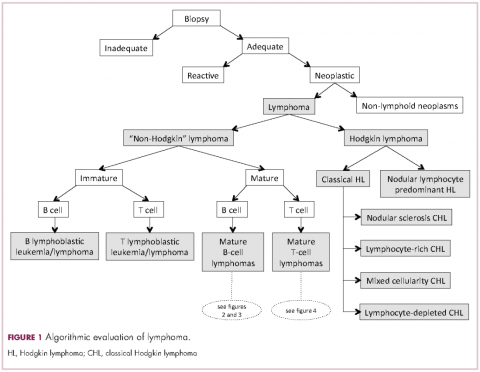
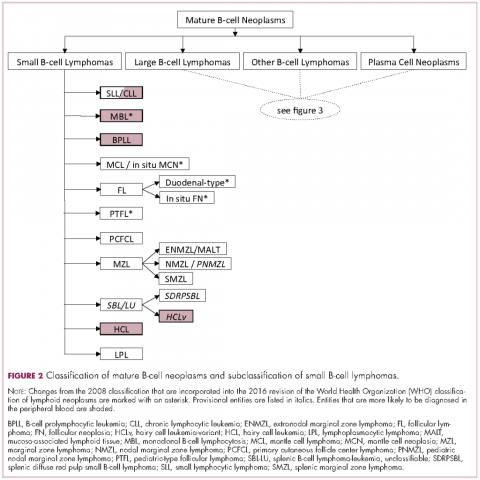
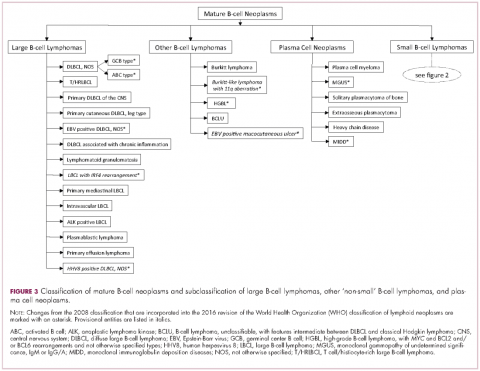
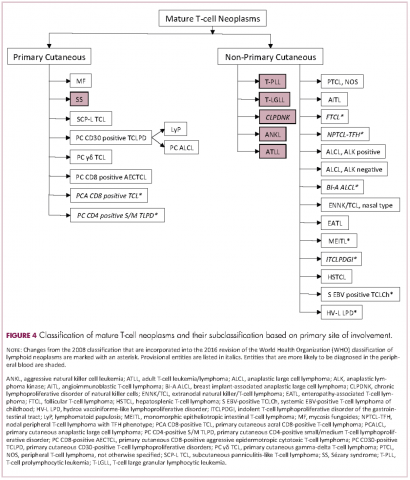
Lymphomas constitute a very heterogeneous group of neoplasms with diverse clinical presentations, prognoses, and responses to therapy. Approximately 80,500 new cases of lymphoma are expected to be diagnosed in the United States in 2017, of which about one quarter will lead to the death of the patient.1 Perhaps more so than any other group of neoplasms, the diagnosis of lymphoma involves the integration of a multiplicity of clinical, histologic and immunophenotypic findings and, on occasion, cytogenetic and molecular results as well. An accurate diagnosis of lymphoma, usually rendered by hematopathologists, allows hematologists/oncologists to treat patients appropriately. Herein we will describe a simplified approach to the diagnosis and classification of lymphomas (Figure 1).
Lymphoma classification
Lymphomas are clonal neoplasms characterized by the expansion of abnormal lymphoid cells that may develop in any organ but commonly involve lymph nodes. The fourth edition of the World Health Organization (WHO) Classification of Tumours of Haematopoietic and Lymphoid tissues, published in 2008, is the official and most current guideline used for diagnosis of lymphoid neoplasms.2 The WHO scheme classifies lymphomas according to the type of cell from which they are derived (mature and immature B cells, T cells, or natural killer (NK) cells, findings determined by their morphology and immunophenotype) and their clinical, cytogenetic, and/or molecular features. This official classification is currently being updated3 and is expected to be published in full in 2017, at which time it is anticipated to include definitions for more than 70 distinct neoplasms.
