
The National Institutes of Health funded the study. The investigators had no disclosures.
FROM GASTROENTEROLOGY
Key clinical point: Minor residual staining that does not obscure the bowel mucosa is adequate for detection of adenomas greater than 5 mm during surveillance or screening colonoscopy.
Major finding: Endoscopists missed about 5% of clinically significant adenomas, regardless of whether the Boston Bowel Preparation Score was 2 (minor residual staining) or 3 (entire mucosa seen well).
Data source: A blinded prospective observational study of 438 men at a single Veterans Affairs center.
Disclosures: The National Institutes of Health funded the study. The investigators had no disclosures.
Drug combo held up in real-world HCV study
A 12-week, ribavirin-free regimen achieved sustained virologic response for 85% of patients with genotype 1 hepatitis C virus (HCV) infection, researchers reported in the February issue of Gastroenterology.
“This represents one of the first applications of a highly effective HCV regimen outside clinical trials,” said Dr. Mark S. Sulkowski of John Hopkins University in Baltimore and his associates. Adding ribavirin to the simeprevir and sofosbuvir combination regimen did not improve sustained virologic response (SVR), but patients were less likely to achieve it if they had cirrhosis, current or prior hepatic decompensation, or a history of failing other protease inhibitors, the investigators said.
Novel hepatitis C therapies have yielded “substantially lower” rates of SVR and more side effects in everyday practice than in clinical trials, the investigators noted. To better understand how some of newest HCV drugs perform in the real world, they conducted an observational cohort study of the safety, tolerability, and efficacy of simeprevir plus sofosbuvir for treating genotype 1 HCV infections in academic and nonacademic settings (HCV-TARGET) (Gastroenterology 2015 doi: 10.1053/j.gastro.2015.10.013).
A total of 836 patients received once-daily simeprevir (150 mg) and sofosbuvir (400 mg), and 169 of them also received ribavirin. Most (61%) patients had genotype 1a infection and were white (76%), male (61%), and cirrhotic (59%); 13% were black. Patients usually were treatment experienced, having failed peginterferon and ribavirin either with (12%) or without (46%) telaprevir or boceprevir, the researchers said.
In all, 675 (84%) patients achieved SVR after 12 weeks of treatment (SVR12; 95% confidence interval, 81%-87%). Adding ribavirin to the combination PI regimen did not improve SVR, regardless of cirrhosis status, genetic subtype, or treatment history. However, crude SVR12 rates were only 75% for patients with hepatic decompensation and 81% for those with cirrhosis, and these patients had significantly lower adjusted odds of achieving SVR, compared with other patients. In hindsight, decompensated and cirrhotic patients might have needed 24 weeks of treatment, as the Food and Drug Administration now recommends based on the COSMOS trial results (Lancet. 2014;384[9956]:1756-65), the investigators said.
The adjusted model did not uncover a link between genotype 1 subtype and SVR, but only about 10% of patients were tested for the Q80K polymorphism, which is more common in genotype 1a infections and is associated with treatment resistance, the investigators noted. Crude SVR12 rates were 92% for patients with genotype 1b infection and 86% for those with 1a infection, they said.
Only 3% of patients stopped treatment; 2% did so because of side effects, and ribavirin did not significantly affect rates of treatment discontinuation, said the investigators. The most common side effects were fatigue, headache, nausea, rash, and insomnia. Serious adverse events affected 5% of patients and included gastrointestinal bleeding (0.5%), hepatic failure or encephalopathy (1.2%), and infections (1.1%).
Taken together, these results show that simeprevir and sofosbuvir effectively translate from the clinical trial setting into clinical practice, said the researchers. “Additional research is needed to understand which patients may benefit from different treatment regimens or longer treatment durations,” they emphasized.
The study was supported by the University of Florida at Gainesville, the University of North Carolina at Chapel Hill, AbbVie, Bristol-Myers Squibb, Gilead, Janssen, Kadmon, Merck, Vertex, and the National Institutes of Health. Dr. Sulkowski reported grants and personal fees from Gilead, Janssen, Achillion, Abbvie, Merck, and Bristol-Myers Squibb. Of 14 coinvestigators, 13 reported financial relationships with a number of pharmaceutical companies.
A 12-week, ribavirin-free regimen achieved sustained virologic response for 85% of patients with genotype 1 hepatitis C virus (HCV) infection, researchers reported in the February issue of Gastroenterology.
“This represents one of the first applications of a highly effective HCV regimen outside clinical trials,” said Dr. Mark S. Sulkowski of John Hopkins University in Baltimore and his associates. Adding ribavirin to the simeprevir and sofosbuvir combination regimen did not improve sustained virologic response (SVR), but patients were less likely to achieve it if they had cirrhosis, current or prior hepatic decompensation, or a history of failing other protease inhibitors, the investigators said.
Novel hepatitis C therapies have yielded “substantially lower” rates of SVR and more side effects in everyday practice than in clinical trials, the investigators noted. To better understand how some of newest HCV drugs perform in the real world, they conducted an observational cohort study of the safety, tolerability, and efficacy of simeprevir plus sofosbuvir for treating genotype 1 HCV infections in academic and nonacademic settings (HCV-TARGET) (Gastroenterology 2015 doi: 10.1053/j.gastro.2015.10.013).
A total of 836 patients received once-daily simeprevir (150 mg) and sofosbuvir (400 mg), and 169 of them also received ribavirin. Most (61%) patients had genotype 1a infection and were white (76%), male (61%), and cirrhotic (59%); 13% were black. Patients usually were treatment experienced, having failed peginterferon and ribavirin either with (12%) or without (46%) telaprevir or boceprevir, the researchers said.
In all, 675 (84%) patients achieved SVR after 12 weeks of treatment (SVR12; 95% confidence interval, 81%-87%). Adding ribavirin to the combination PI regimen did not improve SVR, regardless of cirrhosis status, genetic subtype, or treatment history. However, crude SVR12 rates were only 75% for patients with hepatic decompensation and 81% for those with cirrhosis, and these patients had significantly lower adjusted odds of achieving SVR, compared with other patients. In hindsight, decompensated and cirrhotic patients might have needed 24 weeks of treatment, as the Food and Drug Administration now recommends based on the COSMOS trial results (Lancet. 2014;384[9956]:1756-65), the investigators said.
The adjusted model did not uncover a link between genotype 1 subtype and SVR, but only about 10% of patients were tested for the Q80K polymorphism, which is more common in genotype 1a infections and is associated with treatment resistance, the investigators noted. Crude SVR12 rates were 92% for patients with genotype 1b infection and 86% for those with 1a infection, they said.
Only 3% of patients stopped treatment; 2% did so because of side effects, and ribavirin did not significantly affect rates of treatment discontinuation, said the investigators. The most common side effects were fatigue, headache, nausea, rash, and insomnia. Serious adverse events affected 5% of patients and included gastrointestinal bleeding (0.5%), hepatic failure or encephalopathy (1.2%), and infections (1.1%).
Taken together, these results show that simeprevir and sofosbuvir effectively translate from the clinical trial setting into clinical practice, said the researchers. “Additional research is needed to understand which patients may benefit from different treatment regimens or longer treatment durations,” they emphasized.
The study was supported by the University of Florida at Gainesville, the University of North Carolina at Chapel Hill, AbbVie, Bristol-Myers Squibb, Gilead, Janssen, Kadmon, Merck, Vertex, and the National Institutes of Health. Dr. Sulkowski reported grants and personal fees from Gilead, Janssen, Achillion, Abbvie, Merck, and Bristol-Myers Squibb. Of 14 coinvestigators, 13 reported financial relationships with a number of pharmaceutical companies.
A 12-week, ribavirin-free regimen achieved sustained virologic response for 85% of patients with genotype 1 hepatitis C virus (HCV) infection, researchers reported in the February issue of Gastroenterology.
