User login
Confirm Celiac Diagnosis by Biopsy Before Advising Gluten-Free Diet
SAN DIEGO – An intestinal biopsy is almost always necessary to confirm celiac disease and is a must before committing a patient to the only effective treatment – a lifelong gluten-free diet.
Sticking to such a restricted diet is difficult and expensive, Dr. Sheila Crowe said at the annual meeting of the American College of Physicians. "A lifelong gluten-free diet sounds simple, unless you’re the patient. ... Eating out is very difficult, especially for children and teens who face a lot of peer pressure. And eating gluten free at home is expensive. Studies in the United States, Canada, and the United Kingdom confirm that a lifelong diet of gluten-free foods costs about three times more than a normal diet," said Dr. Crowe, professor in the division of gastroenterology and hepatology at the University of Virginia, Charlottesville.
Because treating celiac disease requires this lifelong commitment, a positive serologic test isn’t enough to rule it in, she said. Nor are any of the available immunologic tests, including the most widely used – tissue transglutaminase IgA (tTG IgA) – specific enough to replace intestinal biopsy as the sole method for reliably diagnosing celiac disease. "A positive tTG test is not enough to place a person on this lifelong treatment without confirmation from an intestinal biopsy," she said. "This is especially important for children, because of the higher likelihood of false positives in that group."
tTG IgA has a very high sensitivity and specificity, but it isn’t perfect, Dr. Crowe said. "If you have a patient with clinical symptoms and the tTG comes back negative, there is still a 10% chance that’s a false negative. Another scenario could be a patient who has an autoimmune disease or a relative with celiac, and is experiencing celiac symptoms. If the tTG came back negative on that person, I would still do an endoscopy."
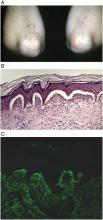
The only possible exception might be a patient with celiac symptoms who already has biopsy-proven dermatitis herpetiformis, with the classic immunofluorescent deposits at the dermal-epidermal junction. "If you biopsy these patients, the intestine will show the changes associated with celiac disease every time," Dr. Crowe said.
Celiac disease is no longer considered a disorder of childhood. "The disease is there lifelong. It appears you cannot suppress the immune response," she said.
The intestine rapidly responds to a gluten-free diet, "But the tendency to have an immunologic response to gluten is always there," Dr. Crowe said.
Relapses are common. Patients are most likely to "fall off" the diet when symptoms begin to abate, she said. They may simply feel "cured" and resume old eating patterns, or they may drop the diet because of changes that can occur as the intestine heals. "If a patient had diarrhea and malabsorption of nutrients, they might find themselves getting constipated and gaining weight on the gluten-free diet, fall off, and get ill again. Even if the intestine is healed, the vast majority of data tell us that patient will relapse," at some point after abandoning the dietary restriction, Dr. Crowe said.
This can lead to the development of refractory celiac disease, in which the intestine fails to recover despite a gluten-free diet. Patients with refractory celiac disease may be unable to fully absorb nutrients and need supplemental feeding methods.
The goal of celiac management is to promote intestinal healing, optimize nutrition, and avoid long-term damage, Dr. Crowe said. "It’s key to bring in a knowledgeable dietitian to help. And I mean knowledgeable – not someone who is going to hand your patient a diet sheet and that’s all."
Many celiac patients are already nutritionally compromised at the time of diagnosis. "This is the time to measure their nutritional parameters," Dr. Crowe said. "They may need supplements. Many are deficient in vitamin D, iron, folate, zinc, or other trace elements."
The risks of untreated disease "are not inconsequential." Patients can develop problems related to nutrient malabsorption, including osteopenia, infertility, miscarriage, and intrauterine growth restriction, and are four times more likely than the general population to develop a malignancy.
Guidelines for managing nutritional status exist, but there are no evidence-based treatment guidelines for celiac disease, Dr. Crowe said. "Aside from nutrition, you have to just advise them to follow good health practices. I have several patients who still smoke, and yet they’re morose about their gluten-free diet. I say, ‘Why are you worried about that? You need to worry about quitting smoking!’ "
Dr. Crowe reported that she receives royalties from a book written for patients with celiac disease.
SAN DIEGO – An intestinal biopsy is almost always necessary to confirm celiac disease and is a must before committing a patient to the only effective treatment – a lifelong gluten-free diet.
Sticking to such a restricted diet is difficult and expensive, Dr. Sheila Crowe said at the annual meeting of the American College of Physicians. "A lifelong gluten-free diet sounds simple, unless you’re the patient. ... Eating out is very difficult, especially for children and teens who face a lot of peer pressure. And eating gluten free at home is expensive. Studies in the United States, Canada, and the United Kingdom confirm that a lifelong diet of gluten-free foods costs about three times more than a normal diet," said Dr. Crowe, professor in the division of gastroenterology and hepatology at the University of Virginia, Charlottesville.
Because treating celiac disease requires this lifelong commitment, a positive serologic test isn’t enough to rule it in, she said. Nor are any of the available immunologic tests, including the most widely used – tissue transglutaminase IgA (tTG IgA) – specific enough to replace intestinal biopsy as the sole method for reliably diagnosing celiac disease. "A positive tTG test is not enough to place a person on this lifelong treatment without confirmation from an intestinal biopsy," she said. "This is especially important for children, because of the higher likelihood of false positives in that group."
tTG IgA has a very high sensitivity and specificity, but it isn’t perfect, Dr. Crowe said. "If you have a patient with clinical symptoms and the tTG comes back negative, there is still a 10% chance that’s a false negative. Another scenario could be a patient who has an autoimmune disease or a relative with celiac, and is experiencing celiac symptoms. If the tTG came back negative on that person, I would still do an endoscopy."
The only possible exception might be a patient with celiac symptoms who already has biopsy-proven dermatitis herpetiformis, with the classic immunofluorescent deposits at the dermal-epidermal junction. "If you biopsy these patients, the intestine will show the changes associated with celiac disease every time," Dr. Crowe said.
Celiac disease is no longer considered a disorder of childhood. "The disease is there lifelong. It appears you cannot suppress the immune response," she said.
The intestine rapidly responds to a gluten-free diet, "But the tendency to have an immunologic response to gluten is always there," Dr. Crowe said.
Relapses are common. Patients are most likely to "fall off" the diet when symptoms begin to abate, she said. They may simply feel "cured" and resume old eating patterns, or they may drop the diet because of changes that can occur as the intestine heals. "If a patient had diarrhea and malabsorption of nutrients, they might find themselves getting constipated and gaining weight on the gluten-free diet, fall off, and get ill again. Even if the intestine is healed, the vast majority of data tell us that patient will relapse," at some point after abandoning the dietary restriction, Dr. Crowe said.
This can lead to the development of refractory celiac disease, in which the intestine fails to recover despite a gluten-free diet. Patients with refractory celiac disease may be unable to fully absorb nutrients and need supplemental feeding methods.
The goal of celiac management is to promote intestinal healing, optimize nutrition, and avoid long-term damage, Dr. Crowe said. "It’s key to bring in a knowledgeable dietitian to help. And I mean knowledgeable – not someone who is going to hand your patient a diet sheet and that’s all."
Many celiac patients are already nutritionally compromised at the time of diagnosis. "This is the time to measure their nutritional parameters," Dr. Crowe said. "They may need supplements. Many are deficient in vitamin D, iron, folate, zinc, or other trace elements."
The risks of untreated disease "are not inconsequential." Patients can develop problems related to nutrient malabsorption, including osteopenia, infertility, miscarriage, and intrauterine growth restriction, and are four times more likely than the general population to develop a malignancy.
Guidelines for managing nutritional status exist, but there are no evidence-based treatment guidelines for celiac disease, Dr. Crowe said. "Aside from nutrition, you have to just advise them to follow good health practices. I have several patients who still smoke, and yet they’re morose about their gluten-free diet. I say, ‘Why are you worried about that? You need to worry about quitting smoking!’ "
Dr. Crowe reported that she receives royalties from a book written for patients with celiac disease.
SAN DIEGO – An intestinal biopsy is almost always necessary to confirm celiac disease and is a must before committing a patient to the only effective treatment – a lifelong gluten-free diet.
Sticking to such a restricted diet is difficult and expensive, Dr. Sheila Crowe said at the annual meeting of the American College of Physicians. "A lifelong gluten-free diet sounds simple, unless you’re the patient. ... Eating out is very difficult, especially for children and teens who face a lot of peer pressure. And eating gluten free at home is expensive. Studies in the United States, Canada, and the United Kingdom confirm that a lifelong diet of gluten-free foods costs about three times more than a normal diet," said Dr. Crowe, professor in the division of gastroenterology and hepatology at the University of Virginia, Charlottesville.
Because treating celiac disease requires this lifelong commitment, a positive serologic test isn’t enough to rule it in, she said. Nor are any of the available immunologic tests, including the most widely used – tissue transglutaminase IgA (tTG IgA) – specific enough to replace intestinal biopsy as the sole method for reliably diagnosing celiac disease. "A positive tTG test is not enough to place a person on this lifelong treatment without confirmation from an intestinal biopsy," she said. "This is especially important for children, because of the higher likelihood of false positives in that group."
tTG IgA has a very high sensitivity and specificity, but it isn’t perfect, Dr. Crowe said. "If you have a patient with clinical symptoms and the tTG comes back negative, there is still a 10% chance that’s a false negative. Another scenario could be a patient who has an autoimmune disease or a relative with celiac, and is experiencing celiac symptoms. If the tTG came back negative on that person, I would still do an endoscopy."
The only possible exception might be a patient with celiac symptoms who already has biopsy-proven dermatitis herpetiformis, with the classic immunofluorescent deposits at the dermal-epidermal junction. "If you biopsy these patients, the intestine will show the changes associated with celiac disease every time," Dr. Crowe said.
Celiac disease is no longer considered a disorder of childhood. "The disease is there lifelong. It appears you cannot suppress the immune response," she said.
The intestine rapidly responds to a gluten-free diet, "But the tendency to have an immunologic response to gluten is always there," Dr. Crowe said.
