Long COVID: Advocating for Patients and Implementing Effective Techniques
Kyle B. Enfield, MD, MS, FSHEA, FCCM
Associate Professor of Medicine
Vice Chair, Quality Improvement and Patient Safety
University of Virginia;
Associate Chief Medical Officer, Critical Care
Department of Medicine
University of Virginia Health System
Charlottesville, VA

Slideshow below.
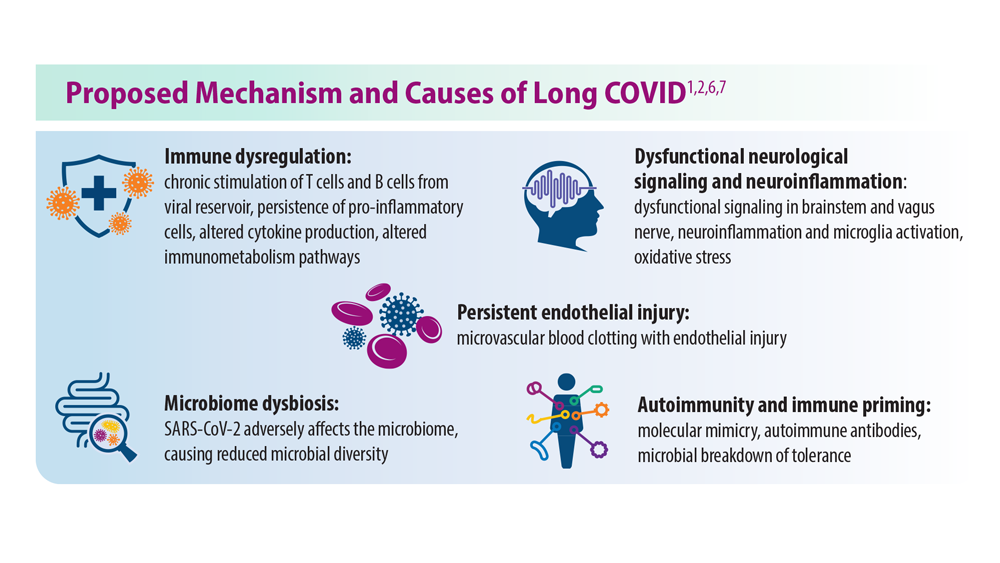
While definitions of postacute sequelae of SARS-CoV-2 (PASC), commonly referred to as long COVID, are heterogeneous, it is internationally recognized that some patients have symptoms that persist after recovery from their acute illness.1,2 Most clinicians agree that this disease manifestation begins at around 60 to 90 days after original COVID-19 infection, based on the World Health Organization (WHO) definition.1 Long COVID has similarities to postviral infections seen in SARS, MERS, Ebola, and West Nile virus.1,3 Theories on its potential cause include ongoing inflammation and autoimmunity, among other theories.1,2
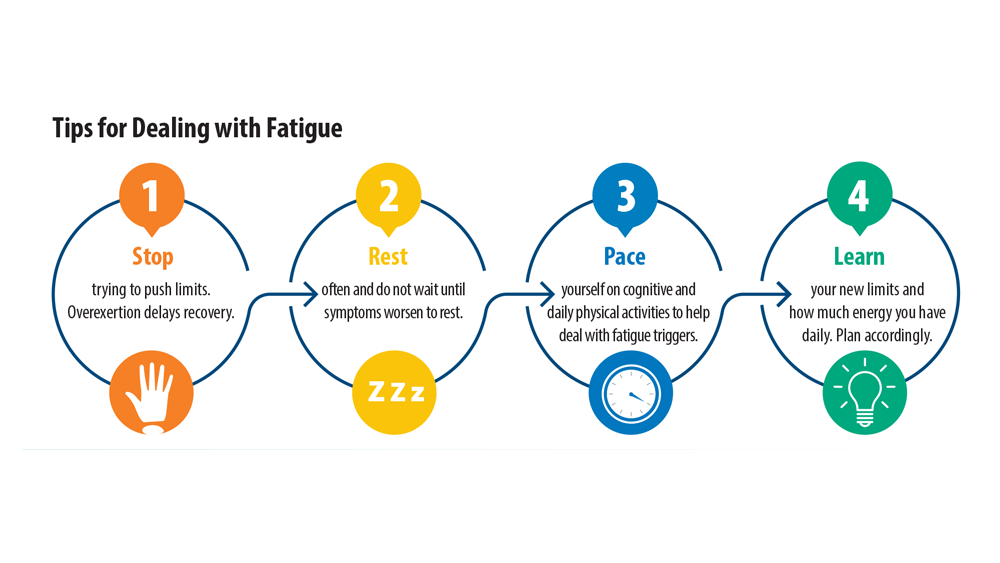
Currently, no FDA-approved treatments are available for long COVID and most patients are receiving variable care with off-label use of drugs.1 Multiple clinical trials are in early stages. Certain nonpharmacological approaches have been effective for 2 common lingering long COVID symptoms: exercise intolerance and fatigue.4 These techniques provide patients with tips to help manage decreased energy levels and provide breathing exercises for patients experiencing exercise intolerance.4
Long COVID is a challenge for the medical community, but progress is being made in pinpointing causes, effective treatments, and techniques to help people who continue to have symptoms after having had COVID-19.1,4
1
-
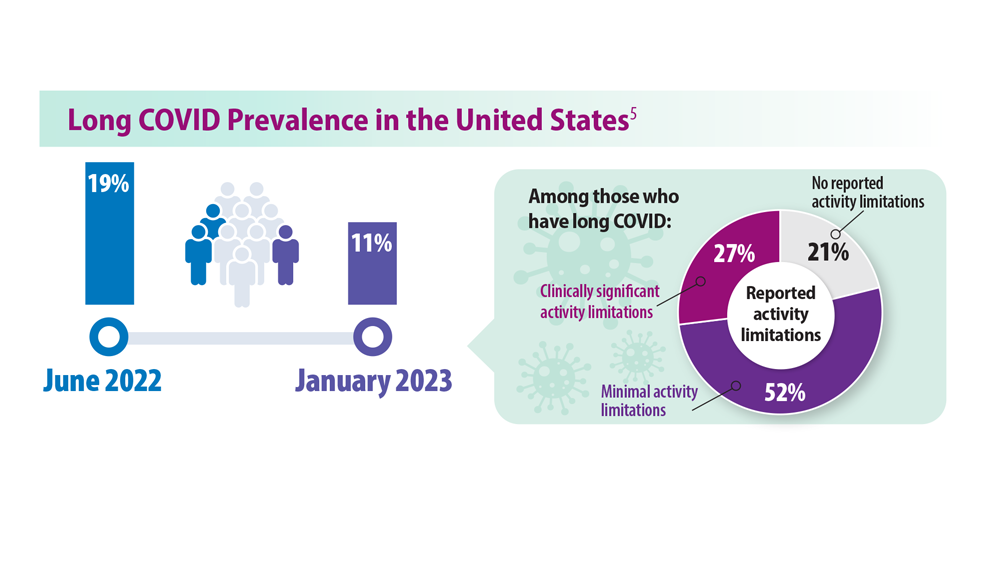
Based on the overall population of patients who had COVID-19.