“This represents one of the first applications of a highly effective HCV regimen outside clinical trials,” said Dr. Mark S. Sulkowski of John Hopkins University in Baltimore and his associates. Adding ribavirin to the simeprevir and sofosbuvir combination regimen did not improve sustained virologic response (SVR), but patients were less likely to achieve it if they had cirrhosis, current or prior hepatic decompensation, or a history of failing other protease inhibitors, the investigators said.
Novel hepatitis C therapies have yielded “substantially lower” rates of SVR and more side effects in everyday practice than in clinical trials, the investigators noted. To better understand how some of newest HCV drugs perform in the real world, they conducted an observational cohort study of the safety, tolerability, and efficacy of simeprevir plus sofosbuvir for treating genotype 1 HCV infections in academic and nonacademic settings (HCV-TARGET) (Gastroenterology 2015 doi: 10.1053/j.gastro.2015.10.013).
A total of 836 patients received once-daily simeprevir (150 mg) and sofosbuvir (400 mg), and 169 of them also received ribavirin. Most (61%) patients had genotype 1a infection and were white (76%), male (61%), and cirrhotic (59%); 13% were black. Patients usually were treatment experienced, having failed peginterferon and ribavirin either with (12%) or without (46%) telaprevir or boceprevir, the researchers said.
In all, 675 (84%) patients achieved SVR after 12 weeks of treatment (SVR12; 95% confidence interval, 81%-87%). Adding ribavirin to the combination PI regimen did not improve SVR, regardless of cirrhosis status, genetic subtype, or treatment history. However, crude SVR12 rates were only 75% for patients with hepatic decompensation and 81% for those with cirrhosis, and these patients had significantly lower adjusted odds of achieving SVR, compared with other patients. In hindsight, decompensated and cirrhotic patients might have needed 24 weeks of treatment, as the Food and Drug Administration now recommends based on the COSMOS trial results (Lancet. 2014;384[9956]:1756-65), the investigators said.
The adjusted model did not uncover a link between genotype 1 subtype and SVR, but only about 10% of patients were tested for the Q80K polymorphism, which is more common in genotype 1a infections and is associated with treatment resistance, the investigators noted. Crude SVR12 rates were 92% for patients with genotype 1b infection and 86% for those with 1a infection, they said.
Only 3% of patients stopped treatment; 2% did so because of side effects, and ribavirin did not significantly affect rates of treatment discontinuation, said the investigators. The most common side effects were fatigue, headache, nausea, rash, and insomnia. Serious adverse events affected 5% of patients and included gastrointestinal bleeding (0.5%), hepatic failure or encephalopathy (1.2%), and infections (1.1%).
Taken together, these results show that simeprevir and sofosbuvir effectively translate from the clinical trial setting into clinical practice, said the researchers. “Additional research is needed to understand which patients may benefit from different treatment regimens or longer treatment durations,” they emphasized.
The study was supported by the University of Florida at Gainesville, the University of North Carolina at Chapel Hill, AbbVie, Bristol-Myers Squibb, Gilead, Janssen, Kadmon, Merck, Vertex, and the National Institutes of Health. Dr. Sulkowski reported grants and personal fees from Gilead, Janssen, Achillion, Abbvie, Merck, and Bristol-Myers Squibb. Of 14 coinvestigators, 13 reported financial relationships with a number of pharmaceutical companies.
FROM GASTROENTEROLOGY
Key clinical point: Twelve weeks of simeprevir and sofosbuvir cured about 85% of real-world patients with genotype 1 hepatitis C virus infection.
Major finding: The unadjusted rate of SVR12 was 85% (95% CI, 82%-88%).
Data source: An analysis of an observational cohort study of protease inhibitor combination regimen with or without ribavirin for 836 patients (HCV-TARGET).
Disclosures: The study was supported by the University of Florida at Gainesville, the University of North Carolina at Chapel Hill, AbbVie, Bristol-Myers Squibb, Gilead, Janssen, Kadmon, Merck, Vertex, and the National Institutes of Health. Dr. Sulkowski reported grants and personal fees from Gilead, Janssen, Achillion, Abbvie, Merck, and Bristol-Myers Squibb. Of 14 coinvestigators, 13 reported financial relationships with a number of pharmaceutical companies.
Factors within VA control could help prevent missed, canceled appointments
Opt-out scheduling protocols and long appointment lead times contributed significantly to missed and canceled colonoscopy appointments at Veterans Health Administration facilities, researchers reported in the February issue of Clinical Gastroenterology and Hepatology.
These factors are within the control of the Veterans Affairs and could be altered to improve productivity and efficiency, said Melissa Partin, Ph.D., of the Center for Chronic Disease Outcomes Research at the Minneapolis Veterans Affairs Health Care System in Minneapolis, and her associates.
Source: American Gastroenterological Association
Missed and canceled medical appointments are always a concern, but particularly so for colonoscopy clinics, where they incur an average daily net loss of $725, the investigators noted. Most clinics have limited colonoscopy capacity, and even a 30-day wait for diagnostic colonoscopy has been linked to “modest but significantly elevated” chances of detecting cancer on exam, they added. To better understand these problems, they separately examined predictors of missed and canceled appointments among 27,994 patients who had positive fecal occult blood tests with diagnostic colonoscopies scheduled at 69 VA facilities between 2009 and 2011 (Clin Gastroenterol Hepatol. 2015 Aug 21. doi: 10.1016/j.cgh.2015.07.051).
Having a life expectancy of 6 months or less and no personal history of polyps best predicted missing an appointment, with odds ratios of 2.74 for each factor, the researchers said. However, only 0.47% of patients had such a short life expectancy. Other significant predictors of missed appointments included being seen at the largest and most complex facilities (odds ratio, 2.69; P = .007), having both psychiatric and substance abuse disorders (OR, 1.82; P less than .0001), and the use of opt-out scheduling, in which patients were automatically scheduled rather than having to schedule appointments themselves (OR, 1.57; P = .02). Canceled appointments also were linked to opt-out scheduling, as well as to older age and having no history of polyps.
Most appointment lead times were 28 days, and each 12-day increase in lead time increased the odds of missing or canceling appointments by about 15% (P less than .0001). The problem could be curtailed by the Veterans Access, Choice and Accountability Act of 2014, which allows those who cannot schedule VA appointments within 30 days to receive care from eligible non–VA providers, the investigators said. “Future research should focus on assessing the effect of the Choice Act on colonoscopy appointment lead time and on developing and evaluating efficient and effective approaches to implementing the other clinic-level changes supported by our findings,” they added.
The study might have oversimplified or missed changes in protocols because it used single-item survey measures at one point in time, the investigators said. For some patients, the first appointment after the fecal occult blood test may have been for another procedure besides colonoscopy, they added. Furthermore, they did not distinguish between appointments canceled by patients versus clinics. “The VHA is a unique context, characterized by a predominantly male, low-income population with high rates of mental health and substance abuse diagnoses. Therefore, our findings may not generalize to other settings,” they added. “However, our findings do have important implications for a substantial population of health providers and consumers in this country, because the VHA is the largest integrated health care system in the United States.”
The study was funded by the Department of Veterans Affairs Clinical Science Service and Health Services Research & Development Service. The investigators had no disclosures.
Opt-out scheduling protocols and long appointment lead times contributed significantly to missed and canceled colonoscopy appointments at Veterans Health Administration facilities, researchers reported in the February issue of Clinical Gastroenterology and Hepatology.
These factors are within the control of the Veterans Affairs and could be altered to improve productivity and efficiency, said Melissa Partin, Ph.D., of the Center for Chronic Disease Outcomes Research at the Minneapolis Veterans Affairs Health Care System in Minneapolis, and her associates.