Relapses are common. Patients are most likely to "fall off" the diet when symptoms begin to abate, she said. They may simply feel "cured" and resume old eating patterns, or they may drop the diet because of changes that can occur as the intestine heals. "If a patient had diarrhea and malabsorption of nutrients, they might find themselves getting constipated and gaining weight on the gluten-free diet, fall off, and get ill again. Even if the intestine is healed, the vast majority of data tell us that patient will relapse," at some point after abandoning the dietary restriction, Dr. Crowe said.
This can lead to the development of refractory celiac disease, in which the intestine fails to recover despite a gluten-free diet. Patients with refractory celiac disease may be unable to fully absorb nutrients and need supplemental feeding methods.
The goal of celiac management is to promote intestinal healing, optimize nutrition, and avoid long-term damage, Dr. Crowe said. "It’s key to bring in a knowledgeable dietitian to help. And I mean knowledgeable – not someone who is going to hand your patient a diet sheet and that’s all."
Many celiac patients are already nutritionally compromised at the time of diagnosis. "This is the time to measure their nutritional parameters," Dr. Crowe said. "They may need supplements. Many are deficient in vitamin D, iron, folate, zinc, or other trace elements."
The risks of untreated disease "are not inconsequential." Patients can develop problems related to nutrient malabsorption, including osteopenia, infertility, miscarriage, and intrauterine growth restriction, and are four times more likely than the general population to develop a malignancy.
Guidelines for managing nutritional status exist, but there are no evidence-based treatment guidelines for celiac disease, Dr. Crowe said. "Aside from nutrition, you have to just advise them to follow good health practices. I have several patients who still smoke, and yet they’re morose about their gluten-free diet. I say, ‘Why are you worried about that? You need to worry about quitting smoking!’ "
Dr. Crowe reported that she receives royalties from a book written for patients with celiac disease.
EXPERT ANALYSIS FROM THE ANNUAL MEETING OF THE AMERICAN COLLEGE OF PHYSICIANS
Confirm Celiac Diagnosis by Biopsy Before Advising Gluten-Free Diet
SAN DIEGO – An intestinal biopsy is almost always necessary to confirm celiac disease and is a must before committing a patient to the only effective treatment – a lifelong gluten-free diet.
Sticking to such a restricted diet is difficult and expensive, Dr. Sheila Crowe said at the annual meeting of the American College of Physicians. "A lifelong gluten-free diet sounds simple, unless you’re the patient. ... Eating out is very difficult, especially for children and teens who face a lot of peer pressure. And eating gluten free at home is expensive. Studies in the United States, Canada, and the United Kingdom confirm that a lifelong diet of gluten-free foods costs about three times more than a normal diet," said Dr. Crowe, professor in the division of gastroenterology and hepatology at the University of Virginia, Charlottesville.
Because treating celiac disease requires this lifelong commitment, a positive serologic test isn’t enough to rule it in, she said. Nor are any of the available immunologic tests, including the most widely used – tissue transglutaminase IgA (tTG IgA) – specific enough to replace intestinal biopsy as the sole method for reliably diagnosing celiac disease. "A positive tTG test is not enough to place a person on this lifelong treatment without confirmation from an intestinal biopsy," she said. "This is especially important for children, because of the higher likelihood of false positives in that group."
tTG IgA has a very high sensitivity and specificity, but it isn’t perfect, Dr. Crowe said. "If you have a patient with clinical symptoms and the tTG comes back negative, there is still a 10% chance that’s a false negative. Another scenario could be a patient who has an autoimmune disease or a relative with celiac, and is experiencing celiac symptoms. If the tTG came back negative on that person, I would still do an endoscopy."
The only possible exception might be a patient with celiac symptoms who already has biopsy-proven dermatitis herpetiformis, with the classic immunofluorescent deposits at the dermal-epidermal junction. "If you biopsy these patients, the intestine will show the changes associated with celiac disease every time," Dr. Crowe said.
Celiac disease is no longer considered a disorder of childhood. "The disease is there lifelong. It appears you cannot suppress the immune response," she said.
The intestine rapidly responds to a gluten-free diet, "But the tendency to have an immunologic response to gluten is always there," Dr. Crowe said.
Relapses are common. Patients are most likely to "fall off" the diet when symptoms begin to abate, she said. They may simply feel "cured" and resume old eating patterns, or they may drop the diet because of changes that can occur as the intestine heals. "If a patient had diarrhea and malabsorption of nutrients, they might find themselves getting constipated and gaining weight on the gluten-free diet, fall off, and get ill again. Even if the intestine is healed, the vast majority of data tell us that patient will relapse," at some point after abandoning the dietary restriction, Dr. Crowe said.
This can lead to the development of refractory celiac disease, in which the intestine fails to recover despite a gluten-free diet. Patients with refractory celiac disease may be unable to fully absorb nutrients and need supplemental feeding methods.
The goal of celiac management is to promote intestinal healing, optimize nutrition, and avoid long-term damage, Dr. Crowe said. "It’s key to bring in a knowledgeable dietitian to help. And I mean knowledgeable – not someone who is going to hand your patient a diet sheet and that’s all."
Many celiac patients are already nutritionally compromised at the time of diagnosis. "This is the time to measure their nutritional parameters," Dr. Crowe said. "They may need supplements. Many are deficient in vitamin D, iron, folate, zinc, or other trace elements."
The risks of untreated disease "are not inconsequential." Patients can develop problems related to nutrient malabsorption, including osteopenia, infertility, miscarriage, and intrauterine growth restriction, and are four times more likely than the general population to develop a malignancy.
Guidelines for managing nutritional status exist, but there are no evidence-based treatment guidelines for celiac disease, Dr. Crowe said. "Aside from nutrition, you have to just advise them to follow good health practices. I have several patients who still smoke, and yet they’re morose about their gluten-free diet. I say, ‘Why are you worried about that? You need to worry about quitting smoking!’ "
Dr. Crowe reported that she receives royalties from a book written for patients with celiac disease.
SAN DIEGO – An intestinal biopsy is almost always necessary to confirm celiac disease and is a must before committing a patient to the only effective treatment – a lifelong gluten-free diet.
Sticking to such a restricted diet is difficult and expensive, Dr. Sheila Crowe said at the annual meeting of the American College of Physicians. "A lifelong gluten-free diet sounds simple, unless you’re the patient. ... Eating out is very difficult, especially for children and teens who face a lot of peer pressure. And eating gluten free at home is expensive. Studies in the United States, Canada, and the United Kingdom confirm that a lifelong diet of gluten-free foods costs about three times more than a normal diet," said Dr. Crowe, professor in the division of gastroenterology and hepatology at the University of Virginia, Charlottesville.
Because treating celiac disease requires this lifelong commitment, a positive serologic test isn’t enough to rule it in, she said. Nor are any of the available immunologic tests, including the most widely used – tissue transglutaminase IgA (tTG IgA) – specific enough to replace intestinal biopsy as the sole method for reliably diagnosing celiac disease. "A positive tTG test is not enough to place a person on this lifelong treatment without confirmation from an intestinal biopsy," she said. "This is especially important for children, because of the higher likelihood of false positives in that group."
tTG IgA has a very high sensitivity and specificity, but it isn’t perfect, Dr. Crowe said. "If you have a patient with clinical symptoms and the tTG comes back negative, there is still a 10% chance that’s a false negative. Another scenario could be a patient who has an autoimmune disease or a relative with celiac, and is experiencing celiac symptoms. If the tTG came back negative on that person, I would still do an endoscopy."
The only possible exception might be a patient with celiac symptoms who already has biopsy-proven dermatitis herpetiformis, with the classic immunofluorescent deposits at the dermal-epidermal junction. "If you biopsy these patients, the intestine will show the changes associated with celiac disease every time," Dr. Crowe said.
Celiac disease is no longer considered a disorder of childhood. "The disease is there lifelong. It appears you cannot suppress the immune response," she said.
The intestine rapidly responds to a gluten-free diet, "But the tendency to have an immunologic response to gluten is always there," Dr. Crowe said.
Relapses are common. Patients are most likely to "fall off" the diet when symptoms begin to abate, she said. They may simply feel "cured" and resume old eating patterns, or they may drop the diet because of changes that can occur as the intestine heals. "If a patient had diarrhea and malabsorption of nutrients, they might find themselves getting constipated and gaining weight on the gluten-free diet, fall off, and get ill again. Even if the intestine is healed, the vast majority of data tell us that patient will relapse," at some point after abandoning the dietary restriction, Dr. Crowe said.
This can lead to the development of refractory celiac disease, in which the intestine fails to recover despite a gluten-free diet. Patients with refractory celiac disease may be unable to fully absorb nutrients and need supplemental feeding methods.
The goal of celiac management is to promote intestinal healing, optimize nutrition, and avoid long-term damage, Dr. Crowe said. "It’s key to bring in a knowledgeable dietitian to help. And I mean knowledgeable – not someone who is going to hand your patient a diet sheet and that’s all."
Many celiac patients are already nutritionally compromised at the time of diagnosis. "This is the time to measure their nutritional parameters," Dr. Crowe said. "They may need supplements. Many are deficient in vitamin D, iron, folate, zinc, or other trace elements."
The risks of untreated disease "are not inconsequential." Patients can develop problems related to nutrient malabsorption, including osteopenia, infertility, miscarriage, and intrauterine growth restriction, and are four times more likely than the general population to develop a malignancy.
Guidelines for managing nutritional status exist, but there are no evidence-based treatment guidelines for celiac disease, Dr. Crowe said. "Aside from nutrition, you have to just advise them to follow good health practices. I have several patients who still smoke, and yet they’re morose about their gluten-free diet. I say, ‘Why are you worried about that? You need to worry about quitting smoking!’ "
Dr. Crowe reported that she receives royalties from a book written for patients with celiac disease.
SAN DIEGO – An intestinal biopsy is almost always necessary to confirm celiac disease and is a must before committing a patient to the only effective treatment – a lifelong gluten-free diet.