Lymphomas are broadly and informally classified as Hodgkin lymphomas (HLs) and non-Hodgkin lymphomas (NHLs), based on the differences these two groups show in their clinical presentation, treatment, prognosis, and proportion of neoplastic cells, among others. NHLs are by far the most common type of lymphomas, accounting for approximately 90% of all new cases of lymphoma in the United States and 70% worldwide.1,2 NHLs are a very heterogeneous group of B-, T-, or NK-cell neoplasms that, in turn, can also be informally subclassified as low-grade (or indolent) or high-grade (or aggressive) according to their predicted clinical behavior. HLs are comparatively rare, less heterogeneous, uniformly of B-cell origin and, in the case of classical Hodgkin lymphoma, highly curable.1,2 It is beyond the scope of this manuscript to outline the features of each of the >70 specific entities, but the reader is referred elsewhere for more detail and encouraged to become familiarized with the complexity, challenges, and beauty of lymphoma diagnosis.2,3
Biopsy procedure
A correct diagnosis begins with an adequate biopsy procedure. It is essential that biopsy specimens for lymphoma evaluation be submitted fresh and unfixed, because some crucial analyses such as flow cytometry or conventional cytogenetics can only be performed on fresh tissue. Indeed, it is important for the hematologist/oncologist and/or surgeon and/or interventional radiologist to converse with the hematopathologist prior to and even during some procedures to ensure the correct processing of the specimen. Also, it is important to limit the compression of the specimen and the excessive use of cauterization during the biopsy procedure, both of which cause artifacts that may render impossible the interpretation of the histopathologic findings.
Given that the diagnosis of lymphoma is based not only on the cytologic details of the lymphoma cells but also on the architectural pattern with which they infiltrate an organ, the larger the biopsy specimen, the easier it will be for a hematopathologist to identify the pattern. In addition, excisional biopsies frequently contain more diagnostic tissue than needle core biopsies and this provides pathologists with the option to submit tissue fragments for ancillary tests that require unfixed tissue as noted above. Needle core biopsies of lymph nodes are increasingly being used because of their association with fewer complications and lower cost than excisional biopsies. However, needle core biopsies provide only a glimpse of the pattern of infiltration and may not be completely representative of the architecture. Therefore, excisional lymph node biopsies of lymph nodes are preferred over needle core biopsies, recognizing that in the setting of deeply seated lymph nodes, needle core biopsies may be the only or the best surgical option.
Clinical presentation
Accurate diagnosis of lymphoma cannot take place in a vacuum. The hematopathologist’s initial approach to the diagnosis of lymphoid processes in tissue biopsies should begin with a thorough review of the clinical history, although some pathology laboratories may not have immediate access to this information. The hematopathologist should evaluate factors such as age, gender, location of the tumor, symptomatology, medications, serology, and prior history of malignancy, immunosuppression or immunodeficiency in every case. Other important but frequently omitted parts of the clinical history are the patient’s occupation, history of exposure to animals, and the presence of tattoos, which may be associated with certain reactive lymphadenopathies.
Histomorphologic evaluation
Despite the plethora of new and increasingly sophisticated tools, histologic and morphologic analysis still remains the cornerstone of diagnosis in hematopathology. However, for the characterization of an increasing number of reactive and neoplastic lymphoid processes, hematopathologists may also require immunophenotypic, molecular, and cytogenetic tests for an accurate diagnosis. Upon review of the clinical information, a microscopic evaluation of the tissue submitted for processing by the histology laboratory will be performed. The results of concurrent flow cytometric evaluation (performed on fresh unfixed material) should also be available in most if not all cases before the H&E-stained slides are available for review. Upon receipt of H&E-stained slides, the hematopathologist will evaluate the quality of the submitted specimen, since many diagnostic difficulties stem from suboptimal techniques related to the biopsy procedure, fixation, processing, cutting, or staining (Figure 1). If deemed suitable for accurate diagnosis, a search for signs of preservation or disruption of the organ that was biopsied will follow. The identification of certain morphologic patterns aids the hematopathologist in answering the first question: “what organ is this and is this consistent with what is indicated on the requisition?” This is usually immediately followed by “is this sufficient and adequate material for a diagnosis?” and “is there any normal architecture?” If the architecture is not normal, “is this alteration due to a reactive or a neoplastic process?” If neoplastic, “is it lymphoma or a non-hematolymphoid neoplasm?”
Both reactive and neoplastic processes have variably unique morphologic features that if properly recognized, guide the subsequent testing. However, some reactive and neoplastic processes can present with overlapping features, and even after extensive immunophenotypic evaluation and the performance of ancillary studies, it may not be possible to conclusively determine its nature. If the lymph node architecture is altered or effaced, the predominant pattern of infiltration (eg, nodular, diffuse, interfollicular, intrasinusoidal) and the degree of alteration of the normal architecture is evaluated, usually at low magnification. When the presence of an infiltrate is recognized, its components must be characterized. If the infiltrate is composed of a homogeneous expansion of lymphoid cells that disrupts or replaces the normal lymphoid architecture, a lymphoma will be suspected or diagnosed. The pattern of distribution of the cells along with their individual morphologic characteristics (ie, size, nuclear shape, chromatin configuration, nucleoli, amount and hue of cytoplasm) are key factors for the diagnosis and classification of the lymphoma that will guide subsequent testing. The immunophenotypic analysis (by immunohistochemistry, flow cytometry or a combination of both) may confirm the reactive or neoplastic nature of the process, and its subclassification. B-cell lymphomas, in particular have variable and distinctive histologic features: as a diffuse infiltrate of large mature lymphoid cells (eg, diffuse large B-cell lymphoma), an expansion of immature lymphoid cells (lymphoblastic lymphoma), and a nodular infiltrate of small, intermediate and/or mature large B cells (eg, follicular lymphoma).