Source: American Gastroenterological Association
Missed and canceled medical appointments are always a concern, but particularly so for colonoscopy clinics, where they incur an average daily net loss of $725, the investigators noted. Most clinics have limited colonoscopy capacity, and even a 30-day wait for diagnostic colonoscopy has been linked to “modest but significantly elevated” chances of detecting cancer on exam, they added. To better understand these problems, they separately examined predictors of missed and canceled appointments among 27,994 patients who had positive fecal occult blood tests with diagnostic colonoscopies scheduled at 69 VA facilities between 2009 and 2011 (Clin Gastroenterol Hepatol. 2015 Aug 21. doi: 10.1016/j.cgh.2015.07.051).
Having a life expectancy of 6 months or less and no personal history of polyps best predicted missing an appointment, with odds ratios of 2.74 for each factor, the researchers said. However, only 0.47% of patients had such a short life expectancy. Other significant predictors of missed appointments included being seen at the largest and most complex facilities (odds ratio, 2.69; P = .007), having both psychiatric and substance abuse disorders (OR, 1.82; P less than .0001), and the use of opt-out scheduling, in which patients were automatically scheduled rather than having to schedule appointments themselves (OR, 1.57; P = .02). Canceled appointments also were linked to opt-out scheduling, as well as to older age and having no history of polyps.
Most appointment lead times were 28 days, and each 12-day increase in lead time increased the odds of missing or canceling appointments by about 15% (P less than .0001). The problem could be curtailed by the Veterans Access, Choice and Accountability Act of 2014, which allows those who cannot schedule VA appointments within 30 days to receive care from eligible non–VA providers, the investigators said. “Future research should focus on assessing the effect of the Choice Act on colonoscopy appointment lead time and on developing and evaluating efficient and effective approaches to implementing the other clinic-level changes supported by our findings,” they added.
The study might have oversimplified or missed changes in protocols because it used single-item survey measures at one point in time, the investigators said. For some patients, the first appointment after the fecal occult blood test may have been for another procedure besides colonoscopy, they added. Furthermore, they did not distinguish between appointments canceled by patients versus clinics. “The VHA is a unique context, characterized by a predominantly male, low-income population with high rates of mental health and substance abuse diagnoses. Therefore, our findings may not generalize to other settings,” they added. “However, our findings do have important implications for a substantial population of health providers and consumers in this country, because the VHA is the largest integrated health care system in the United States.”
The study was funded by the Department of Veterans Affairs Clinical Science Service and Health Services Research & Development Service. The investigators had no disclosures.
Opt-out scheduling protocols and long appointment lead times contributed significantly to missed and canceled colonoscopy appointments at Veterans Health Administration facilities, researchers reported in the February issue of Clinical Gastroenterology and Hepatology.
These factors are within the control of the Veterans Affairs and could be altered to improve productivity and efficiency, said Melissa Partin, Ph.D., of the Center for Chronic Disease Outcomes Research at the Minneapolis Veterans Affairs Health Care System in Minneapolis, and her associates.
Source: American Gastroenterological Association
Missed and canceled medical appointments are always a concern, but particularly so for colonoscopy clinics, where they incur an average daily net loss of $725, the investigators noted. Most clinics have limited colonoscopy capacity, and even a 30-day wait for diagnostic colonoscopy has been linked to “modest but significantly elevated” chances of detecting cancer on exam, they added. To better understand these problems, they separately examined predictors of missed and canceled appointments among 27,994 patients who had positive fecal occult blood tests with diagnostic colonoscopies scheduled at 69 VA facilities between 2009 and 2011 (Clin Gastroenterol Hepatol. 2015 Aug 21. doi: 10.1016/j.cgh.2015.07.051).
Having a life expectancy of 6 months or less and no personal history of polyps best predicted missing an appointment, with odds ratios of 2.74 for each factor, the researchers said. However, only 0.47% of patients had such a short life expectancy. Other significant predictors of missed appointments included being seen at the largest and most complex facilities (odds ratio, 2.69; P = .007), having both psychiatric and substance abuse disorders (OR, 1.82; P less than .0001), and the use of opt-out scheduling, in which patients were automatically scheduled rather than having to schedule appointments themselves (OR, 1.57; P = .02). Canceled appointments also were linked to opt-out scheduling, as well as to older age and having no history of polyps.
Most appointment lead times were 28 days, and each 12-day increase in lead time increased the odds of missing or canceling appointments by about 15% (P less than .0001). The problem could be curtailed by the Veterans Access, Choice and Accountability Act of 2014, which allows those who cannot schedule VA appointments within 30 days to receive care from eligible non–VA providers, the investigators said. “Future research should focus on assessing the effect of the Choice Act on colonoscopy appointment lead time and on developing and evaluating efficient and effective approaches to implementing the other clinic-level changes supported by our findings,” they added.
The study might have oversimplified or missed changes in protocols because it used single-item survey measures at one point in time, the investigators said. For some patients, the first appointment after the fecal occult blood test may have been for another procedure besides colonoscopy, they added. Furthermore, they did not distinguish between appointments canceled by patients versus clinics. “The VHA is a unique context, characterized by a predominantly male, low-income population with high rates of mental health and substance abuse diagnoses. Therefore, our findings may not generalize to other settings,” they added. “However, our findings do have important implications for a substantial population of health providers and consumers in this country, because the VHA is the largest integrated health care system in the United States.”
The study was funded by the Department of Veterans Affairs Clinical Science Service and Health Services Research & Development Service. The investigators had no disclosures.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: Opt-out scheduling practices and long appointment lead times predicted missed and canceled colonoscopies at the VA.
Major finding: Estimated ratios for these predictors ranged between 1.12 and 1.57, and all were statistically significant.
Data source: An analysis of data from 27,994 patients who had positive fecal occult blood tests with diagnostic colonoscopies scheduled at 69 VA facilities between 2009 and 2011.
Disclosures: The study was funded by the Department of Veterans Affairs Clinical Science Service and Health Services Research and Development Service. The investigators had no disclosures.
FDA approves new treatment for chronic HCV genotypes 1 and 4
The U.S. Food and Drug Administration has approved Zepatier (elbasvir and grazoprevir) with or without ribavirin for the treatment of chronic hepatitis C virus genotypes 1 and 4 infections in adults.
Zepatier, marketed by Merck, was granted breakthrough therapy designation for the treatment of chronic HCV genotype 1 infection in patients with end stage renal disease on hemodialysis and for the treatment of chronic HCV genotype 4 infection. Breakthrough therapy designation is a program designed to expedite the development and review of drugs that are intended to treat a serious condition and preliminary clinical evidence indicates that the drug may demonstrate substantial improvement over available therapy on a clinically significant endpoint.
“Today’s approval provides another oral treatment option for patients with genotypes 1 and 4 HCV infections without requiring use of interferon,” said Dr. Edward Cox, director of the Office of Antimicrobial Products in the FDA’s Center for Drug Evaluation and Research in a statement.
The safety and efficacy of Zepatier with or without ribavirin was evaluated in clinical trials of 1,373 participants with chronic HCV genotype 1 or 4 infections with and without cirrhosis. The participants received Zepatier with or without ribavirin once daily for 12 or 16 weeks. The studies were designed to measure whether a participant’s hepatitis C virus was no longer detected in the blood 12 weeks after finishing treatment (sustained virologic response), suggesting a participant’s infection had been cured.
The overall sustained virologic response rates ranged from 94% to 97% in genotype 1–infected subjects and from 97% to 100% in genotype 4–infected subjects across trials for the approved treatment regimens.
The FDA recommends clinicians screen genotype 1a–infected patients for certain viral genetic variations prior to starting treatment with Zepatier to determine dosage regimen and duration. Zepatier carries a warning alerting patients and health care providers that elevations of liver enzymes to greater than five times the upper limit of normal occurred in approximately 1% of clinical trial participants, generally at or after treatment week 8. The FDA says liver-related blood tests should be performed prior to starting therapy and at certain times during treatment.
Zepatier is not intended for patients with moderate or severe liver impairment.