Sticking to such a restricted diet is difficult and expensive, Dr. Sheila Crowe said at the annual meeting of the American College of Physicians. "A lifelong gluten-free diet sounds simple, unless you’re the patient. ... Eating out is very difficult, especially for children and teens who face a lot of peer pressure. And eating gluten free at home is expensive. Studies in the United States, Canada, and the United Kingdom confirm that a lifelong diet of gluten-free foods costs about three times more than a normal diet," said Dr. Crowe, professor in the division of gastroenterology and hepatology at the University of Virginia, Charlottesville.
Because treating celiac disease requires this lifelong commitment, a positive serologic test isn’t enough to rule it in, she said. Nor are any of the available immunologic tests, including the most widely used – tissue transglutaminase IgA (tTG IgA) – specific enough to replace intestinal biopsy as the sole method for reliably diagnosing celiac disease. "A positive tTG test is not enough to place a person on this lifelong treatment without confirmation from an intestinal biopsy," she said. "This is especially important for children, because of the higher likelihood of false positives in that group."
tTG IgA has a very high sensitivity and specificity, but it isn’t perfect, Dr. Crowe said. "If you have a patient with clinical symptoms and the tTG comes back negative, there is still a 10% chance that’s a false negative. Another scenario could be a patient who has an autoimmune disease or a relative with celiac, and is experiencing celiac symptoms. If the tTG came back negative on that person, I would still do an endoscopy."
The only possible exception might be a patient with celiac symptoms who already has biopsy-proven dermatitis herpetiformis, with the classic immunofluorescent deposits at the dermal-epidermal junction. "If you biopsy these patients, the intestine will show the changes associated with celiac disease every time," Dr. Crowe said.
Celiac disease is no longer considered a disorder of childhood. "The disease is there lifelong. It appears you cannot suppress the immune response," she said.
The intestine rapidly responds to a gluten-free diet, "But the tendency to have an immunologic response to gluten is always there," Dr. Crowe said.
Relapses are common. Patients are most likely to "fall off" the diet when symptoms begin to abate, she said. They may simply feel "cured" and resume old eating patterns, or they may drop the diet because of changes that can occur as the intestine heals. "If a patient had diarrhea and malabsorption of nutrients, they might find themselves getting constipated and gaining weight on the gluten-free diet, fall off, and get ill again. Even if the intestine is healed, the vast majority of data tell us that patient will relapse," at some point after abandoning the dietary restriction, Dr. Crowe said.
This can lead to the development of refractory celiac disease, in which the intestine fails to recover despite a gluten-free diet. Patients with refractory celiac disease may be unable to fully absorb nutrients and need supplemental feeding methods.
The goal of celiac management is to promote intestinal healing, optimize nutrition, and avoid long-term damage, Dr. Crowe said. "It’s key to bring in a knowledgeable dietitian to help. And I mean knowledgeable – not someone who is going to hand your patient a diet sheet and that’s all."
Many celiac patients are already nutritionally compromised at the time of diagnosis. "This is the time to measure their nutritional parameters," Dr. Crowe said. "They may need supplements. Many are deficient in vitamin D, iron, folate, zinc, or other trace elements."
The risks of untreated disease "are not inconsequential." Patients can develop problems related to nutrient malabsorption, including osteopenia, infertility, miscarriage, and intrauterine growth restriction, and are four times more likely than the general population to develop a malignancy.
Guidelines for managing nutritional status exist, but there are no evidence-based treatment guidelines for celiac disease, Dr. Crowe said. "Aside from nutrition, you have to just advise them to follow good health practices. I have several patients who still smoke, and yet they’re morose about their gluten-free diet. I say, ‘Why are you worried about that? You need to worry about quitting smoking!’ "
Dr. Crowe reported that she receives royalties from a book written for patients with celiac disease.
EXPERT ANALYSIS FROM THE ANNUAL MEETING OF THE AMERICAN COLLEGE OF PHYSICIANS
Confirm Celiac Diagnosis by Biopsy Before Advising Gluten-Free Diet
SAN DIEGO – An intestinal biopsy is almost always necessary to confirm celiac disease and is a must before committing a patient to the only effective treatment – a lifelong gluten-free diet.
Sticking to such a restricted diet is difficult and expensive, Dr. Sheila Crowe said at the annual meeting of the American College of Physicians. "A lifelong gluten-free diet sounds simple, unless you’re the patient. ... Eating out is very difficult, especially for children and teens who face a lot of peer pressure. And eating gluten free at home is expensive. Studies in the United States, Canada, and the United Kingdom confirm that a lifelong diet of gluten-free foods costs about three times more than a normal diet," said Dr. Crowe, professor in the division of gastroenterology and hepatology at the University of Virginia, Charlottesville.
Because treating celiac disease requires this lifelong commitment, a positive serologic test isn’t enough to rule it in, she said. Nor are any of the available immunologic tests, including the most widely used – tissue transglutaminase IgA (tTG IgA) – specific enough to replace intestinal biopsy as the sole method for reliably diagnosing celiac disease. "A positive tTG test is not enough to place a person on this lifelong treatment without confirmation from an intestinal biopsy," she said. "This is especially important for children, because of the higher likelihood of false positives in that group."
tTG IgA has a very high sensitivity and specificity, but it isn’t perfect, Dr. Crowe said. "If you have a patient with clinical symptoms and the tTG comes back negative, there is still a 10% chance that’s a false negative. Another scenario could be a patient who has an autoimmune disease or a relative with celiac, and is experiencing celiac symptoms. If the tTG came back negative on that person, I would still do an endoscopy."
The only possible exception might be a patient with celiac symptoms who already has biopsy-proven dermatitis herpetiformis, with the classic immunofluorescent deposits at the dermal-epidermal junction. "If you biopsy these patients, the intestine will show the changes associated with celiac disease every time," Dr. Crowe said.
Celiac disease is no longer considered a disorder of childhood. "The disease is there lifelong. It appears you cannot suppress the immune response," she said.
The intestine rapidly responds to a gluten-free diet, "But the tendency to have an immunologic response to gluten is always there," Dr. Crowe said.
Relapses are common. Patients are most likely to "fall off" the diet when symptoms begin to abate, she said. They may simply feel "cured" and resume old eating patterns, or they may drop the diet because of changes that can occur as the intestine heals. "If a patient had diarrhea and malabsorption of nutrients, they might find themselves getting constipated and gaining weight on the gluten-free diet, fall off, and get ill again. Even if the intestine is healed, the vast majority of data tell us that patient will relapse," at some point after abandoning the dietary restriction, Dr. Crowe said.
This can lead to the development of refractory celiac disease, in which the intestine fails to recover despite a gluten-free diet. Patients with refractory celiac disease may be unable to fully absorb nutrients and need supplemental feeding methods.
The goal of celiac management is to promote intestinal healing, optimize nutrition, and avoid long-term damage, Dr. Crowe said. "It’s key to bring in a knowledgeable dietitian to help. And I mean knowledgeable – not someone who is going to hand your patient a diet sheet and that’s all."
Many celiac patients are already nutritionally compromised at the time of diagnosis. "This is the time to measure their nutritional parameters," Dr. Crowe said. "They may need supplements. Many are deficient in vitamin D, iron, folate, zinc, or other trace elements."
The risks of untreated disease "are not inconsequential." Patients can develop problems related to nutrient malabsorption, including osteopenia, infertility, miscarriage, and intrauterine growth restriction, and are four times more likely than the general population to develop a malignancy.
Guidelines for managing nutritional status exist, but there are no evidence-based treatment guidelines for celiac disease, Dr. Crowe said. "Aside from nutrition, you have to just advise them to follow good health practices. I have several patients who still smoke, and yet they’re morose about their gluten-free diet. I say, ‘Why are you worried about that? You need to worry about quitting smoking!’ "
Dr. Crowe reported that she receives royalties from a book written for patients with celiac disease.
SAN DIEGO – An intestinal biopsy is almost always necessary to confirm celiac disease and is a must before committing a patient to the only effective treatment – a lifelong gluten-free diet.
Sticking to such a restricted diet is difficult and expensive, Dr. Sheila Crowe said at the annual meeting of the American College of Physicians. "A lifelong gluten-free diet sounds simple, unless you’re the patient. ... Eating out is very difficult, especially for children and teens who face a lot of peer pressure. And eating gluten free at home is expensive. Studies in the United States, Canada, and the United Kingdom confirm that a lifelong diet of gluten-free foods costs about three times more than a normal diet," said Dr. Crowe, professor in the division of gastroenterology and hepatology at the University of Virginia, Charlottesville.
Because treating celiac disease requires this lifelong commitment, a positive serologic test isn’t enough to rule it in, she said. Nor are any of the available immunologic tests, including the most widely used – tissue transglutaminase IgA (tTG IgA) – specific enough to replace intestinal biopsy as the sole method for reliably diagnosing celiac disease. "A positive tTG test is not enough to place a person on this lifelong treatment without confirmation from an intestinal biopsy," she said. "This is especially important for children, because of the higher likelihood of false positives in that group."
tTG IgA has a very high sensitivity and specificity, but it isn’t perfect, Dr. Crowe said. "If you have a patient with clinical symptoms and the tTG comes back negative, there is still a 10% chance that’s a false negative. Another scenario could be a patient who has an autoimmune disease or a relative with celiac, and is experiencing celiac symptoms. If the tTG came back negative on that person, I would still do an endoscopy."
The only possible exception might be a patient with celiac symptoms who already has biopsy-proven dermatitis herpetiformis, with the classic immunofluorescent deposits at the dermal-epidermal junction. "If you biopsy these patients, the intestine will show the changes associated with celiac disease every time," Dr. Crowe said.
Celiac disease is no longer considered a disorder of childhood. "The disease is there lifelong. It appears you cannot suppress the immune response," she said.
The intestine rapidly responds to a gluten-free diet, "But the tendency to have an immunologic response to gluten is always there," Dr. Crowe said.