Mature T-cell lymphomas may display similar histologic, features but they can be quite heterogeneous with an infiltrate composed of one predominant cell type or a mixture of small, medium-sized, and large atypical lymphoid cells (on occasion with abundant clear cytoplasm) and a variable number of eosinophils, plasma cells, macrophages (including granulomas), and B cells. HLs most commonly efface the lymph node architecture with a nodular or diffuse infiltrate variably composed of reactive lymphocytes, granulocytes, macrophages, and plasma cells and usually a minority of large neoplastic cells (Hodgkin/Reed-Sternberg cells and/or lymphocyte predominant cells).
Once the H&E-stained slides are evaluated and a diagnosis of lymphoma is suspected or established, the hematopathologist will attempt to determine whether it has mature or immature features, and whether low- or high-grade morphologic characteristics are present. The maturity of lymphoid cells is generally determined by the nature of the chromatin, which if “fine” and homogeneous (with or without a conspicuous nucleolus) will usually, but not always, be considered immature, whereas clumped, vesicular or hyperchromatic chromatin is generally, but not always, associated with maturity. If the chromatin displays immature features, the differential diagnosis will mainly include B- and T-lymphoblastic lymphomas, but also blastoid variants of mature neoplasm such as mantle cell lymphoma, and follicular lymphoma, as well as high-grade B-cell lymphomas. Features associated with low-grade lymphomas (eg, follicular lymphoma, small lymphocytic lymphoma/chronic lymphocytic leukemia, marginal zone lymphoma, lymphoplasmacytic lymphoma) include small cell morphology, mature chromatin, absence of a significant number of mitoses or apoptotic cells, and a low proliferation index as shown by immunohistochemistry for Ki67. High-grade lymphomas, such as lymphoblastic lymphoma, Burkitt lymphoma, or certain large B-cell lymphomas tend to show opposite features, and some of the mature entities are frequently associated with MYC rearrangements. Of note, immature lymphomas tend to be clinically high grade, but not all clinically high-grade lymphomas are immature. Conversely, the majority of low-grade lymphomas are usually mature.
Immunophenotypic evaluation
Immunophenotypic evaluation is essential because the lineage of lymphoma cells cannot be determined by morphology alone. The immunophenotype is the combination of proteins/markers (eg, CD20, CD3, TdT) expressed by cells. Usually, it is evaluated by immunohistochemistry and/or flow cytometry, which help determine the proportion of lymphoid cells that express a certain marker and its location and intensity within the cells. While immunohistochemistry is normally performed on formalin-fixed and paraffin-embedded tissue, flow cytometry can be evaluated only on fresh unfixed tissue. Flow cytometry has the advantage over immunohistochemistry of being faster and better at simultaneously identifying coexpression of multiple markers on multiple cell populations. However, certain markers can only be evaluated by immunohistochemistry.
The immunophenotypic analysis will in most cases reveal whether the lymphomas is of B-, T- or NK-cell origin, and whether a lymphoma subtype associated immunophenotype is present. Typical pan B-cell antigens include PAX5, CD19, and CD79a (CD20 is less broadly expressed throughout B-cell differentiation, although it is usually evident in most mature B-cell lymphomas), and typical pan T-cell antigens include CD2, CD5, and CD7. The immature or mature nature of a lymphoma can also be confirmed by evaluation of the immunophenotype. Immature lymphomas commonly express one or more of TdT, CD10, or CD34; T-lymphoblastic lymphoma cells may also coexpress CD1a. The majority of NHLs and all HLs are derived from (or reflect) B cells at different stages of maturation. Mature B-cell lymphomas are the most common type of lymphoma and typically, but not always, express pan B-cell markers as well as surface membrane immunoglobulin, with the latter also most useful in assessing clonality via a determination of light chain restriction. Some mature B-cell lymphomas tend to acquire markers that are either never physiologically expressed by normal mature B cells (eg, cyclin D1 in mantle cell lymphoma, or BCL2 in germinal center B cells in follicular lymphoma) or only expressed in a minor fraction (eg, CD5 that is characteristically expressed in small lymphocytic and mantle cell lymphoma). The most common mature B-cell lymphomas include diffuse large B-cell lymphoma, follicular lymphoma, small lymphocytic lymphoma, mantle cell lymphoma, marginal zone lymphoma, Burkitt lymphoma, and lymphoplasmacytic lymphoma (Figures 2 and 3). Classical HLs are also lymphomas of B-cell origin that demonstrate diminished preservation of their B-cell immunophenotype (as evidenced by the dim expression of PAX5 but absence of most other pan B-cell antigens), expression of CD30, variable expression of CD15, and loss of CD45 (Figure 1). In contrast, nodular lymphocyte predominant HL shows a preserved B-cell immunophenotypic program and expression of CD45, typically without CD30 and CD15. Of note, the evaluation of the immunophenotype of the neoplastic cells in HL is routinely assessed by immunohistochemistry because most flow cytometry laboratories cannot reliably detect and characterize the low numbers of these cells.