On Twitter @richpizzi
The U.S. Food and Drug Administration has approved Zepatier (elbasvir and grazoprevir) with or without ribavirin for the treatment of chronic hepatitis C virus genotypes 1 and 4 infections in adults.
Zepatier, marketed by Merck, was granted breakthrough therapy designation for the treatment of chronic HCV genotype 1 infection in patients with end stage renal disease on hemodialysis and for the treatment of chronic HCV genotype 4 infection. Breakthrough therapy designation is a program designed to expedite the development and review of drugs that are intended to treat a serious condition and preliminary clinical evidence indicates that the drug may demonstrate substantial improvement over available therapy on a clinically significant endpoint.
“Today’s approval provides another oral treatment option for patients with genotypes 1 and 4 HCV infections without requiring use of interferon,” said Dr. Edward Cox, director of the Office of Antimicrobial Products in the FDA’s Center for Drug Evaluation and Research in a statement.
The safety and efficacy of Zepatier with or without ribavirin was evaluated in clinical trials of 1,373 participants with chronic HCV genotype 1 or 4 infections with and without cirrhosis. The participants received Zepatier with or without ribavirin once daily for 12 or 16 weeks. The studies were designed to measure whether a participant’s hepatitis C virus was no longer detected in the blood 12 weeks after finishing treatment (sustained virologic response), suggesting a participant’s infection had been cured.
The overall sustained virologic response rates ranged from 94% to 97% in genotype 1–infected subjects and from 97% to 100% in genotype 4–infected subjects across trials for the approved treatment regimens.
The FDA recommends clinicians screen genotype 1a–infected patients for certain viral genetic variations prior to starting treatment with Zepatier to determine dosage regimen and duration. Zepatier carries a warning alerting patients and health care providers that elevations of liver enzymes to greater than five times the upper limit of normal occurred in approximately 1% of clinical trial participants, generally at or after treatment week 8. The FDA says liver-related blood tests should be performed prior to starting therapy and at certain times during treatment.
Zepatier is not intended for patients with moderate or severe liver impairment.
On Twitter @richpizzi
The U.S. Food and Drug Administration has approved Zepatier (elbasvir and grazoprevir) with or without ribavirin for the treatment of chronic hepatitis C virus genotypes 1 and 4 infections in adults.
Zepatier, marketed by Merck, was granted breakthrough therapy designation for the treatment of chronic HCV genotype 1 infection in patients with end stage renal disease on hemodialysis and for the treatment of chronic HCV genotype 4 infection. Breakthrough therapy designation is a program designed to expedite the development and review of drugs that are intended to treat a serious condition and preliminary clinical evidence indicates that the drug may demonstrate substantial improvement over available therapy on a clinically significant endpoint.
“Today’s approval provides another oral treatment option for patients with genotypes 1 and 4 HCV infections without requiring use of interferon,” said Dr. Edward Cox, director of the Office of Antimicrobial Products in the FDA’s Center for Drug Evaluation and Research in a statement.
The safety and efficacy of Zepatier with or without ribavirin was evaluated in clinical trials of 1,373 participants with chronic HCV genotype 1 or 4 infections with and without cirrhosis. The participants received Zepatier with or without ribavirin once daily for 12 or 16 weeks. The studies were designed to measure whether a participant’s hepatitis C virus was no longer detected in the blood 12 weeks after finishing treatment (sustained virologic response), suggesting a participant’s infection had been cured.
The overall sustained virologic response rates ranged from 94% to 97% in genotype 1–infected subjects and from 97% to 100% in genotype 4–infected subjects across trials for the approved treatment regimens.
The FDA recommends clinicians screen genotype 1a–infected patients for certain viral genetic variations prior to starting treatment with Zepatier to determine dosage regimen and duration. Zepatier carries a warning alerting patients and health care providers that elevations of liver enzymes to greater than five times the upper limit of normal occurred in approximately 1% of clinical trial participants, generally at or after treatment week 8. The FDA says liver-related blood tests should be performed prior to starting therapy and at certain times during treatment.
Zepatier is not intended for patients with moderate or severe liver impairment.
On Twitter @richpizzi
Hepatitis C incidence rising in hemodialysis patients
Incidence of newly acquired hepatitis C virus has increased recently in patients undergoing hemodialysis, according to a health advisory from the Centers for Disease Control and Prevention.
In 2014 and 2015, 36 cases of HCV infection were reported to the CDC from 19 clinics in eight states. While investigation is ongoing, HCV transmission between patients has been confirmed in at least nine facilities, and in several facilities, lapses in infection control were also identified. Better screening and awareness of HCV infection potential may also play a role in the increased disease incidence.
The CDC recommends that dialysis facilities assess current infection control practices, environmental cleaning, and disinfection practices to evaluate adherence to standards, address any gaps, screen patients for HCV, and to report all HCV infections to the CDC promptly.
“Dialysis facilities should actively assess and continuously improve their infection control, environmental cleaning and disinfection, and HCV screening practices, whether or not they are aware of infections in their clinic. Any case of new HCV infection in a patient undergoing hemodialysis is likely to be a health care–associated infection and should be reported to public health authorities in a timely manner,” the CDC said
Find the full health advisory on the CDC website.
Incidence of newly acquired hepatitis C virus has increased recently in patients undergoing hemodialysis, according to a health advisory from the Centers for Disease Control and Prevention.
In 2014 and 2015, 36 cases of HCV infection were reported to the CDC from 19 clinics in eight states. While investigation is ongoing, HCV transmission between patients has been confirmed in at least nine facilities, and in several facilities, lapses in infection control were also identified. Better screening and awareness of HCV infection potential may also play a role in the increased disease incidence.
The CDC recommends that dialysis facilities assess current infection control practices, environmental cleaning, and disinfection practices to evaluate adherence to standards, address any gaps, screen patients for HCV, and to report all HCV infections to the CDC promptly.
“Dialysis facilities should actively assess and continuously improve their infection control, environmental cleaning and disinfection, and HCV screening practices, whether or not they are aware of infections in their clinic. Any case of new HCV infection in a patient undergoing hemodialysis is likely to be a health care–associated infection and should be reported to public health authorities in a timely manner,” the CDC said
Find the full health advisory on the CDC website.
Incidence of newly acquired hepatitis C virus has increased recently in patients undergoing hemodialysis, according to a health advisory from the Centers for Disease Control and Prevention.
In 2014 and 2015, 36 cases of HCV infection were reported to the CDC from 19 clinics in eight states. While investigation is ongoing, HCV transmission between patients has been confirmed in at least nine facilities, and in several facilities, lapses in infection control were also identified. Better screening and awareness of HCV infection potential may also play a role in the increased disease incidence.
The CDC recommends that dialysis facilities assess current infection control practices, environmental cleaning, and disinfection practices to evaluate adherence to standards, address any gaps, screen patients for HCV, and to report all HCV infections to the CDC promptly.
“Dialysis facilities should actively assess and continuously improve their infection control, environmental cleaning and disinfection, and HCV screening practices, whether or not they are aware of infections in their clinic. Any case of new HCV infection in a patient undergoing hemodialysis is likely to be a health care–associated infection and should be reported to public health authorities in a timely manner,” the CDC said
Find the full health advisory on the CDC website.
Trial results for hepatitis C antivirals not generalizable to coinfected HIV/HCV patients
Clinical trial results for direct-acting antivirals against hepatitis C virus (HCV) may have limited generalizability for patients coinfected with HIV and HCV, a recent study suggested.
A research team led by Dr. Marina Klein of the division of infectious diseases at McGill University Health Centre, Montreal, determined it was important to assess the generalizability of clinical trial results for direct-acting antivirals (DAA) since the drugs have been described as revolutionary treatments for HCV. Clinical trial results have generally shown that DAAs are well tolerated, more conveniently dosed, and highly efficacious, compared with earlier, interferon-based HCV therapies.
