Nivolumab is a full human immunoglobulin antibody to the programmed cell death 1 (PD-1) immune checkpoint receptor on T cells. This programmed cell death inhibitor is a targeted immunotherapy used to treat patients with melanoma, among other malignancies.1 More recently, nivolumab has been used for advanced non-small-cell lung cancer (NSCLC) after failure of previous chemotherapeutic agents. It was approved by the US Food and Drug Administration for the NSCLC indication in 2015.2
PD-1 inhibitors are efficacious in treating advanced malignancies, although their immune-mediated functions can lead to undesirable side effects. Patients treated with nivolumab have been reported to develop thyroid disease,1,3,4 diabetes,3 hypophysitis,1,3 hypopituitarism,3 and pneumonitis,4,2 as well as other autoimmune conditions.3 Although nivolumab is often used to treat skin diseases such as melanoma, it can have many cutaneous side effects including pruritus,1,3-6 rash,1,3,4,6,7 vitiligo,1,3,7,6 mouth sores,3 injection site reactions,3,6 and alopecia.5 Herein, we describe a patient who was treated with nivolumab and developed an exacerbation of pre-existing psoriasis.
Case presentation and summary
A 57-year-old man with metastatic NSCLC and a history of plaque psoriasis presented to the dermatology clinic for evaluation of new lesions on his palms and soles. The patient had been previously treated with numerous therapies for NSCLC, including chemotherapy and radiation. Previous chemotherapeutic agents included the cisplatin plus etoposide combination, with doxetaxel and pemetrexed. The patient was not able to tolerate the chemotherapy and instead opted for hospice care. After several months, he chose to restart therapy, and was started on the programmed cell death (PD)-1 inhibitor, nivolumab, at a dose of 3 mg/kg for a total of 6 cycles. He received his first dose 5 weeks before his current presentation to the clinic, and his second dose 2 weeks before.
The patient reported a 20-year history of plaque psoriasis, characterized by psoriatic plaques on the elbows and shins and for which he was treated with topical therapies with good effect. Every few months, he would develop one or two small plaques of psoriasis on his palms and soles. The lesions were inconsequential to the patient, as he never experienced more than one or two small palmoplantar lesions at a time. One week after his second cycle of nivolumab, the patient developed an eruption of lesions on his palms and soles. He observed that the lesions seemed to be similar to his previous palmoplantar psoriatic plaques but with significantly greater skin involvement. The patient denied any new-onset joint pain.
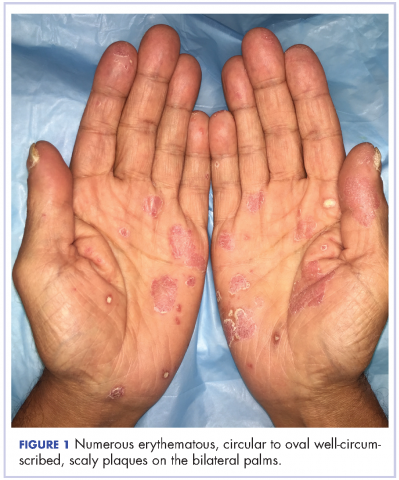
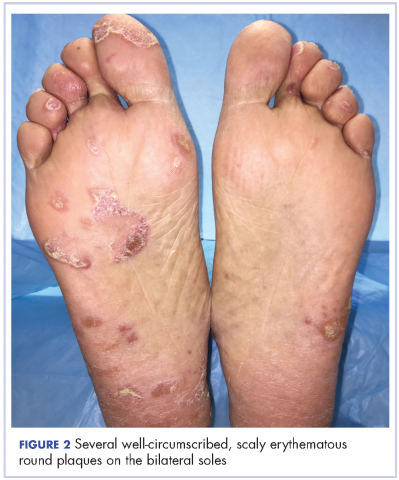
The results of a physical examination revealed a cachectic man in no acute distress, with more than 30 erythematous circular to oval circumscribed plaques with yellow to whitish scales on the bilateral palms (Figure 1) and soles (Figure 2).