-
-
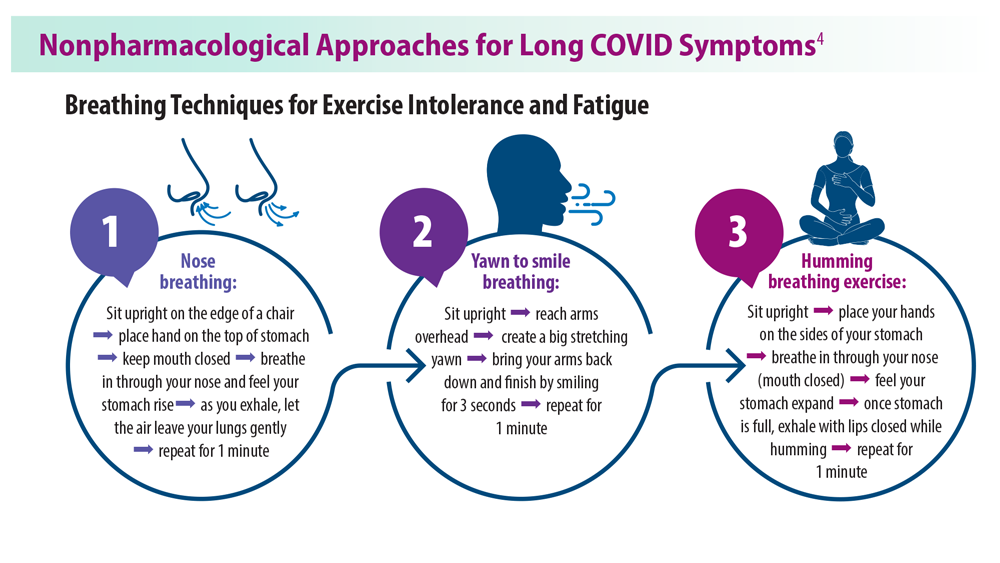
The UK's National Institute for Health and Care Excellence (NICE) recommends against a graded exercise program for people recovering from COVID-19. Graded exercise programs are structured exercise programs that gradually increase the amount of physical activity.
-
-
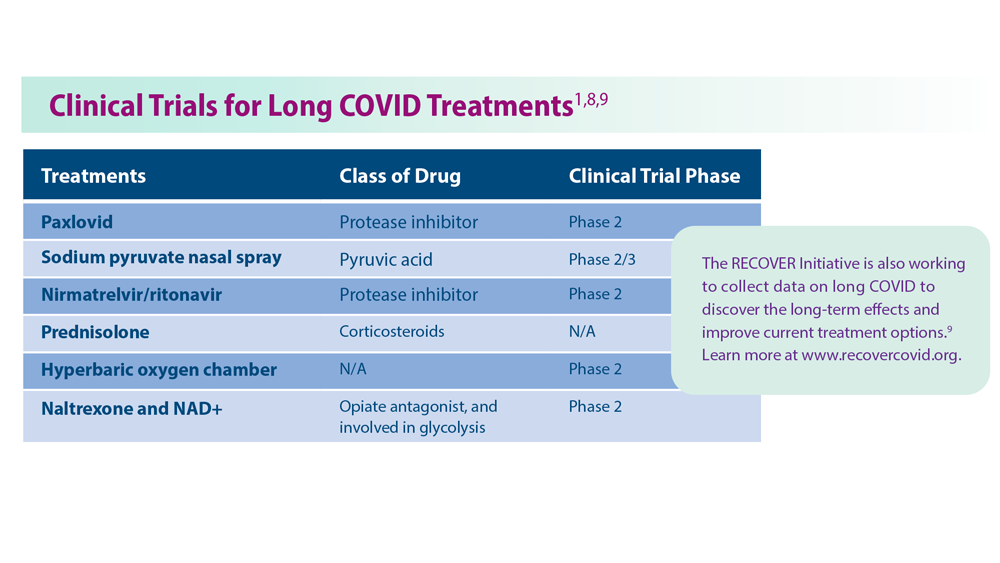
Based on a ClinicalTrials.gov search of treatment: recruiting, active, not recruiting, completed, enrolling by invitation studies; Long+COVID. Results as of August 2, 2023.
N/A, not available; NAD+, nicotinamide adenine dinucleotide.