The Canadian researchers noted, however, that DAA trials have included relatively small numbers of participants (subgroups ranging from 6 to 60) and have applied very strict eligibility criteria, likely excluding a substantial segment of the coinfected patient population.
To assess DAA trial generalizability, investigators applied the eligibility criteria from five efficacy trials evaluating simeprevir; sofosbuvir; ombitasvir, paritaprevir/ritonavir, and dasabuvir (3D); sofosbuvir/ledipasvir; and daclatasvir/sofosbuvir to the Canadian Co-Infection Cohort, representing about 23% of the total coinfected Canadian population in care.
The results confirmed the researchers’ suspicions, as only 5.9% of coinfected patients in the cohort would have been eligible for enrollment in the simeprevir trial, 9.8% in the sofosbuvir trial, 6.3% in the 3D trial, and 8.1% in the sofosbuvir/ledipasvir trial. Eligibility into the daclatasvir/sofosbuvir study was more inclusive – 43% of the cohort would have been eligible. The most exclusive eligibility criteria across all trials, with the exception of the daclatasvir/sofosbuvir study, were restriction to specific antiretroviral therapies (63%-79%) and active illicit drug use (53%-55%).
The exclusions “appeared to be related to improving treatment outcomes by not including those at higher risk of poor adherence and reinfection – individuals for whom real world data is urgently needed,” Dr. Klein and her coauthors concluded.
Read the full report in Clinical Infectious Diseases (Clin Infect Dis. 2016 Jan 6. doi: 10.1093/cid/civ1222).
On Twitter @richpizzi
Clinical trial results for direct-acting antivirals against hepatitis C virus (HCV) may have limited generalizability for patients coinfected with HIV and HCV, a recent study suggested.
A research team led by Dr. Marina Klein of the division of infectious diseases at McGill University Health Centre, Montreal, determined it was important to assess the generalizability of clinical trial results for direct-acting antivirals (DAA) since the drugs have been described as revolutionary treatments for HCV. Clinical trial results have generally shown that DAAs are well tolerated, more conveniently dosed, and highly efficacious, compared with earlier, interferon-based HCV therapies.
The Canadian researchers noted, however, that DAA trials have included relatively small numbers of participants (subgroups ranging from 6 to 60) and have applied very strict eligibility criteria, likely excluding a substantial segment of the coinfected patient population.
To assess DAA trial generalizability, investigators applied the eligibility criteria from five efficacy trials evaluating simeprevir; sofosbuvir; ombitasvir, paritaprevir/ritonavir, and dasabuvir (3D); sofosbuvir/ledipasvir; and daclatasvir/sofosbuvir to the Canadian Co-Infection Cohort, representing about 23% of the total coinfected Canadian population in care.
The results confirmed the researchers’ suspicions, as only 5.9% of coinfected patients in the cohort would have been eligible for enrollment in the simeprevir trial, 9.8% in the sofosbuvir trial, 6.3% in the 3D trial, and 8.1% in the sofosbuvir/ledipasvir trial. Eligibility into the daclatasvir/sofosbuvir study was more inclusive – 43% of the cohort would have been eligible. The most exclusive eligibility criteria across all trials, with the exception of the daclatasvir/sofosbuvir study, were restriction to specific antiretroviral therapies (63%-79%) and active illicit drug use (53%-55%).
The exclusions “appeared to be related to improving treatment outcomes by not including those at higher risk of poor adherence and reinfection – individuals for whom real world data is urgently needed,” Dr. Klein and her coauthors concluded.
Read the full report in Clinical Infectious Diseases (Clin Infect Dis. 2016 Jan 6. doi: 10.1093/cid/civ1222).
On Twitter @richpizzi
Clinical trial results for direct-acting antivirals against hepatitis C virus (HCV) may have limited generalizability for patients coinfected with HIV and HCV, a recent study suggested.
A research team led by Dr. Marina Klein of the division of infectious diseases at McGill University Health Centre, Montreal, determined it was important to assess the generalizability of clinical trial results for direct-acting antivirals (DAA) since the drugs have been described as revolutionary treatments for HCV. Clinical trial results have generally shown that DAAs are well tolerated, more conveniently dosed, and highly efficacious, compared with earlier, interferon-based HCV therapies.
The Canadian researchers noted, however, that DAA trials have included relatively small numbers of participants (subgroups ranging from 6 to 60) and have applied very strict eligibility criteria, likely excluding a substantial segment of the coinfected patient population.
To assess DAA trial generalizability, investigators applied the eligibility criteria from five efficacy trials evaluating simeprevir; sofosbuvir; ombitasvir, paritaprevir/ritonavir, and dasabuvir (3D); sofosbuvir/ledipasvir; and daclatasvir/sofosbuvir to the Canadian Co-Infection Cohort, representing about 23% of the total coinfected Canadian population in care.
The results confirmed the researchers’ suspicions, as only 5.9% of coinfected patients in the cohort would have been eligible for enrollment in the simeprevir trial, 9.8% in the sofosbuvir trial, 6.3% in the 3D trial, and 8.1% in the sofosbuvir/ledipasvir trial. Eligibility into the daclatasvir/sofosbuvir study was more inclusive – 43% of the cohort would have been eligible. The most exclusive eligibility criteria across all trials, with the exception of the daclatasvir/sofosbuvir study, were restriction to specific antiretroviral therapies (63%-79%) and active illicit drug use (53%-55%).
The exclusions “appeared to be related to improving treatment outcomes by not including those at higher risk of poor adherence and reinfection – individuals for whom real world data is urgently needed,” Dr. Klein and her coauthors concluded.
Read the full report in Clinical Infectious Diseases (Clin Infect Dis. 2016 Jan 6. doi: 10.1093/cid/civ1222).
On Twitter @richpizzi
FROM CLINICAL INFECTIOUS DISEASES
Nonalcoholic fatty liver disease will keep rising ‘in near term’
Nonalcoholic fatty liver disease (NAFLD) almost tripled among United States veterans in a recent 9-year period, investigators reported in the February issue of Clinical Gastroenterology and Hepatology.
The trend “was evident in all racial groups, across all age groups, and in both genders,” said Dr. Fasiha Kanwal of the Michael E. DeBakey Veterans Affairs Medical Center and Baylor College of Medicine, both in Houston, and her associates. The increasing prevalence of NAFLD “is likely generalizable to nonveterans,” and will probably persist because of a “fairly steady” 2%-3% overall annual incidence and a steeper rise among younger individuals, they added. “Nonalcoholic fatty liver disease will continue to remain a major public health problem in the United States, at least in the near and intermediate future.”
Although NAFLD is the leading cause of chronic liver failure in the United States, few studies have examined its incidence or prevalence over time, which are key to predicting future disease burden. Therefore, the investigators analyzed data for more than 9.78 million patients who visited the VA at least once between 2003 and 2011. They defined NAFLD as at least two elevated alanine aminotransferase (ALT) values (greater than 40 IU/mL) separated by at least 6 months, with no history of positive serology for hepatitis B surface antigen or hepatitis C virus RNA, and no alcohol-related ICD-9 codes or positive AUDIT-C scores within a year of elevated ALT levels (Clin Gastroenterol Hepatol. 2015 Aug 7. doi: 10.1016/j.cgh.2015.08.010).
During the study period, more than 1.3 million patients, or 13.6%, met the definition of NAFLD, said the researchers. Age-adjusted incidence rates dropped slightly from 3.16% in 2003 to 2.5% in 2011, ranging between 2.3% and 2.7% in most years. Prevalence, however, rose from 6.3% in 2003 (95% confidence interval, 6.26%-6.3%) to 17.6% in 2011 (95% CI, 17.58%-17.65%), a 2.8-fold increase. Moreover, about one in five patients with NAFLD who visited the VA in 2011 was at risk for advanced fibrosis.
