RSV Updates: Prophylaxis Approval and Hospitalization for Severe RSV
Riddhi Upadhyay, MD
Attending Staff Physician
Division of Critical Care
Geisinger Community Medical Center
Scranton, PA
Dr. Upadhyay has no relevant financial disclosures.

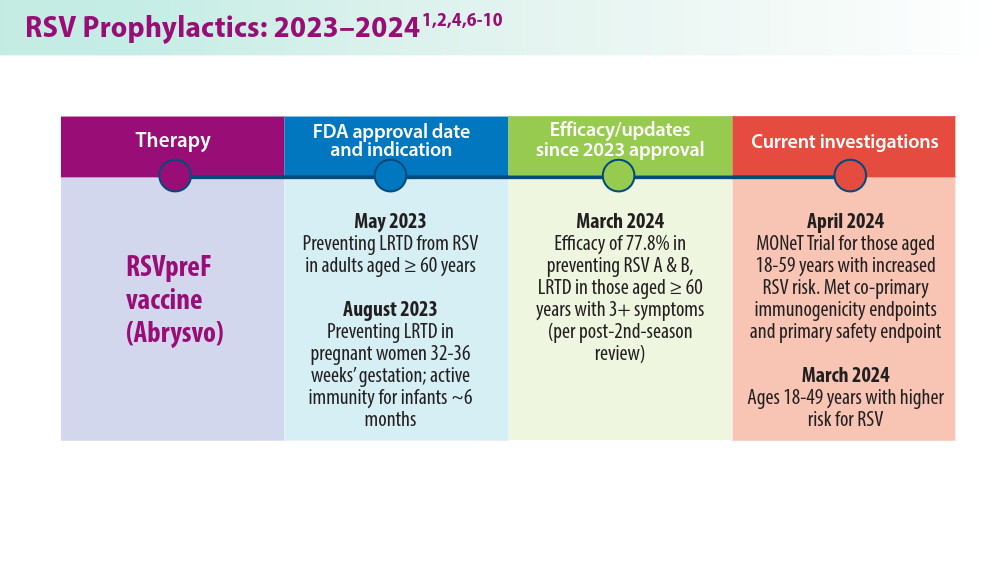
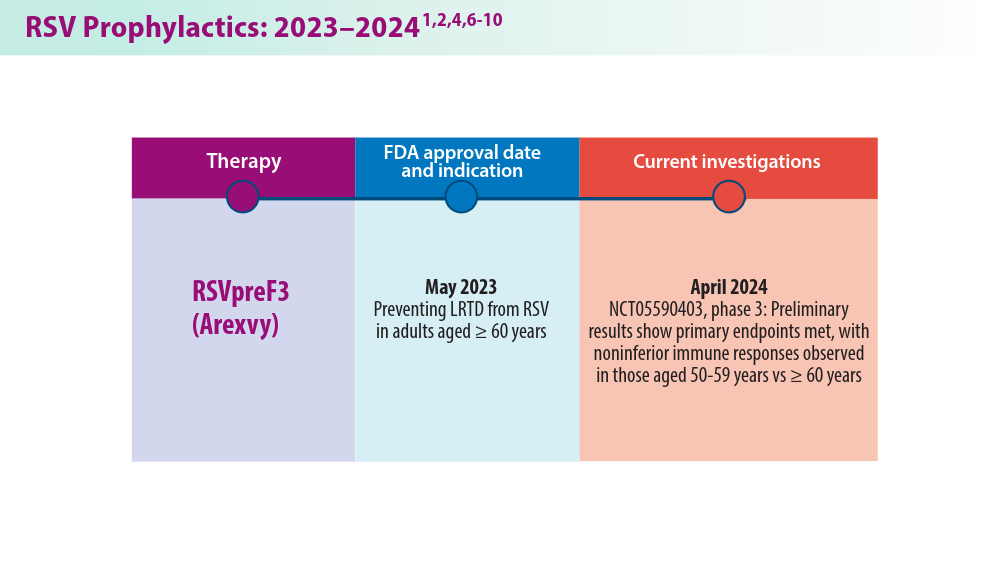
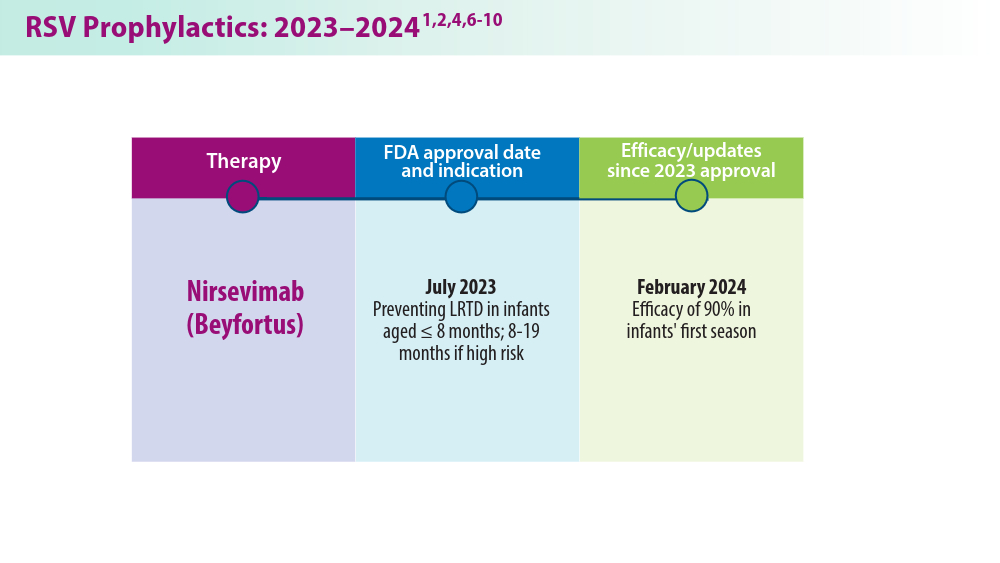
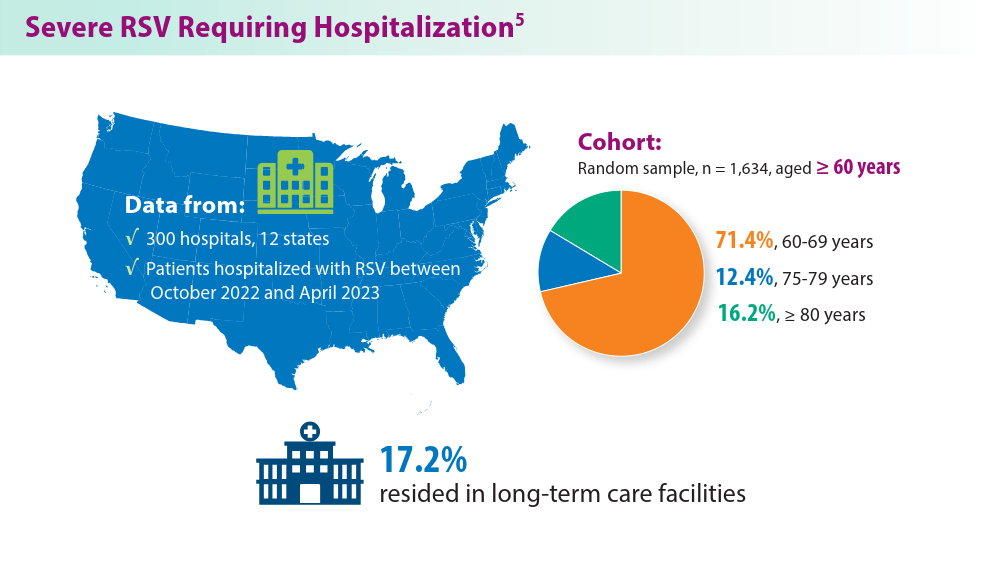
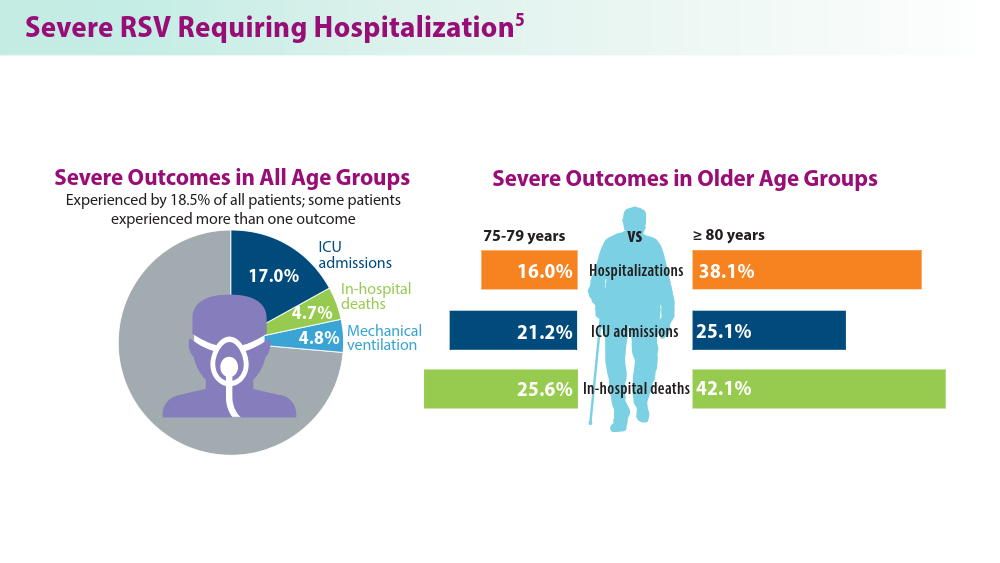
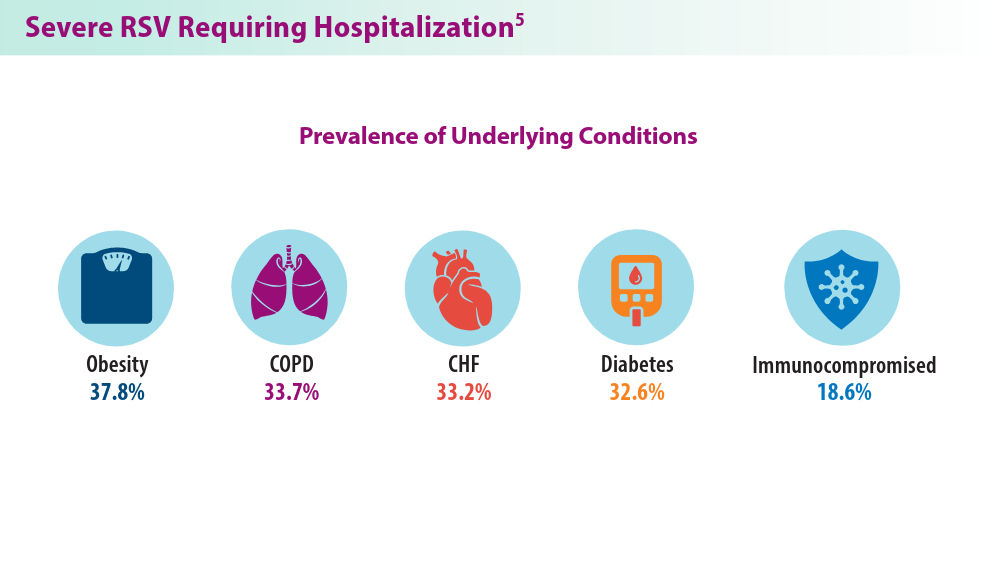
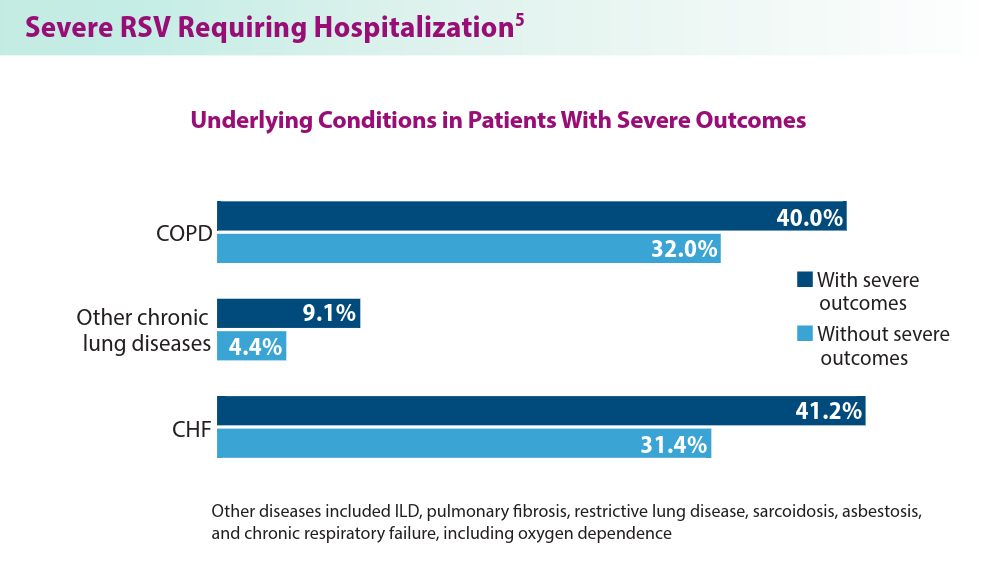
In 2023, significant progress was made in preventing RSV lower respiratory tract disease (LRTD) with the FDA approval of 3 vaccines and a monoclonal antibody. Published efficacy rates and ongoing trials, like the MONeT (RSV IMmunizatiON Study for AdulTs with a Higher Risk of Severe Illness) trial for high-risk 18- to 59-year-olds, continue to advance RSV prophylaxis.1 Early 2024 results showed that the RSVpreF vaccine (Abrysvo) effectively protected against RSV A and B, with a 77.8% effectiveness in preventing RSV LRTD in adults aged ≥ 60 years in its second season.2 The CDC reported nirsevimab was 90% effective in preventing RSV hospitalization in infants during their first RSV season.3,4 Further, results from a study published in June 2023 identified obesity, COPD, and congestive heart failure (CHF) as common comorbidities in patients who were ≥ 60 years and hospitalized with RSV. The study also found that those aged ≥ 75 years experienced worse outcomes.5 This data aids in performing risk assessments for patients with RSV by age and comorbidities. Ongoing research for preventing RSV in different populations with various risks and comorbidities is imperative. Additional FDA approvals will help protect more individuals from this potentially life-threatening disease.
1