Relapses are common. Patients are most likely to "fall off" the diet when symptoms begin to abate, she said. They may simply feel "cured" and resume old eating patterns, or they may drop the diet because of changes that can occur as the intestine heals. "If a patient had diarrhea and malabsorption of nutrients, they might find themselves getting constipated and gaining weight on the gluten-free diet, fall off, and get ill again. Even if the intestine is healed, the vast majority of data tell us that patient will relapse," at some point after abandoning the dietary restriction, Dr. Crowe said.
This can lead to the development of refractory celiac disease, in which the intestine fails to recover despite a gluten-free diet. Patients with refractory celiac disease may be unable to fully absorb nutrients and need supplemental feeding methods.
The goal of celiac management is to promote intestinal healing, optimize nutrition, and avoid long-term damage, Dr. Crowe said. "It’s key to bring in a knowledgeable dietitian to help. And I mean knowledgeable – not someone who is going to hand your patient a diet sheet and that’s all."
Many celiac patients are already nutritionally compromised at the time of diagnosis. "This is the time to measure their nutritional parameters," Dr. Crowe said. "They may need supplements. Many are deficient in vitamin D, iron, folate, zinc, or other trace elements."
The risks of untreated disease "are not inconsequential." Patients can develop problems related to nutrient malabsorption, including osteopenia, infertility, miscarriage, and intrauterine growth restriction, and are four times more likely than the general population to develop a malignancy.
Guidelines for managing nutritional status exist, but there are no evidence-based treatment guidelines for celiac disease, Dr. Crowe said. "Aside from nutrition, you have to just advise them to follow good health practices. I have several patients who still smoke, and yet they’re morose about their gluten-free diet. I say, ‘Why are you worried about that? You need to worry about quitting smoking!’ "
Dr. Crowe reported that she receives royalties from a book written for patients with celiac disease.
SAN DIEGO – An intestinal biopsy is almost always necessary to confirm celiac disease and is a must before committing a patient to the only effective treatment – a lifelong gluten-free diet.
Sticking to such a restricted diet is difficult and expensive, Dr. Sheila Crowe said at the annual meeting of the American College of Physicians. "A lifelong gluten-free diet sounds simple, unless you’re the patient. ... Eating out is very difficult, especially for children and teens who face a lot of peer pressure. And eating gluten free at home is expensive. Studies in the United States, Canada, and the United Kingdom confirm that a lifelong diet of gluten-free foods costs about three times more than a normal diet," said Dr. Crowe, professor in the division of gastroenterology and hepatology at the University of Virginia, Charlottesville.
Because treating celiac disease requires this lifelong commitment, a positive serologic test isn’t enough to rule it in, she said. Nor are any of the available immunologic tests, including the most widely used – tissue transglutaminase IgA (tTG IgA) – specific enough to replace intestinal biopsy as the sole method for reliably diagnosing celiac disease. "A positive tTG test is not enough to place a person on this lifelong treatment without confirmation from an intestinal biopsy," she said. "This is especially important for children, because of the higher likelihood of false positives in that group."
tTG IgA has a very high sensitivity and specificity, but it isn’t perfect, Dr. Crowe said. "If you have a patient with clinical symptoms and the tTG comes back negative, there is still a 10% chance that’s a false negative. Another scenario could be a patient who has an autoimmune disease or a relative with celiac, and is experiencing celiac symptoms. If the tTG came back negative on that person, I would still do an endoscopy."
The only possible exception might be a patient with celiac symptoms who already has biopsy-proven dermatitis herpetiformis, with the classic immunofluorescent deposits at the dermal-epidermal junction. "If you biopsy these patients, the intestine will show the changes associated with celiac disease every time," Dr. Crowe said.
Celiac disease is no longer considered a disorder of childhood. "The disease is there lifelong. It appears you cannot suppress the immune response," she said.
The intestine rapidly responds to a gluten-free diet, "But the tendency to have an immunologic response to gluten is always there," Dr. Crowe said.
Relapses are common. Patients are most likely to "fall off" the diet when symptoms begin to abate, she said. They may simply feel "cured" and resume old eating patterns, or they may drop the diet because of changes that can occur as the intestine heals. "If a patient had diarrhea and malabsorption of nutrients, they might find themselves getting constipated and gaining weight on the gluten-free diet, fall off, and get ill again. Even if the intestine is healed, the vast majority of data tell us that patient will relapse," at some point after abandoning the dietary restriction, Dr. Crowe said.
This can lead to the development of refractory celiac disease, in which the intestine fails to recover despite a gluten-free diet. Patients with refractory celiac disease may be unable to fully absorb nutrients and need supplemental feeding methods.
The goal of celiac management is to promote intestinal healing, optimize nutrition, and avoid long-term damage, Dr. Crowe said. "It’s key to bring in a knowledgeable dietitian to help. And I mean knowledgeable – not someone who is going to hand your patient a diet sheet and that’s all."
Many celiac patients are already nutritionally compromised at the time of diagnosis. "This is the time to measure their nutritional parameters," Dr. Crowe said. "They may need supplements. Many are deficient in vitamin D, iron, folate, zinc, or other trace elements."
The risks of untreated disease "are not inconsequential." Patients can develop problems related to nutrient malabsorption, including osteopenia, infertility, miscarriage, and intrauterine growth restriction, and are four times more likely than the general population to develop a malignancy.
Guidelines for managing nutritional status exist, but there are no evidence-based treatment guidelines for celiac disease, Dr. Crowe said. "Aside from nutrition, you have to just advise them to follow good health practices. I have several patients who still smoke, and yet they’re morose about their gluten-free diet. I say, ‘Why are you worried about that? You need to worry about quitting smoking!’ "
Dr. Crowe reported that she receives royalties from a book written for patients with celiac disease.
EXPERT ANALYSIS FROM THE ANNUAL MEETING OF THE AMERICAN COLLEGE OF PHYSICIANS
Allopurinol Still Deemed First Line for Gout
SAN DIEGO – Allopurinol should remain the first-line agent for gout prophylaxis, despite some competition from a newer drug, febuxostat, according to Dr. John Pendleton.
Allopurinol is generally very effective, can be safely titrated for those with renal impairment, and is the most cost-effective option, he said at the annual meeting of the American College of Physicians.
"I still recommend allopurinol as the initial treatment, because you don’t need a 24-hour urine collection to give it; it’s effective in both overproducers and underexcretors [of uric acid]; it can be taken just once a day; and it’s safe and effective for those with mild renal insufficiency when the dose is adjusted," said Dr. Pendleton, an internist in Roanoke, Va.
Price is also an important factor, he noted. "Generic allopurinol costs about $15 per month. The brand name costs about $43 per month. But the price for febuxostat comes in at about $156 per month."
With either drug, the treatment goal should be to lower uric acid levels to be 6 mg/dL, said Dr. Pendleton, referring to a retrospective study of 276 patients with recurrent gout attacks. "This study noted that among the 81 patients with a uric acid of less than 6 mg/dL, 88% had no recurrent attacks during the 3-year observational period" (Arthritis Rheum. 2004;51:321-5).
Allopurinol has been the "standby drug" for gouty arthritis for 60 years, and still performs admirably, Dr. Pendleton said. Although most initial doses range from 50 to 300 mg/day, "some recent studies suggest that only 25% of patients will reach the uric acid target on that regimen. For many patients, we need to increase the dose to get that level down."
The dose should be incrementally increased every 3-4 weeks to reach the uric acid target level; doses of up to 800 mg/day are approved for this indication. "But if you’re not able to achieve this desired level by pushing the dose close to 800 mg, I would consider trying febuxostat."
Febuxostat, a xanthine oxidase inhibitor, is more selective and potent than allopurinol. "It’s metabolized in the liver and very little of the active drug is excreted renally, raising the possibility that it might be safer in patients with mild to moderate renal insufficiency," Dr. Pendleton said.
It’s not easy to fully compare the two, because all three of the studies on the basis of which febuxostat was approved used a fixed-dose allopurinol regimen. "None of them allowed the total upward titration of allopurinol for a fair comparison," Dr. Pendleton pointed out.
The studies concluded that 40 mg of febuxostat was as effective as 300 mg of allopurinol. "The higher dose [of febuxostat 80 mg] seemed to be more effective than 400 mg allopurinol, but again, the studies did not allow for an upward titration" of the comparator, he said.
Although none of the patients in those trials had a creatinine level of more than 2.5 mg/dL, "a short-term study suggests that febuxostat dosing would not need to be adjusted even with a very low creatinine clearance [of 10-29 mL/min]. But just the same I would be very careful in that setting," Dr. Pendleton said (Am. J. Ther. 2005;12:22-34).
Febuxostat also appears to be safe for patients with mild hepatic dysfunction, with an adverse event profile similar to that of allopurinol. However, febuxostat is contraindicated in patients who are taking azathioprine, mercaptopurine, and theophylline.
Probenecid could be another option for some patients, especially those who underexcrete uric acid. Although probenecid has a somewhat better adverse event profile than allopurinol, it requires a 24-hour urine collection to rule out overproduction of uric acid and it seems to increase the risk of kidney stones. The drug is not as effective in patients with a low creatinine clearance (less than 60 mL/min), and "seems to have no effect at all at a creatinine level of 30 mL/min or lower," Dr. Pendleton said. Probenecid also must be taken three times a day – another drawback, in his opinion.
Dr. Pendleton said that he had no relevant financial disclosures.
SAN DIEGO – Allopurinol should remain the first-line agent for gout prophylaxis, despite some competition from a newer drug, febuxostat, according to Dr. John Pendleton.
Allopurinol is generally very effective, can be safely titrated for those with renal impairment, and is the most cost-effective option, he said at the annual meeting of the American College of Physicians.
"I still recommend allopurinol as the initial treatment, because you don’t need a 24-hour urine collection to give it; it’s effective in both overproducers and underexcretors [of uric acid]; it can be taken just once a day; and it’s safe and effective for those with mild renal insufficiency when the dose is adjusted," said Dr. Pendleton, an internist in Roanoke, Va.