Among individuals who were younger than 45 years, the incidence of NAFLD rose from 2.3 to 4.3 cases per 100 persons (annual percentage change, 7.4%; 95% CI, 5.7% to 9.2%), the researchers also found. “Although recent studies show that the rate of increase in both obesity and diabetes, which are both major risk factors for NAFLD, may be slowing down in the U.S., this may not be the case in the VA, where the prevalence of obesity and diabetes is in fact higher than in the U.S. population,” they said.
In general, the findings mirror a recent analysis of the National Health and Nutrition Examination Survey (Aliment Pharmacol Ther. 2015 Jan;41[1]:65-76), according to the investigators. “The VA is the largest integrated health care system in the United States,” they added. “We believe that the sheer size of the veteran cohort, combined with a complete dearth of information regarding the burden of NAFLD in the VA, renders our findings highly significant. Furthermore, the VA is in a unique position to test and implement systemic changes in medical care delivery to improve the health care of NAFLD patients.”
The study was partially supported by the Michael E. DeBakey Veterans Affairs Medical Center. The researchers had no disclosures.
Kanwal and colleagues present an interesting study assessing the trends in the incidence and prevalence of NAFLD in the United States. Findings suggest that the annual incidence of NAFLD has generally been stable (2.2%-3.2%), while the prevalence of NAFLD has increased by 2.8-fold (6.3%-17.6%). These findings are consistent with the literature and provide additional evidence supporting the increasing burden of NAFLD. Although an important study, there are some limitations to the study design. First, the diagnosis of NAFLD was solely based on elevated liver enzymes, which can underestimate the true incidence and prevalence of NAFLD. In fact, in a recent meta-analysis, NAFLD prevalence based on liver enzymes was 13%, while NAFLD prevalence based on radiologic diagnosis was 25% (Hepatology. 2015 Dec 28. doi: 10.1002/hep.28431. [Epub ahead of print]). Second, the study subjects came from the VA system, which may not be representative of the U.S. population (Patrick AFB, FL: Defense Equal Opportunity Management Institute, 2010). This is important because sex-specific differences in the prevalence of NAFLD have been reported (Hepatology. 2015 Dec 28. doi: 10.1002/hep.28431. [Epub ahead of print]). Nevertheless, these limitations do not minimize the important contribution of this study. There appears to be an alarming increase in the burden of NAFLD within all the racial and age groups in the U.S. Further, this increase in the incidence and prevalence of NAFLD is especially significant among the younger age groups (less than 45 years). This finding is in contrast to others who have reported a higher prevalence in older subjects (Presented at AASLD 2015. San Francisco. Abstract #534). If confirmed, this younger cohort of patients with NAFLD can fuel the future burden of liver disease for the next few decades (JAMA. 2012;307:491-7). Given the current lack of an effective treatment for NAFLD, a national strategy to deal with this important and rising cause of chronic liver disease is urgently needed.
Dr. Zobair M. Younossi, MPH, FACG, AGAF, FAASLD, is chairman, department of medicine, Inova Fairfax Hospital; vice president for research, Inova Health System; professor of medicine, VCU-Inova Campus and Beatty Center for Integrated Research, Falls Church, Va. He has consulted for Gilead, AbbVie, Intercept, BMS, and GSK.
Kanwal and colleagues present an interesting study assessing the trends in the incidence and prevalence of NAFLD in the United States. Findings suggest that the annual incidence of NAFLD has generally been stable (2.2%-3.2%), while the prevalence of NAFLD has increased by 2.8-fold (6.3%-17.6%). These findings are consistent with the literature and provide additional evidence supporting the increasing burden of NAFLD. Although an important study, there are some limitations to the study design. First, the diagnosis of NAFLD was solely based on elevated liver enzymes, which can underestimate the true incidence and prevalence of NAFLD. In fact, in a recent meta-analysis, NAFLD prevalence based on liver enzymes was 13%, while NAFLD prevalence based on radiologic diagnosis was 25% (Hepatology. 2015 Dec 28. doi: 10.1002/hep.28431. [Epub ahead of print]). Second, the study subjects came from the VA system, which may not be representative of the U.S. population (Patrick AFB, FL: Defense Equal Opportunity Management Institute, 2010). This is important because sex-specific differences in the prevalence of NAFLD have been reported (Hepatology. 2015 Dec 28. doi: 10.1002/hep.28431. [Epub ahead of print]). Nevertheless, these limitations do not minimize the important contribution of this study. There appears to be an alarming increase in the burden of NAFLD within all the racial and age groups in the U.S. Further, this increase in the incidence and prevalence of NAFLD is especially significant among the younger age groups (less than 45 years). This finding is in contrast to others who have reported a higher prevalence in older subjects (Presented at AASLD 2015. San Francisco. Abstract #534). If confirmed, this younger cohort of patients with NAFLD can fuel the future burden of liver disease for the next few decades (JAMA. 2012;307:491-7). Given the current lack of an effective treatment for NAFLD, a national strategy to deal with this important and rising cause of chronic liver disease is urgently needed.
Dr. Zobair M. Younossi, MPH, FACG, AGAF, FAASLD, is chairman, department of medicine, Inova Fairfax Hospital; vice president for research, Inova Health System; professor of medicine, VCU-Inova Campus and Beatty Center for Integrated Research, Falls Church, Va. He has consulted for Gilead, AbbVie, Intercept, BMS, and GSK.
Kanwal and colleagues present an interesting study assessing the trends in the incidence and prevalence of NAFLD in the United States. Findings suggest that the annual incidence of NAFLD has generally been stable (2.2%-3.2%), while the prevalence of NAFLD has increased by 2.8-fold (6.3%-17.6%). These findings are consistent with the literature and provide additional evidence supporting the increasing burden of NAFLD. Although an important study, there are some limitations to the study design. First, the diagnosis of NAFLD was solely based on elevated liver enzymes, which can underestimate the true incidence and prevalence of NAFLD. In fact, in a recent meta-analysis, NAFLD prevalence based on liver enzymes was 13%, while NAFLD prevalence based on radiologic diagnosis was 25% (Hepatology. 2015 Dec 28. doi: 10.1002/hep.28431. [Epub ahead of print]). Second, the study subjects came from the VA system, which may not be representative of the U.S. population (Patrick AFB, FL: Defense Equal Opportunity Management Institute, 2010). This is important because sex-specific differences in the prevalence of NAFLD have been reported (Hepatology. 2015 Dec 28. doi: 10.1002/hep.28431. [Epub ahead of print]). Nevertheless, these limitations do not minimize the important contribution of this study. There appears to be an alarming increase in the burden of NAFLD within all the racial and age groups in the U.S. Further, this increase in the incidence and prevalence of NAFLD is especially significant among the younger age groups (less than 45 years). This finding is in contrast to others who have reported a higher prevalence in older subjects (Presented at AASLD 2015. San Francisco. Abstract #534). If confirmed, this younger cohort of patients with NAFLD can fuel the future burden of liver disease for the next few decades (JAMA. 2012;307:491-7). Given the current lack of an effective treatment for NAFLD, a national strategy to deal with this important and rising cause of chronic liver disease is urgently needed.
Dr. Zobair M. Younossi, MPH, FACG, AGAF, FAASLD, is chairman, department of medicine, Inova Fairfax Hospital; vice president for research, Inova Health System; professor of medicine, VCU-Inova Campus and Beatty Center for Integrated Research, Falls Church, Va. He has consulted for Gilead, AbbVie, Intercept, BMS, and GSK.
Nonalcoholic fatty liver disease (NAFLD) almost tripled among United States veterans in a recent 9-year period, investigators reported in the February issue of Clinical Gastroenterology and Hepatology.
