Health officials from 23 states in which contaminated methylprednisolone acetate products have been shipped continue to watch for additional cases of fungal meningitis tied to epidural injections given with the contaminated medication.*
As of Oct. 10, the outbreak is responsible for 137 cases and 12 deaths in 10 states, including Florida (6 cases, including one death), Indiana (15 cases), Tennessee (44 cases, including six deaths), Maryland (9 cases, including one death), Michigan, (28 cases, including three deaths), North Carolina (2 cases), Virginia (27 cases, including one death), Ohio (1 case), New Jersey (2 cases), and Minnesota (3 cases). The cases have all been linked to epidural injections with methylprednisolone acetate compounded by the New England Compounding Center (NECC) in Framingham, Mass. Production lots of the steroid at doses of 40 mg/mL and 80 mg/mL have been recalled, as well as all other products currently in circulation that were compounded at and distributed from its facility in Framingham.
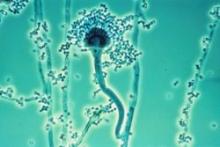
The Food and Drug Administration found fungal contamination by direct microscopic examination of foreign matter taken from a sealed vial of the steroid collected from NECC, which voluntarily shut down its operations Oct. 3. The agency is in the process of conducting additional microbial testing to confirm the exact species of the fungus and is working closely with Centers of Disease Control and Prevention and state authorities to determine whether this sample taken from the product matches the organism found in patients. So far, cultures of cerebrospinal fluid (CSF) and histopathologic analysis of specimens have indicated fungal infection in nine patients, including Aspergillus and Exserohilum species.
The CDC notes that "Infected patients have presented approximately 1-4 weeks following their injection with a variety of symptoms, including fever, new or worsening headache, nausea, and new neurological deficit (consistent with deep brain stroke). Some of these patients’ symptoms were very mild in nature. CSF obtained from these patients has typically shown elevated white cell count (with a predominance of neutrophils), low glucose, and elevated protein."
"Physicians should contact patients who have had an injection (e.g., spinal, joint) using any of the three lots of methylprednisolone acetate ... to determine if they are having any symptoms. Although all cases detected to date occurred after injections with products from these three lots, out of an abundance of caution, CDC and FDA recommend that health care professionals cease use of any product produced by the New England Compounding Center until further information is available," the CDC said in a statement. The potentially contaminated injections were given starting May 21, 2012.
The CDC has provided instructions for clinicians on diagnostic testing and specimen submission.
The 23 states that received the implicated lots of medication are California, Connecticut, Florida, Georgia, Idaho, Illinois, Indiana, Maryland, Michigan, Minnesota, North Carolina, New Hampshire, New Jersey, Nevada, New York, Ohio, Pennsylvania, Rhode Island, South Carolina, Tennessee, Texas, Virginia, and West Virginia.
All reports of complaints or problems associated with these products should be made at the MedWatch website, the FDA’s voluntary reporting program, or by calling 800-FDA-1088.
* Story updated October 10, 2012.