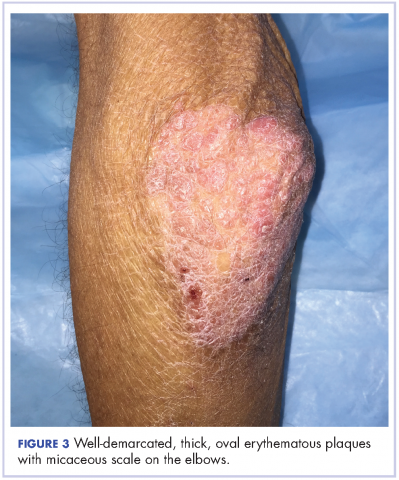
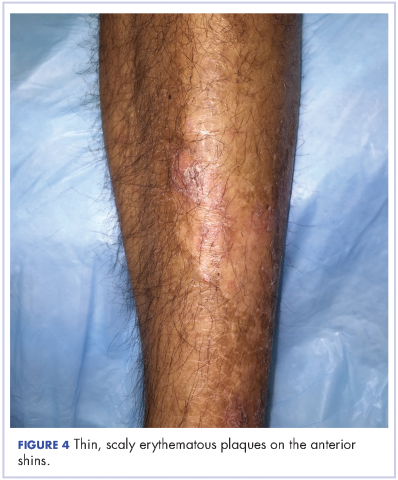
The patient also had well-demarcated, thick oval erythematous plaques with micaceous scales on the bilateral elbows (Figure 3), and thin scaly erythematous plaques on the anterior shins (Figure 4). There were no psoriatic plaques on the remainder of the trunk or extremities. Mucosal surfaces, scalp, and nails were uninvolved.
A clinical diagnosis of exacerbation of pre-existing psoriasis owing to nivolumab therapy was made. The patient was started on clobetasol 0.05% ointment twice daily under occlusion with plastic wrap to the affected areas, and he was continued on nivolumab for his NSCLC.
Discussion
Treatment with nivolumab can lead to a range of autoimmune side effects, and as shown in this case, psoriasis is one of the cutaneous findings that could be exacerbated by treatment with nivolumab. To date, two cases of exacerbation of psoriasis in patients treated with nivolumab for melanoma have been reported in the literature.8,9 In the first case, the patient had well-controlled plaque psoriasis at baseline and he subsequently developed psoriatic plaques on the trunk and extremities after the second infusion of nivolumab for metastatic melanoma. A biopsy showed regular acanthosis with hyperkeratosis and parakeratosis in addition to dilated vessels in the papillary dermis.8 In the second case, the patient had a history of psoriasis vulgaris with no active lesions. Three weeks after his first course of nivolumab for metastatic oral mucosal melanoma, he developed new, well-circumscribed erythematous scaly plaques on the trunk and extremities that were clinically diagnosed as psoriasis.9 In a third case, a patient without a prior history of psoriasis experienced a psoriasiform eruption on the trunk and extremities after the fourth dose of nivolumab for oral mucosal melanoma.10 Thus, our case is the third reported case of exacerbation of preexisting psoriasis in a patient treated with nivolumab. Furthermore, our patient is the first reported case of a patient treated with nivolumab for NSCLC to develop this adverse event. Whereas the previously reported cases were characterized by widespread trunk and extremity involvement, our patient developed focal exacerbation of the palmoplantar areas.
Additional studies are needed to more clearly characterize the specific cutaneous toxicities of nivolumab and to determine if particular skin reactions may indicate a better response to the anticancer agent. Side effects such as psoriasis can often be managed with topical therapies and may not require withdrawal of the medication. We encourage the collaboration of dermatologists and oncologists to enhance the diagnosis and management of these cutaneous side effects in cancer patients.