The trend “was evident in all racial groups, across all age groups, and in both genders,” said Dr. Fasiha Kanwal of the Michael E. DeBakey Veterans Affairs Medical Center and Baylor College of Medicine, both in Houston, and her associates. The increasing prevalence of NAFLD “is likely generalizable to nonveterans,” and will probably persist because of a “fairly steady” 2%-3% overall annual incidence and a steeper rise among younger individuals, they added. “Nonalcoholic fatty liver disease will continue to remain a major public health problem in the United States, at least in the near and intermediate future.”
Although NAFLD is the leading cause of chronic liver failure in the United States, few studies have examined its incidence or prevalence over time, which are key to predicting future disease burden. Therefore, the investigators analyzed data for more than 9.78 million patients who visited the VA at least once between 2003 and 2011. They defined NAFLD as at least two elevated alanine aminotransferase (ALT) values (greater than 40 IU/mL) separated by at least 6 months, with no history of positive serology for hepatitis B surface antigen or hepatitis C virus RNA, and no alcohol-related ICD-9 codes or positive AUDIT-C scores within a year of elevated ALT levels (Clin Gastroenterol Hepatol. 2015 Aug 7. doi: 10.1016/j.cgh.2015.08.010).
During the study period, more than 1.3 million patients, or 13.6%, met the definition of NAFLD, said the researchers. Age-adjusted incidence rates dropped slightly from 3.16% in 2003 to 2.5% in 2011, ranging between 2.3% and 2.7% in most years. Prevalence, however, rose from 6.3% in 2003 (95% confidence interval, 6.26%-6.3%) to 17.6% in 2011 (95% CI, 17.58%-17.65%), a 2.8-fold increase. Moreover, about one in five patients with NAFLD who visited the VA in 2011 was at risk for advanced fibrosis.
Among individuals who were younger than 45 years, the incidence of NAFLD rose from 2.3 to 4.3 cases per 100 persons (annual percentage change, 7.4%; 95% CI, 5.7% to 9.2%), the researchers also found. “Although recent studies show that the rate of increase in both obesity and diabetes, which are both major risk factors for NAFLD, may be slowing down in the U.S., this may not be the case in the VA, where the prevalence of obesity and diabetes is in fact higher than in the U.S. population,” they said.
In general, the findings mirror a recent analysis of the National Health and Nutrition Examination Survey (Aliment Pharmacol Ther. 2015 Jan;41[1]:65-76), according to the investigators. “The VA is the largest integrated health care system in the United States,” they added. “We believe that the sheer size of the veteran cohort, combined with a complete dearth of information regarding the burden of NAFLD in the VA, renders our findings highly significant. Furthermore, the VA is in a unique position to test and implement systemic changes in medical care delivery to improve the health care of NAFLD patients.”
The study was partially supported by the Michael E. DeBakey Veterans Affairs Medical Center. The researchers had no disclosures.
Nonalcoholic fatty liver disease (NAFLD) almost tripled among United States veterans in a recent 9-year period, investigators reported in the February issue of Clinical Gastroenterology and Hepatology.
The trend “was evident in all racial groups, across all age groups, and in both genders,” said Dr. Fasiha Kanwal of the Michael E. DeBakey Veterans Affairs Medical Center and Baylor College of Medicine, both in Houston, and her associates. The increasing prevalence of NAFLD “is likely generalizable to nonveterans,” and will probably persist because of a “fairly steady” 2%-3% overall annual incidence and a steeper rise among younger individuals, they added. “Nonalcoholic fatty liver disease will continue to remain a major public health problem in the United States, at least in the near and intermediate future.”
Although NAFLD is the leading cause of chronic liver failure in the United States, few studies have examined its incidence or prevalence over time, which are key to predicting future disease burden. Therefore, the investigators analyzed data for more than 9.78 million patients who visited the VA at least once between 2003 and 2011. They defined NAFLD as at least two elevated alanine aminotransferase (ALT) values (greater than 40 IU/mL) separated by at least 6 months, with no history of positive serology for hepatitis B surface antigen or hepatitis C virus RNA, and no alcohol-related ICD-9 codes or positive AUDIT-C scores within a year of elevated ALT levels (Clin Gastroenterol Hepatol. 2015 Aug 7. doi: 10.1016/j.cgh.2015.08.010).
During the study period, more than 1.3 million patients, or 13.6%, met the definition of NAFLD, said the researchers. Age-adjusted incidence rates dropped slightly from 3.16% in 2003 to 2.5% in 2011, ranging between 2.3% and 2.7% in most years. Prevalence, however, rose from 6.3% in 2003 (95% confidence interval, 6.26%-6.3%) to 17.6% in 2011 (95% CI, 17.58%-17.65%), a 2.8-fold increase. Moreover, about one in five patients with NAFLD who visited the VA in 2011 was at risk for advanced fibrosis.
Among individuals who were younger than 45 years, the incidence of NAFLD rose from 2.3 to 4.3 cases per 100 persons (annual percentage change, 7.4%; 95% CI, 5.7% to 9.2%), the researchers also found. “Although recent studies show that the rate of increase in both obesity and diabetes, which are both major risk factors for NAFLD, may be slowing down in the U.S., this may not be the case in the VA, where the prevalence of obesity and diabetes is in fact higher than in the U.S. population,” they said.
In general, the findings mirror a recent analysis of the National Health and Nutrition Examination Survey (Aliment Pharmacol Ther. 2015 Jan;41[1]:65-76), according to the investigators. “The VA is the largest integrated health care system in the United States,” they added. “We believe that the sheer size of the veteran cohort, combined with a complete dearth of information regarding the burden of NAFLD in the VA, renders our findings highly significant. Furthermore, the VA is in a unique position to test and implement systemic changes in medical care delivery to improve the health care of NAFLD patients.”
The study was partially supported by the Michael E. DeBakey Veterans Affairs Medical Center. The researchers had no disclosures.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: The prevalence of nonalcoholic fatty liver disease has risen substantially since 2003, and will probably keep increasing in the near term.
Major finding: Prevalence among veterans rose about 2.8 times between 2003 and 2011, mirroring trends reported in the general population.
Data source: An analysis of data from 9.78 million Veterans Affairs patients.
Disclosures: The study was partially supported by the Michael E. DeBakey Veterans Affairs Medical Center. The researchers had no disclosures.
Hepatitis C linked to Parkinson’s disease
Hepatitis C infection may increase the risk of Parkinson’s disease, according to a nationwide population-based study.
Researchers analyzed 10 years of data from the Taiwan National Health Insurance Research Database, which included 49,967 patients with viral hepatitis – 35,619 with hepatitis B infection, 10,286 with hepatitis C, and 4,062 with both – and 199,868 noninfected controls.
According to a paper published online Dec. 23 in Neurology, individuals with hepatitis C infection had a 29% greater incidence of Parkinson’s disease after adjustment for confounders such as sex, age, heart disease, stroke, and head injury (hazard ratio, 1.29; 95% confidence interval, 1.06-1.56).
There were no significant associations between hepatitis B or coinfection, and Parkinson’s disease risk (Neurology. 2016;86:1-7).
Age was the most common risk factor for Parkinson’s disease across all cohorts, and in the control group comorbidities such as hyperlipidemia, hypertension, ischemic heart disease, diabetes, and head injury all were associated with a significant increase in the risk of Parkinson’s disease.
Among individuals with hepatitis C infection, however, only ischemic heart disease and head injury remained significantly associated with Parkinson’s disease risk.
The possibility of an association between hepatitis C infection and Parkinson’s disease has emerged recently with evidence showing that the virus is neurotropic and can replicate in the central nervous system, reported Dr. Hsin-Hsi Tsai of the National Taiwan University Hospital, Taipei, and coauthors.
“Parkinsonism is rarely a described feature in patients with HCV. However, a recent study has discovered that HCV can induce dopaminergic neuron death, suggesting a possible association between HCV infection and” Parkinson’s disease, the authors wrote.
The study also showed that the association between hepatitis C infection and Parkinson’s disease was even more significant in individuals younger than 65 years old, who had a 61% greater risk of developing the neurodegenerative disease.