Price is also an important factor, he noted. "Generic allopurinol costs about $15 per month. The brand name costs about $43 per month. But the price for febuxostat comes in at about $156 per month."
With either drug, the treatment goal should be to lower uric acid levels to be 6 mg/dL, said Dr. Pendleton, referring to a retrospective study of 276 patients with recurrent gout attacks. "This study noted that among the 81 patients with a uric acid of less than 6 mg/dL, 88% had no recurrent attacks during the 3-year observational period" (Arthritis Rheum. 2004;51:321-5).
Allopurinol has been the "standby drug" for gouty arthritis for 60 years, and still performs admirably, Dr. Pendleton said. Although most initial doses range from 50 to 300 mg/day, "some recent studies suggest that only 25% of patients will reach the uric acid target on that regimen. For many patients, we need to increase the dose to get that level down."
The dose should be incrementally increased every 3-4 weeks to reach the uric acid target level; doses of up to 800 mg/day are approved for this indication. "But if you’re not able to achieve this desired level by pushing the dose close to 800 mg, I would consider trying febuxostat."
Febuxostat, a xanthine oxidase inhibitor, is more selective and potent than allopurinol. "It’s metabolized in the liver and very little of the active drug is excreted renally, raising the possibility that it might be safer in patients with mild to moderate renal insufficiency," Dr. Pendleton said.
It’s not easy to fully compare the two, because all three of the studies on the basis of which febuxostat was approved used a fixed-dose allopurinol regimen. "None of them allowed the total upward titration of allopurinol for a fair comparison," Dr. Pendleton pointed out.
The studies concluded that 40 mg of febuxostat was as effective as 300 mg of allopurinol. "The higher dose [of febuxostat 80 mg] seemed to be more effective than 400 mg allopurinol, but again, the studies did not allow for an upward titration" of the comparator, he said.
Although none of the patients in those trials had a creatinine level of more than 2.5 mg/dL, "a short-term study suggests that febuxostat dosing would not need to be adjusted even with a very low creatinine clearance [of 10-29 mL/min]. But just the same I would be very careful in that setting," Dr. Pendleton said (Am. J. Ther. 2005;12:22-34).
Febuxostat also appears to be safe for patients with mild hepatic dysfunction, with an adverse event profile similar to that of allopurinol. However, febuxostat is contraindicated in patients who are taking azathioprine, mercaptopurine, and theophylline.
Probenecid could be another option for some patients, especially those who underexcrete uric acid. Although probenecid has a somewhat better adverse event profile than allopurinol, it requires a 24-hour urine collection to rule out overproduction of uric acid and it seems to increase the risk of kidney stones. The drug is not as effective in patients with a low creatinine clearance (less than 60 mL/min), and "seems to have no effect at all at a creatinine level of 30 mL/min or lower," Dr. Pendleton said. Probenecid also must be taken three times a day – another drawback, in his opinion.
Dr. Pendleton said that he had no relevant financial disclosures.
SAN DIEGO – Allopurinol should remain the first-line agent for gout prophylaxis, despite some competition from a newer drug, febuxostat, according to Dr. John Pendleton.
Allopurinol is generally very effective, can be safely titrated for those with renal impairment, and is the most cost-effective option, he said at the annual meeting of the American College of Physicians.
"I still recommend allopurinol as the initial treatment, because you don’t need a 24-hour urine collection to give it; it’s effective in both overproducers and underexcretors [of uric acid]; it can be taken just once a day; and it’s safe and effective for those with mild renal insufficiency when the dose is adjusted," said Dr. Pendleton, an internist in Roanoke, Va.
Price is also an important factor, he noted. "Generic allopurinol costs about $15 per month. The brand name costs about $43 per month. But the price for febuxostat comes in at about $156 per month."
With either drug, the treatment goal should be to lower uric acid levels to be 6 mg/dL, said Dr. Pendleton, referring to a retrospective study of 276 patients with recurrent gout attacks. "This study noted that among the 81 patients with a uric acid of less than 6 mg/dL, 88% had no recurrent attacks during the 3-year observational period" (Arthritis Rheum. 2004;51:321-5).
Allopurinol has been the "standby drug" for gouty arthritis for 60 years, and still performs admirably, Dr. Pendleton said. Although most initial doses range from 50 to 300 mg/day, "some recent studies suggest that only 25% of patients will reach the uric acid target on that regimen. For many patients, we need to increase the dose to get that level down."
The dose should be incrementally increased every 3-4 weeks to reach the uric acid target level; doses of up to 800 mg/day are approved for this indication. "But if you’re not able to achieve this desired level by pushing the dose close to 800 mg, I would consider trying febuxostat."
Febuxostat, a xanthine oxidase inhibitor, is more selective and potent than allopurinol. "It’s metabolized in the liver and very little of the active drug is excreted renally, raising the possibility that it might be safer in patients with mild to moderate renal insufficiency," Dr. Pendleton said.
It’s not easy to fully compare the two, because all three of the studies on the basis of which febuxostat was approved used a fixed-dose allopurinol regimen. "None of them allowed the total upward titration of allopurinol for a fair comparison," Dr. Pendleton pointed out.
The studies concluded that 40 mg of febuxostat was as effective as 300 mg of allopurinol. "The higher dose [of febuxostat 80 mg] seemed to be more effective than 400 mg allopurinol, but again, the studies did not allow for an upward titration" of the comparator, he said.
Although none of the patients in those trials had a creatinine level of more than 2.5 mg/dL, "a short-term study suggests that febuxostat dosing would not need to be adjusted even with a very low creatinine clearance [of 10-29 mL/min]. But just the same I would be very careful in that setting," Dr. Pendleton said (Am. J. Ther. 2005;12:22-34).
Febuxostat also appears to be safe for patients with mild hepatic dysfunction, with an adverse event profile similar to that of allopurinol. However, febuxostat is contraindicated in patients who are taking azathioprine, mercaptopurine, and theophylline.
Probenecid could be another option for some patients, especially those who underexcrete uric acid. Although probenecid has a somewhat better adverse event profile than allopurinol, it requires a 24-hour urine collection to rule out overproduction of uric acid and it seems to increase the risk of kidney stones. The drug is not as effective in patients with a low creatinine clearance (less than 60 mL/min), and "seems to have no effect at all at a creatinine level of 30 mL/min or lower," Dr. Pendleton said. Probenecid also must be taken three times a day – another drawback, in his opinion.
Dr. Pendleton said that he had no relevant financial disclosures.
EXPERT ANALYSIS FROM THE ANNUAL MEETING OF THE AMERICAN COLLEGE OF PHYSICIANS
Allopurinol Still Deemed First Line for Gout
SAN DIEGO – Allopurinol should remain the first-line agent for gout prophylaxis, despite some competition from a newer drug, febuxostat, according to Dr. John Pendleton.
Allopurinol is generally very effective, can be safely titrated for those with renal impairment, and is the most cost-effective option, he said at the annual meeting of the American College of Physicians.
"I still recommend allopurinol as the initial treatment, because you don’t need a 24-hour urine collection to give it; it’s effective in both overproducers and underexcretors [of uric acid]; it can be taken just once a day; and it’s safe and effective for those with mild renal insufficiency when the dose is adjusted," said Dr. Pendleton, an internist in Roanoke, Va.
Price is also an important factor, he noted. "Generic allopurinol costs about $15 per month. The brand name costs about $43 per month. But the price for febuxostat comes in at about $156 per month."
With either drug, the treatment goal should be to lower uric acid levels to be 6 mg/dL, said Dr. Pendleton, referring to a retrospective study of 276 patients with recurrent gout attacks. "This study noted that among the 81 patients with a uric acid of less than 6 mg/dL, 88% had no recurrent attacks during the 3-year observational period" (Arthritis Rheum. 2004;51:321-5).
Allopurinol has been the "standby drug" for gouty arthritis for 60 years, and still performs admirably, Dr. Pendleton said. Although most initial doses range from 50 to 300 mg/day, "some recent studies suggest that only 25% of patients will reach the uric acid target on that regimen. For many patients, we need to increase the dose to get that level down."
The dose should be incrementally increased every 3-4 weeks to reach the uric acid target level; doses of up to 800 mg/day are approved for this indication. "But if you’re not able to achieve this desired level by pushing the dose close to 800 mg, I would consider trying febuxostat."
Febuxostat, a xanthine oxidase inhibitor, is more selective and potent than allopurinol. "It’s metabolized in the liver and very little of the active drug is excreted renally, raising the possibility that it might be safer in patients with mild to moderate renal insufficiency," Dr. Pendleton said.
It’s not easy to fully compare the two, because all three of the studies on the basis of which febuxostat was approved used a fixed-dose allopurinol regimen. "None of them allowed the total upward titration of allopurinol for a fair comparison," Dr. Pendleton pointed out.
The studies concluded that 40 mg of febuxostat was as effective as 300 mg of allopurinol. "The higher dose [of febuxostat 80 mg] seemed to be more effective than 400 mg allopurinol, but again, the studies did not allow for an upward titration" of the comparator, he said.
Although none of the patients in those trials had a creatinine level of more than 2.5 mg/dL, "a short-term study suggests that febuxostat dosing would not need to be adjusted even with a very low creatinine clearance [of 10-29 mL/min]. But just the same I would be very careful in that setting," Dr. Pendleton said (Am. J. Ther. 2005;12:22-34).
Febuxostat also appears to be safe for patients with mild hepatic dysfunction, with an adverse event profile similar to that of allopurinol. However, febuxostat is contraindicated in patients who are taking azathioprine, mercaptopurine, and theophylline.
Probenecid could be another option for some patients, especially those who underexcrete uric acid. Although probenecid has a somewhat better adverse event profile than allopurinol, it requires a 24-hour urine collection to rule out overproduction of uric acid and it seems to increase the risk of kidney stones. The drug is not as effective in patients with a low creatinine clearance (less than 60 mL/min), and "seems to have no effect at all at a creatinine level of 30 mL/min or lower," Dr. Pendleton said. Probenecid also must be taken three times a day – another drawback, in his opinion.