Mature T-cell lymphomas generally express one or more T-cell markers, and tend to display a T-helper (CD4-positive) or cytotoxic (CD8-positive) immunophenotype and may show loss of markers expressed by most normal T-cells (eg, CD5, CD7; Figure 4). However, a subset of them may express markers not commonly detected in normal T cells, such as ALK. NK-cell lymphomas lack surface CD3 (expressing only cytoplasmic CD3) and CD5 but express some pan T-cell antigens (such as CD2 and CD7) as well as CD16 and/or CD56.
Patients with primary or acquired immune dysfunction are at risk for development of lymphoma and other less clearly defined lymphoproliferative disorders, the majority of which are associated with infection of the lymphoid cells with Epstein-Barr virus (EBV). Therefore, evaluation with chromogenic in situ hybridization for an EBV-encoded early RNA (EBER1) is routinely performed in these cases; it is thus essential that the hematopathologist be informed of the altered immune system of the patient. If lymphoma develops, they may be morphologically similar to those that appear in immunocompetent patients, which specifically in the post-transplant setting are known as monomorphic post-transplant lymphoproliferative disorders (PTLD). If the PTLD does not meet the criteria for any of the recognized types of lymphoma, it may be best characterized as a polymorphic PTLD.
Once the lineage (B-, T-, or NK-cell) of the mature lymphoma has been established, the sum (and on occasion the gestalt) of the clinical, morphologic, immunophenotypic and other findings will be considered for the subclassification of the neoplasm.
Cytogenetic and molecular evaluation
If the morphologic and immunophenotypic analysis is inconclusive or nondiagnostic, then molecular and/or cytogenetic testing may further aid in the characterization of the process. Some of available molecular tests include analyses for the rearrangements of the variable region of the immunoglobulin (IG) or T-cell receptor (TCR) genes and for mutations on specific genes. The identification of specific mutations not only confirms the clonal nature of the process but, on occasion, it may also help subclassify the lymphoma, whereas IG or TCR rearrangement studies are used to establish whether a lymphoid expansion is polyclonal or monoclonal. The molecular findings should not be evaluated in isolation, because not all monoclonal rearrangements are diagnostic of lymphoma, and not all lymphomas will show a monoclonal rearrangement. Other methodologies that can aid in the identification of a clonal process or specific genetic abnormalities include metaphase cytogenetics (karyotyping) and fluorescence in situ hybridization (FISH). If any cytogenetic abnormalities are found in sufficient numbers (and constitutional abnormalities are excluded), their identification indicates the presence of a clonal process. Also, some cytogenetic abnormalities are characteristic of certain lymphomas. However, they may be neither 100% diagnostically sensitive nor diagnostically specific, for example, the hallmark t(14;18)/IGH-BCL2 is not present in all follicular lymphomas and not all lymphomas with this translocation are follicular lymphomas. Whereas FISH is generally performed on a minimum of 200 cells, compared with typically 20 metaphase by “conventional” karyotyping, and is therefore considered to have higher analytical sensitivity, it evaluates only for the presence or absence of the abnormality being investigated with a given set of probes, and therefore other abnormalities, if present, will not be identified. The value of FISH cytogenetic studies is perhaps best illustrated in the need to diagnose double hit lymphomas, amongst other scenarios. The detection of certain mutations can aid in the diagnosis of certain lymphomas, such as MYD88 in lymphoplasmacytic lymphoma, prognosis of others, such as in follicular lymphoma and identify pathways that may be precisely therapeutically targeted.
Final remarks
The diagnosis of lymphoma can be complex and usually requires the hematopathologist to integrate multiple parameters. The classification of lymphomas is not static, and new entities or variants are continuously described, and the facets of well-known ones refined. While such changes are often to the chagrin of hematologists/oncologists and hematopathologists alike, we should embrace the incorporation of nascent and typically cool data into our practice, as more therapeutically relevant entities are molded.