“Some of the risk factors for HCV infection, such as illicit drug use and associated behaviors, may be confounding factors in this age group,” the authors wrote, although they pointed out that, in Taiwan, use of intravenous drugs was not known to be a risk factor for infection. Commenting on a possible mechanism for the association between hepatitis C infection and Parkinson’s disease, Dr. Tsai and associates suggested the hepatitis C virus could be a possible viral candidate for triggering the neuroinflammation that is a characteristic feature of the disease.
“An earlier imaging study that involved using magnetic resonance spectroscopy to investigate the cerebral effect of HCV showed that chronic HCV infection was associated with elevated choline/creatinine ratios, a biomarker indicating inflammatory and infective conditions, in the basal ganglia and white matter,” they wrote.
The study was supported by the Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence, China Medical University Hospital, Academia Sinica Taiwan Biobank, Stroke Biosignature Project, NRPB Stroke Clinical Trial Consortium, the Tseng-Lien Lin Foundation, the Taiwan Brain Disease Foundation, the Katsuzo and Kiyo Aoshima Memorial Funds, and CMU under the Aim for Top University Plan of the Ministry of Education. There were no conflicts of interest declared.
Hepatitis C infection may increase the risk of Parkinson’s disease, according to a nationwide population-based study.
Researchers analyzed 10 years of data from the Taiwan National Health Insurance Research Database, which included 49,967 patients with viral hepatitis – 35,619 with hepatitis B infection, 10,286 with hepatitis C, and 4,062 with both – and 199,868 noninfected controls.
According to a paper published online Dec. 23 in Neurology, individuals with hepatitis C infection had a 29% greater incidence of Parkinson’s disease after adjustment for confounders such as sex, age, heart disease, stroke, and head injury (hazard ratio, 1.29; 95% confidence interval, 1.06-1.56).
There were no significant associations between hepatitis B or coinfection, and Parkinson’s disease risk (Neurology. 2016;86:1-7).
Age was the most common risk factor for Parkinson’s disease across all cohorts, and in the control group comorbidities such as hyperlipidemia, hypertension, ischemic heart disease, diabetes, and head injury all were associated with a significant increase in the risk of Parkinson’s disease.
Among individuals with hepatitis C infection, however, only ischemic heart disease and head injury remained significantly associated with Parkinson’s disease risk.
The possibility of an association between hepatitis C infection and Parkinson’s disease has emerged recently with evidence showing that the virus is neurotropic and can replicate in the central nervous system, reported Dr. Hsin-Hsi Tsai of the National Taiwan University Hospital, Taipei, and coauthors.
“Parkinsonism is rarely a described feature in patients with HCV. However, a recent study has discovered that HCV can induce dopaminergic neuron death, suggesting a possible association between HCV infection and” Parkinson’s disease, the authors wrote.
The study also showed that the association between hepatitis C infection and Parkinson’s disease was even more significant in individuals younger than 65 years old, who had a 61% greater risk of developing the neurodegenerative disease.
“Some of the risk factors for HCV infection, such as illicit drug use and associated behaviors, may be confounding factors in this age group,” the authors wrote, although they pointed out that, in Taiwan, use of intravenous drugs was not known to be a risk factor for infection. Commenting on a possible mechanism for the association between hepatitis C infection and Parkinson’s disease, Dr. Tsai and associates suggested the hepatitis C virus could be a possible viral candidate for triggering the neuroinflammation that is a characteristic feature of the disease.
“An earlier imaging study that involved using magnetic resonance spectroscopy to investigate the cerebral effect of HCV showed that chronic HCV infection was associated with elevated choline/creatinine ratios, a biomarker indicating inflammatory and infective conditions, in the basal ganglia and white matter,” they wrote.
The study was supported by the Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence, China Medical University Hospital, Academia Sinica Taiwan Biobank, Stroke Biosignature Project, NRPB Stroke Clinical Trial Consortium, the Tseng-Lien Lin Foundation, the Taiwan Brain Disease Foundation, the Katsuzo and Kiyo Aoshima Memorial Funds, and CMU under the Aim for Top University Plan of the Ministry of Education. There were no conflicts of interest declared.
Hepatitis C infection may increase the risk of Parkinson’s disease, according to a nationwide population-based study.
Researchers analyzed 10 years of data from the Taiwan National Health Insurance Research Database, which included 49,967 patients with viral hepatitis – 35,619 with hepatitis B infection, 10,286 with hepatitis C, and 4,062 with both – and 199,868 noninfected controls.
According to a paper published online Dec. 23 in Neurology, individuals with hepatitis C infection had a 29% greater incidence of Parkinson’s disease after adjustment for confounders such as sex, age, heart disease, stroke, and head injury (hazard ratio, 1.29; 95% confidence interval, 1.06-1.56).
There were no significant associations between hepatitis B or coinfection, and Parkinson’s disease risk (Neurology. 2016;86:1-7).
Age was the most common risk factor for Parkinson’s disease across all cohorts, and in the control group comorbidities such as hyperlipidemia, hypertension, ischemic heart disease, diabetes, and head injury all were associated with a significant increase in the risk of Parkinson’s disease.
Among individuals with hepatitis C infection, however, only ischemic heart disease and head injury remained significantly associated with Parkinson’s disease risk.
The possibility of an association between hepatitis C infection and Parkinson’s disease has emerged recently with evidence showing that the virus is neurotropic and can replicate in the central nervous system, reported Dr. Hsin-Hsi Tsai of the National Taiwan University Hospital, Taipei, and coauthors.
“Parkinsonism is rarely a described feature in patients with HCV. However, a recent study has discovered that HCV can induce dopaminergic neuron death, suggesting a possible association between HCV infection and” Parkinson’s disease, the authors wrote.
The study also showed that the association between hepatitis C infection and Parkinson’s disease was even more significant in individuals younger than 65 years old, who had a 61% greater risk of developing the neurodegenerative disease.
“Some of the risk factors for HCV infection, such as illicit drug use and associated behaviors, may be confounding factors in this age group,” the authors wrote, although they pointed out that, in Taiwan, use of intravenous drugs was not known to be a risk factor for infection. Commenting on a possible mechanism for the association between hepatitis C infection and Parkinson’s disease, Dr. Tsai and associates suggested the hepatitis C virus could be a possible viral candidate for triggering the neuroinflammation that is a characteristic feature of the disease.
“An earlier imaging study that involved using magnetic resonance spectroscopy to investigate the cerebral effect of HCV showed that chronic HCV infection was associated with elevated choline/creatinine ratios, a biomarker indicating inflammatory and infective conditions, in the basal ganglia and white matter,” they wrote.
The study was supported by the Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence, China Medical University Hospital, Academia Sinica Taiwan Biobank, Stroke Biosignature Project, NRPB Stroke Clinical Trial Consortium, the Tseng-Lien Lin Foundation, the Taiwan Brain Disease Foundation, the Katsuzo and Kiyo Aoshima Memorial Funds, and CMU under the Aim for Top University Plan of the Ministry of Education. There were no conflicts of interest declared.
FROM NEUROLOGY
Key clinical point: Hepatitis C infection is associated with an increased risk of Parkinson’s disease.
Major finding: Individuals with hepatitis C infection had a 29% greater incidence of Parkinson’s disease.
Data source: Analysis of data from the Taiwan National Health Insurance Research Database, including 49,967 patients with viral hepatitis and 199,868 noninfected controls.
Disclosures: The study was supported by the Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence, China Medical University Hospital, Academia Sinica Taiwan Biobank, Stroke Biosignature Project, NRPB Stroke Clinical Trial Consortium, the Tseng-Lien Lin Foundation, the Taiwan Brain Disease Foundation, the Katsuzo and Kiyo Aoshima Memorial Funds, and CMU under the Aim for Top University Plan of the Ministry of Education. There were no conflicts of interest declared.