Dr. Pendleton said that he had no relevant financial disclosures.
SAN DIEGO – Allopurinol should remain the first-line agent for gout prophylaxis, despite some competition from a newer drug, febuxostat, according to Dr. John Pendleton.
Allopurinol is generally very effective, can be safely titrated for those with renal impairment, and is the most cost-effective option, he said at the annual meeting of the American College of Physicians.
"I still recommend allopurinol as the initial treatment, because you don’t need a 24-hour urine collection to give it; it’s effective in both overproducers and underexcretors [of uric acid]; it can be taken just once a day; and it’s safe and effective for those with mild renal insufficiency when the dose is adjusted," said Dr. Pendleton, an internist in Roanoke, Va.
Price is also an important factor, he noted. "Generic allopurinol costs about $15 per month. The brand name costs about $43 per month. But the price for febuxostat comes in at about $156 per month."
With either drug, the treatment goal should be to lower uric acid levels to be 6 mg/dL, said Dr. Pendleton, referring to a retrospective study of 276 patients with recurrent gout attacks. "This study noted that among the 81 patients with a uric acid of less than 6 mg/dL, 88% had no recurrent attacks during the 3-year observational period" (Arthritis Rheum. 2004;51:321-5).
Allopurinol has been the "standby drug" for gouty arthritis for 60 years, and still performs admirably, Dr. Pendleton said. Although most initial doses range from 50 to 300 mg/day, "some recent studies suggest that only 25% of patients will reach the uric acid target on that regimen. For many patients, we need to increase the dose to get that level down."
The dose should be incrementally increased every 3-4 weeks to reach the uric acid target level; doses of up to 800 mg/day are approved for this indication. "But if you’re not able to achieve this desired level by pushing the dose close to 800 mg, I would consider trying febuxostat."
Febuxostat, a xanthine oxidase inhibitor, is more selective and potent than allopurinol. "It’s metabolized in the liver and very little of the active drug is excreted renally, raising the possibility that it might be safer in patients with mild to moderate renal insufficiency," Dr. Pendleton said.
It’s not easy to fully compare the two, because all three of the studies on the basis of which febuxostat was approved used a fixed-dose allopurinol regimen. "None of them allowed the total upward titration of allopurinol for a fair comparison," Dr. Pendleton pointed out.
The studies concluded that 40 mg of febuxostat was as effective as 300 mg of allopurinol. "The higher dose [of febuxostat 80 mg] seemed to be more effective than 400 mg allopurinol, but again, the studies did not allow for an upward titration" of the comparator, he said.
Although none of the patients in those trials had a creatinine level of more than 2.5 mg/dL, "a short-term study suggests that febuxostat dosing would not need to be adjusted even with a very low creatinine clearance [of 10-29 mL/min]. But just the same I would be very careful in that setting," Dr. Pendleton said (Am. J. Ther. 2005;12:22-34).
Febuxostat also appears to be safe for patients with mild hepatic dysfunction, with an adverse event profile similar to that of allopurinol. However, febuxostat is contraindicated in patients who are taking azathioprine, mercaptopurine, and theophylline.
Probenecid could be another option for some patients, especially those who underexcrete uric acid. Although probenecid has a somewhat better adverse event profile than allopurinol, it requires a 24-hour urine collection to rule out overproduction of uric acid and it seems to increase the risk of kidney stones. The drug is not as effective in patients with a low creatinine clearance (less than 60 mL/min), and "seems to have no effect at all at a creatinine level of 30 mL/min or lower," Dr. Pendleton said. Probenecid also must be taken three times a day – another drawback, in his opinion.
Dr. Pendleton said that he had no relevant financial disclosures.
SAN DIEGO – Allopurinol should remain the first-line agent for gout prophylaxis, despite some competition from a newer drug, febuxostat, according to Dr. John Pendleton.
Allopurinol is generally very effective, can be safely titrated for those with renal impairment, and is the most cost-effective option, he said at the annual meeting of the American College of Physicians.
"I still recommend allopurinol as the initial treatment, because you don’t need a 24-hour urine collection to give it; it’s effective in both overproducers and underexcretors [of uric acid]; it can be taken just once a day; and it’s safe and effective for those with mild renal insufficiency when the dose is adjusted," said Dr. Pendleton, an internist in Roanoke, Va.
Price is also an important factor, he noted. "Generic allopurinol costs about $15 per month. The brand name costs about $43 per month. But the price for febuxostat comes in at about $156 per month."
With either drug, the treatment goal should be to lower uric acid levels to be 6 mg/dL, said Dr. Pendleton, referring to a retrospective study of 276 patients with recurrent gout attacks. "This study noted that among the 81 patients with a uric acid of less than 6 mg/dL, 88% had no recurrent attacks during the 3-year observational period" (Arthritis Rheum. 2004;51:321-5).
Allopurinol has been the "standby drug" for gouty arthritis for 60 years, and still performs admirably, Dr. Pendleton said. Although most initial doses range from 50 to 300 mg/day, "some recent studies suggest that only 25% of patients will reach the uric acid target on that regimen. For many patients, we need to increase the dose to get that level down."
The dose should be incrementally increased every 3-4 weeks to reach the uric acid target level; doses of up to 800 mg/day are approved for this indication. "But if you’re not able to achieve this desired level by pushing the dose close to 800 mg, I would consider trying febuxostat."
Febuxostat, a xanthine oxidase inhibitor, is more selective and potent than allopurinol. "It’s metabolized in the liver and very little of the active drug is excreted renally, raising the possibility that it might be safer in patients with mild to moderate renal insufficiency," Dr. Pendleton said.
It’s not easy to fully compare the two, because all three of the studies on the basis of which febuxostat was approved used a fixed-dose allopurinol regimen. "None of them allowed the total upward titration of allopurinol for a fair comparison," Dr. Pendleton pointed out.
The studies concluded that 40 mg of febuxostat was as effective as 300 mg of allopurinol. "The higher dose [of febuxostat 80 mg] seemed to be more effective than 400 mg allopurinol, but again, the studies did not allow for an upward titration" of the comparator, he said.
Although none of the patients in those trials had a creatinine level of more than 2.5 mg/dL, "a short-term study suggests that febuxostat dosing would not need to be adjusted even with a very low creatinine clearance [of 10-29 mL/min]. But just the same I would be very careful in that setting," Dr. Pendleton said (Am. J. Ther. 2005;12:22-34).
Febuxostat also appears to be safe for patients with mild hepatic dysfunction, with an adverse event profile similar to that of allopurinol. However, febuxostat is contraindicated in patients who are taking azathioprine, mercaptopurine, and theophylline.
Probenecid could be another option for some patients, especially those who underexcrete uric acid. Although probenecid has a somewhat better adverse event profile than allopurinol, it requires a 24-hour urine collection to rule out overproduction of uric acid and it seems to increase the risk of kidney stones. The drug is not as effective in patients with a low creatinine clearance (less than 60 mL/min), and "seems to have no effect at all at a creatinine level of 30 mL/min or lower," Dr. Pendleton said. Probenecid also must be taken three times a day – another drawback, in his opinion.
Dr. Pendleton said that he had no relevant financial disclosures.
EXPERT ANALYSIS FROM THE ANNUAL MEETING OF THE AMERICAN COLLEGE OF PHYSICIANS
Malaria Prophylaxis: Destination Designates Drug
SAN DIEGO – A very small insect causes more than 1 million deaths each year, and in some countries, ranks second only to HIV/AIDS in associated mortality rates.
But malaria doesn’t just affect people who live in endemic regions, Dr. Loren Miller said at the annual meeting of the American College of Physicians. Americans who travel to those countries for business or pleasure are also at risk, and should discuss malaria prophylaxis with their health care providers.
Prophylaxis is generally very safe and extremely effective, but choosing the right agent is key, said Dr. Miller, director of the infection control program at Harbor-UCLA Medical Center, Torrance, Calif. Two of the most common agents – chloroquine and mefloquine – are no longer effective in many parts of Mexico, Central and South America, the Middle East, sub-Saharan Africa, India, and Southeast Asia.
In chloroquine-susceptible regions, the drug is a good choice, but still has limitations. "It has to be started 1-2 weeks before travel, so it’s not useful in people who come to the travel clinic and tell you they’re leaving in a few days," Dr. Miller said. The drug also has to be continued for a month after travel. It’s generally well tolerated, although it can exacerbate psoriasis.
Mefloquine can also be an option in countries of susceptible malaria strains. It too, must be started well in advance of travel [1-3 weeks] and continued for 4 weeks afterward. Adverse effects include the rare possibility of psychiatric symptoms, and exacerbation of seizure disorders and cardiac conduction abnormalities. "The lay press really has it in for this drug," he added. "There have been anecdotal reports of patients having hallucinations after getting it, and patients may come to you having read about this in a travel magazine. But in my experience, these are very rare."
The combination of atovaquone/proguanil is another option. "This can be started just 1 day before travel and it has to be continued for 7 days afterward. It’s very well tolerated, although contraindicated in those with a creatinine clearance of less than 30 mL/minute."
The drug is very expensive, running about $56 per week and, like all antimalarials, isn’t usually covered by insurance.
"The poor man’s alternative is doxycycline," Dr. Miller said. This must be started 1-2 weeks early and continued 2-4 weeks after travel. "This drug can cause photosensitivity, which you need to talk about because these people are going to tropical areas – and it can cause vaginal candidiasis."
Many experts consider primaquine to be a second-line agent. It’s most effective against Plasmodium vivax. A glucose-6-phosphate dehydrogenase deficiency test is necessary for blacks, Asians, and patients of Mediterranean descent, because the drug can cause acute hemolysis in deficient patients. "Since this test takes a while to come back, primaquine is not recommended for patients who want to travel soon," Dr. Miller said.
Immigrants who want to return to their native countries might claim they don’t need malarial prophylaxis because of exposure during childhood. Two studies have come to different conclusions about this idea, Dr. Miller said.
