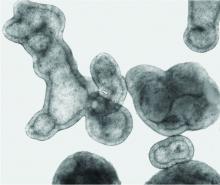
Lung organoids
Dr. Antony’s pulmospheres emanate not from stem cells but from primary tissue obtained from diseased lung. “We reconstitute the lung cells in single-cell suspensions, and then we allow them to come back together to form lung tissue,” she said. The pulmospheres take about 3 days to grow.
In a study published 5 years ago of pulmospheres of 20 patients with IPF and 9 control subjects, Dr. Antony and colleagues quantitated invasiveness and found “remarkable” differences in the invasiveness of IPF pulmospheres following exposure to the Food and Drug Administration–approved antifibrotic drugs nintedanib and pirfenidone. Some pulmospheres responded to one or the other drug, some to both, and two to neither – findings that Dr. Antony said offer hope for the goals of personalizing therapy and assessing new drugs.
Moreover, clinical disease progression correlated with invasiveness of the pulmospheres, showing that the organoid-like structures “do give us a model that [reflects] what’s happening in the clinical setting,” she said. (Lung tissue for the study was obtained via video-assisted thoracic surgery biopsy of IPF patients and from failed donor lung explants, but bronchoscopic forceps biopsies have become a useful method for obtaining tissue.)
The pulmospheres are not yet in clinical use, Dr. Antony said, but her lab is testing other fibrosis modifiers and continuing to use the model as a research tool.
One state to the east, at Vanderbilt University, Nashville, Tenn., Amanda Linkous, PhD, grows “branching lung organoids” and brain organoids to study the biology of small cell lung cancer (SCLC).
“We want to understand how [SCLC] cells change in the primary organ site, compared with metastatic sites like the brain. ... Are different transcription factors expressed [for instance] depending on where the tumor is growing?” said Dr. Linkous, scientific center manager of the National Cancer Institute’s Center for Systems Biology of SCLC at Vanderbilt. “Then we hope to start drug screening within the next year.”
Her lung organoids take shape from either human embryonic stem cells or iPSCs. Within commercially available media, the cells mature through several stages of differentiation, forming definitive endoderm, anterior foregut endoderm, and then circular lung bud structures – the latter of which are then placed into droplets of Matrigel, an extracellular matrix gel.
“In the Matrigel droplets, the lung bud cells will develop proximal and distal-like branching structures that express things like EPCAM, MUC1, SOX2, SOX9, and NKX2.1 – key markers that you should see in a more mature lung microenvironment,” she said. Tumor cells from established SCLC cell lines will then easily invade the branching lung organoid.
Dr. Linkous said she has found her organoid models highly reproducible and values their long-lasting nature – especially for future drug screening. “We can keep organoids going for months at a time,” said Dr. Linkous, a research associate professor in Vanderbilt’s department of biochemistry.
Like Dr. Antony, she envisions personalizing treatment in the future. “SCLC is a very heterogeneous tumor with many different cell types, so what works for one patient may not work well at all for another patient,” she said.
As recently as 5 years ago, “many in the cancer field would have been resistant to moving away from mouse models,” Dr. Linkous noted. “But preclinical studies in mice often don’t pan out in the clinic ... so we’re moving toward a human microenvironment to study human disease.”
The greatest challenge, Dr. Linkous and Dr. Antony said, lies in integrating both vascular blood flow and air into these models. “We just don’t have that combination as of yet,” Dr. Antony said.