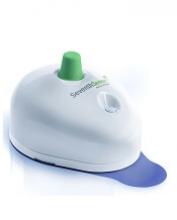
The US Food and Drug Administration (FDA) has granted 510(k) clearance for a push-button blood collection device called TAP.
It provides “virtually painless, simple, and fast” blood collection, according to Seventh Sense Biosystems, Inc., the company marketing the device.
The FDA clearance allows healthcare workers to use TAP only for the collection of capillary blood for hemoglobin A1c testing.
However, Seventh Sense Biosystems said it is working with the FDA to expand the use of TAP to add additional tests, as well as at-home blood collection.
How TAP works
The TAP device is placed on a patient’s upper arm, and blood collection starts with the press of a button. The process typically takes 2 to 3 minutes.
When TAP is placed on the arm, the base of the device generates a seal with the skin to create a vacuum. When the user presses the button, TAP deploys microneedles that puncture the skin above the dermis.
The vacuum draws capillary blood out of the micropunctures and through microfluidic channels into a collection chamber prefilled with lithium heparin.
TAP collects up to 100 μL of blood. To transfer the blood to an analyzer, a precision pipette is placed in an access port at the bottom of the device.
Seventh Sense Biosystems said it will launch TAP over the coming months. For up-to-date information, visit www.7sbio.com.