A 2005 study compared 99 Europeans traveling to malaria-endemic regions with 252 Africans returning home to such areas. The immigrants had lived in Europe for an average of 4 or more years. "Of those who contracted malaria, the Africans had lower mean parasite densities, less severe disease, and accelerated parasite clearance," Dr. Miller said (Am. J. Trop. Med. Hyg. 2005;72:21-25).
The second study, conducted in 2004, found no significant clinical differences between 93 immigrants returning to malaria-endemic countries and 167 to nonendemic countries. Of those who contracted the disease, "There was a trend toward less ICU admission in the previously exposed group, but it wasn’t significant (4% vs. 11%). So clearly, these people can get malaria, they can go to the ICU for it, and if they are in the ICU, there is a chance of dying from it. If someone is returning to an endemic area, I would absolutely recommend prophylaxis" (QJM 2004;10:645-9).
Dr. Miller divides his vaccination protocol into three parts, according to destination. "If they’re going to Central America, the Caribbean or the mid-East, they get chloroquine. If they’re going to the area of the Thai-Burma border, they get atovaquone/proguanil or doxycycline. If they’re going anywhere else, they get mefloquine or, if they can’t take that, either doxycycline or atovaquone/proguanil."
The Centers for Disease Control and Prevention’s Yellow Book notes which antimalarials are effective in any given country. For that resource, go to www.cdc.gov/travel.
Dr. Miller reported having financial relationships with Pfizer, Cubist Pharmaceuticals, GlaxoSmithKline, and Merck. He has been a consultant with Theravance.
SAN DIEGO – A very small insect causes more than 1 million deaths each year, and in some countries, ranks second only to HIV/AIDS in associated mortality rates.
But malaria doesn’t just affect people who live in endemic regions, Dr. Loren Miller said at the annual meeting of the American College of Physicians. Americans who travel to those countries for business or pleasure are also at risk, and should discuss malaria prophylaxis with their health care providers.
Prophylaxis is generally very safe and extremely effective, but choosing the right agent is key, said Dr. Miller, director of the infection control program at Harbor-UCLA Medical Center, Torrance, Calif. Two of the most common agents – chloroquine and mefloquine – are no longer effective in many parts of Mexico, Central and South America, the Middle East, sub-Saharan Africa, India, and Southeast Asia.
In chloroquine-susceptible regions, the drug is a good choice, but still has limitations. "It has to be started 1-2 weeks before travel, so it’s not useful in people who come to the travel clinic and tell you they’re leaving in a few days," Dr. Miller said. The drug also has to be continued for a month after travel. It’s generally well tolerated, although it can exacerbate psoriasis.
Mefloquine can also be an option in countries of susceptible malaria strains. It too, must be started well in advance of travel [1-3 weeks] and continued for 4 weeks afterward. Adverse effects include the rare possibility of psychiatric symptoms, and exacerbation of seizure disorders and cardiac conduction abnormalities. "The lay press really has it in for this drug," he added. "There have been anecdotal reports of patients having hallucinations after getting it, and patients may come to you having read about this in a travel magazine. But in my experience, these are very rare."
The combination of atovaquone/proguanil is another option. "This can be started just 1 day before travel and it has to be continued for 7 days afterward. It’s very well tolerated, although contraindicated in those with a creatinine clearance of less than 30 mL/minute."
The drug is very expensive, running about $56 per week and, like all antimalarials, isn’t usually covered by insurance.
"The poor man’s alternative is doxycycline," Dr. Miller said. This must be started 1-2 weeks early and continued 2-4 weeks after travel. "This drug can cause photosensitivity, which you need to talk about because these people are going to tropical areas – and it can cause vaginal candidiasis."
Many experts consider primaquine to be a second-line agent. It’s most effective against Plasmodium vivax. A glucose-6-phosphate dehydrogenase deficiency test is necessary for blacks, Asians, and patients of Mediterranean descent, because the drug can cause acute hemolysis in deficient patients. "Since this test takes a while to come back, primaquine is not recommended for patients who want to travel soon," Dr. Miller said.
Immigrants who want to return to their native countries might claim they don’t need malarial prophylaxis because of exposure during childhood. Two studies have come to different conclusions about this idea, Dr. Miller said.
A 2005 study compared 99 Europeans traveling to malaria-endemic regions with 252 Africans returning home to such areas. The immigrants had lived in Europe for an average of 4 or more years. "Of those who contracted malaria, the Africans had lower mean parasite densities, less severe disease, and accelerated parasite clearance," Dr. Miller said (Am. J. Trop. Med. Hyg. 2005;72:21-25).
The second study, conducted in 2004, found no significant clinical differences between 93 immigrants returning to malaria-endemic countries and 167 to nonendemic countries. Of those who contracted the disease, "There was a trend toward less ICU admission in the previously exposed group, but it wasn’t significant (4% vs. 11%). So clearly, these people can get malaria, they can go to the ICU for it, and if they are in the ICU, there is a chance of dying from it. If someone is returning to an endemic area, I would absolutely recommend prophylaxis" (QJM 2004;10:645-9).
Dr. Miller divides his vaccination protocol into three parts, according to destination. "If they’re going to Central America, the Caribbean or the mid-East, they get chloroquine. If they’re going to the area of the Thai-Burma border, they get atovaquone/proguanil or doxycycline. If they’re going anywhere else, they get mefloquine or, if they can’t take that, either doxycycline or atovaquone/proguanil."
The Centers for Disease Control and Prevention’s Yellow Book notes which antimalarials are effective in any given country. For that resource, go to www.cdc.gov/travel.
Dr. Miller reported having financial relationships with Pfizer, Cubist Pharmaceuticals, GlaxoSmithKline, and Merck. He has been a consultant with Theravance.
SAN DIEGO – A very small insect causes more than 1 million deaths each year, and in some countries, ranks second only to HIV/AIDS in associated mortality rates.
But malaria doesn’t just affect people who live in endemic regions, Dr. Loren Miller said at the annual meeting of the American College of Physicians. Americans who travel to those countries for business or pleasure are also at risk, and should discuss malaria prophylaxis with their health care providers.
Prophylaxis is generally very safe and extremely effective, but choosing the right agent is key, said Dr. Miller, director of the infection control program at Harbor-UCLA Medical Center, Torrance, Calif. Two of the most common agents – chloroquine and mefloquine – are no longer effective in many parts of Mexico, Central and South America, the Middle East, sub-Saharan Africa, India, and Southeast Asia.
In chloroquine-susceptible regions, the drug is a good choice, but still has limitations. "It has to be started 1-2 weeks before travel, so it’s not useful in people who come to the travel clinic and tell you they’re leaving in a few days," Dr. Miller said. The drug also has to be continued for a month after travel. It’s generally well tolerated, although it can exacerbate psoriasis.
Mefloquine can also be an option in countries of susceptible malaria strains. It too, must be started well in advance of travel [1-3 weeks] and continued for 4 weeks afterward. Adverse effects include the rare possibility of psychiatric symptoms, and exacerbation of seizure disorders and cardiac conduction abnormalities. "The lay press really has it in for this drug," he added. "There have been anecdotal reports of patients having hallucinations after getting it, and patients may come to you having read about this in a travel magazine. But in my experience, these are very rare."
The combination of atovaquone/proguanil is another option. "This can be started just 1 day before travel and it has to be continued for 7 days afterward. It’s very well tolerated, although contraindicated in those with a creatinine clearance of less than 30 mL/minute."
The drug is very expensive, running about $56 per week and, like all antimalarials, isn’t usually covered by insurance.
"The poor man’s alternative is doxycycline," Dr. Miller said. This must be started 1-2 weeks early and continued 2-4 weeks after travel. "This drug can cause photosensitivity, which you need to talk about because these people are going to tropical areas – and it can cause vaginal candidiasis."
Many experts consider primaquine to be a second-line agent. It’s most effective against Plasmodium vivax. A glucose-6-phosphate dehydrogenase deficiency test is necessary for blacks, Asians, and patients of Mediterranean descent, because the drug can cause acute hemolysis in deficient patients. "Since this test takes a while to come back, primaquine is not recommended for patients who want to travel soon," Dr. Miller said.
Immigrants who want to return to their native countries might claim they don’t need malarial prophylaxis because of exposure during childhood. Two studies have come to different conclusions about this idea, Dr. Miller said.
A 2005 study compared 99 Europeans traveling to malaria-endemic regions with 252 Africans returning home to such areas. The immigrants had lived in Europe for an average of 4 or more years. "Of those who contracted malaria, the Africans had lower mean parasite densities, less severe disease, and accelerated parasite clearance," Dr. Miller said (Am. J. Trop. Med. Hyg. 2005;72:21-25).
The second study, conducted in 2004, found no significant clinical differences between 93 immigrants returning to malaria-endemic countries and 167 to nonendemic countries. Of those who contracted the disease, "There was a trend toward less ICU admission in the previously exposed group, but it wasn’t significant (4% vs. 11%). So clearly, these people can get malaria, they can go to the ICU for it, and if they are in the ICU, there is a chance of dying from it. If someone is returning to an endemic area, I would absolutely recommend prophylaxis" (QJM 2004;10:645-9).
Dr. Miller divides his vaccination protocol into three parts, according to destination. "If they’re going to Central America, the Caribbean or the mid-East, they get chloroquine. If they’re going to the area of the Thai-Burma border, they get atovaquone/proguanil or doxycycline. If they’re going anywhere else, they get mefloquine or, if they can’t take that, either doxycycline or atovaquone/proguanil."
The Centers for Disease Control and Prevention’s Yellow Book notes which antimalarials are effective in any given country. For that resource, go to www.cdc.gov/travel.
Dr. Miller reported having financial relationships with Pfizer, Cubist Pharmaceuticals, GlaxoSmithKline, and Merck. He has been a consultant with Theravance.
FROM THE ANNUAL MEETING OF THE AMERICAN COLLEGE OF PHYSICIANS
Malaria Prophylaxis: Destination Designates Drug
SAN DIEGO – A very small insect causes more than 1 million deaths each year, and in some countries, ranks second only to HIV/AIDS in associated mortality rates.
But malaria doesn’t just affect people who live in endemic regions, Dr. Loren Miller said at the annual meeting of the American College of Physicians. Americans who travel to those countries for business or pleasure are also at risk, and should discuss malaria prophylaxis with their health care providers.
Prophylaxis is generally very safe and extremely effective, but choosing the right agent is key, said Dr. Miller, director of the infection control program at Harbor-UCLA Medical Center, Torrance, Calif. Two of the most common agents – chloroquine and mefloquine – are no longer effective in many parts of Mexico, Central and South America, the Middle East, sub-Saharan Africa, India, and Southeast Asia.
In chloroquine-susceptible regions, the drug is a good choice, but still has limitations. "It has to be started 1-2 weeks before travel, so it’s not useful in people who come to the travel clinic and tell you they’re leaving in a few days," Dr. Miller said. The drug also has to be continued for a month after travel. It’s generally well tolerated, although it can exacerbate psoriasis.
Mefloquine can also be an option in countries of susceptible malaria strains. It too, must be started well in advance of travel [1-3 weeks] and continued for 4 weeks afterward. Adverse effects include the rare possibility of psychiatric symptoms, and exacerbation of seizure disorders and cardiac conduction abnormalities. "The lay press really has it in for this drug," he added. "There have been anecdotal reports of patients having hallucinations after getting it, and patients may come to you having read about this in a travel magazine. But in my experience, these are very rare."
The combination of atovaquone/proguanil is another option. "This can be started just 1 day before travel and it has to be continued for 7 days afterward. It’s very well tolerated, although contraindicated in those with a creatinine clearance of less than 30 mL/minute."
The drug is very expensive, running about $56 per week and, like all antimalarials, isn’t usually covered by insurance.
"The poor man’s alternative is doxycycline," Dr. Miller said. This must be started 1-2 weeks early and continued 2-4 weeks after travel. "This drug can cause photosensitivity, which you need to talk about because these people are going to tropical areas – and it can cause vaginal candidiasis."
Many experts consider primaquine to be a second-line agent. It’s most effective against Plasmodium vivax. A glucose-6-phosphate dehydrogenase deficiency test is necessary for blacks, Asians, and patients of Mediterranean descent, because the drug can cause acute hemolysis in deficient patients. "Since this test takes a while to come back, primaquine is not recommended for patients who want to travel soon," Dr. Miller said.
Immigrants who want to return to their native countries might claim they don’t need malarial prophylaxis because of exposure during childhood. Two studies have come to different conclusions about this idea, Dr. Miller said.
A 2005 study compared 99 Europeans traveling to malaria-endemic regions with 252 Africans returning home to such areas. The immigrants had lived in Europe for an average of 4 or more years. "Of those who contracted malaria, the Africans had lower mean parasite densities, less severe disease, and accelerated parasite clearance," Dr. Miller said (Am. J. Trop. Med. Hyg. 2005;72:21-25).
The second study, conducted in 2004, found no significant clinical differences between 93 immigrants returning to malaria-endemic countries and 167 to nonendemic countries. Of those who contracted the disease, "There was a trend toward less ICU admission in the previously exposed group, but it wasn’t significant (4% vs. 11%). So clearly, these people can get malaria, they can go to the ICU for it, and if they are in the ICU, there is a chance of dying from it. If someone is returning to an endemic area, I would absolutely recommend prophylaxis" (QJM 2004;10:645-9).
Dr. Miller divides his vaccination protocol into three parts, according to destination. "If they’re going to Central America, the Caribbean or the mid-East, they get chloroquine. If they’re going to the area of the Thai-Burma border, they get atovaquone/proguanil or doxycycline. If they’re going anywhere else, they get mefloquine or, if they can’t take that, either doxycycline or atovaquone/proguanil."
The Centers for Disease Control and Prevention’s Yellow Book notes which antimalarials are effective in any given country. For that resource, go to www.cdc.gov/travel.
Dr. Miller reported having financial relationships with Pfizer, Cubist Pharmaceuticals, GlaxoSmithKline, and Merck. He has been a consultant with Theravance.
SAN DIEGO – A very small insect causes more than 1 million deaths each year, and in some countries, ranks second only to HIV/AIDS in associated mortality rates.
But malaria doesn’t just affect people who live in endemic regions, Dr. Loren Miller said at the annual meeting of the American College of Physicians. Americans who travel to those countries for business or pleasure are also at risk, and should discuss malaria prophylaxis with their health care providers.
Prophylaxis is generally very safe and extremely effective, but choosing the right agent is key, said Dr. Miller, director of the infection control program at Harbor-UCLA Medical Center, Torrance, Calif. Two of the most common agents – chloroquine and mefloquine – are no longer effective in many parts of Mexico, Central and South America, the Middle East, sub-Saharan Africa, India, and Southeast Asia.
In chloroquine-susceptible regions, the drug is a good choice, but still has limitations. "It has to be started 1-2 weeks before travel, so it’s not useful in people who come to the travel clinic and tell you they’re leaving in a few days," Dr. Miller said. The drug also has to be continued for a month after travel. It’s generally well tolerated, although it can exacerbate psoriasis.
Mefloquine can also be an option in countries of susceptible malaria strains. It too, must be started well in advance of travel [1-3 weeks] and continued for 4 weeks afterward. Adverse effects include the rare possibility of psychiatric symptoms, and exacerbation of seizure disorders and cardiac conduction abnormalities. "The lay press really has it in for this drug," he added. "There have been anecdotal reports of patients having hallucinations after getting it, and patients may come to you having read about this in a travel magazine. But in my experience, these are very rare."
The combination of atovaquone/proguanil is another option. "This can be started just 1 day before travel and it has to be continued for 7 days afterward. It’s very well tolerated, although contraindicated in those with a creatinine clearance of less than 30 mL/minute."
The drug is very expensive, running about $56 per week and, like all antimalarials, isn’t usually covered by insurance.
"The poor man’s alternative is doxycycline," Dr. Miller said. This must be started 1-2 weeks early and continued 2-4 weeks after travel. "This drug can cause photosensitivity, which you need to talk about because these people are going to tropical areas – and it can cause vaginal candidiasis."
Many experts consider primaquine to be a second-line agent. It’s most effective against Plasmodium vivax. A glucose-6-phosphate dehydrogenase deficiency test is necessary for blacks, Asians, and patients of Mediterranean descent, because the drug can cause acute hemolysis in deficient patients. "Since this test takes a while to come back, primaquine is not recommended for patients who want to travel soon," Dr. Miller said.
Immigrants who want to return to their native countries might claim they don’t need malarial prophylaxis because of exposure during childhood. Two studies have come to different conclusions about this idea, Dr. Miller said.
A 2005 study compared 99 Europeans traveling to malaria-endemic regions with 252 Africans returning home to such areas. The immigrants had lived in Europe for an average of 4 or more years. "Of those who contracted malaria, the Africans had lower mean parasite densities, less severe disease, and accelerated parasite clearance," Dr. Miller said (Am. J. Trop. Med. Hyg. 2005;72:21-25).
The second study, conducted in 2004, found no significant clinical differences between 93 immigrants returning to malaria-endemic countries and 167 to nonendemic countries. Of those who contracted the disease, "There was a trend toward less ICU admission in the previously exposed group, but it wasn’t significant (4% vs. 11%). So clearly, these people can get malaria, they can go to the ICU for it, and if they are in the ICU, there is a chance of dying from it. If someone is returning to an endemic area, I would absolutely recommend prophylaxis" (QJM 2004;10:645-9).
Dr. Miller divides his vaccination protocol into three parts, according to destination. "If they’re going to Central America, the Caribbean or the mid-East, they get chloroquine. If they’re going to the area of the Thai-Burma border, they get atovaquone/proguanil or doxycycline. If they’re going anywhere else, they get mefloquine or, if they can’t take that, either doxycycline or atovaquone/proguanil."
The Centers for Disease Control and Prevention’s Yellow Book notes which antimalarials are effective in any given country. For that resource, go to www.cdc.gov/travel.
Dr. Miller reported having financial relationships with Pfizer, Cubist Pharmaceuticals, GlaxoSmithKline, and Merck. He has been a consultant with Theravance.
SAN DIEGO – A very small insect causes more than 1 million deaths each year, and in some countries, ranks second only to HIV/AIDS in associated mortality rates.
But malaria doesn’t just affect people who live in endemic regions, Dr. Loren Miller said at the annual meeting of the American College of Physicians. Americans who travel to those countries for business or pleasure are also at risk, and should discuss malaria prophylaxis with their health care providers.
Prophylaxis is generally very safe and extremely effective, but choosing the right agent is key, said Dr. Miller, director of the infection control program at Harbor-UCLA Medical Center, Torrance, Calif. Two of the most common agents – chloroquine and mefloquine – are no longer effective in many parts of Mexico, Central and South America, the Middle East, sub-Saharan Africa, India, and Southeast Asia.
In chloroquine-susceptible regions, the drug is a good choice, but still has limitations. "It has to be started 1-2 weeks before travel, so it’s not useful in people who come to the travel clinic and tell you they’re leaving in a few days," Dr. Miller said. The drug also has to be continued for a month after travel. It’s generally well tolerated, although it can exacerbate psoriasis.
Mefloquine can also be an option in countries of susceptible malaria strains. It too, must be started well in advance of travel [1-3 weeks] and continued for 4 weeks afterward. Adverse effects include the rare possibility of psychiatric symptoms, and exacerbation of seizure disorders and cardiac conduction abnormalities. "The lay press really has it in for this drug," he added. "There have been anecdotal reports of patients having hallucinations after getting it, and patients may come to you having read about this in a travel magazine. But in my experience, these are very rare."
The combination of atovaquone/proguanil is another option. "This can be started just 1 day before travel and it has to be continued for 7 days afterward. It’s very well tolerated, although contraindicated in those with a creatinine clearance of less than 30 mL/minute."
The drug is very expensive, running about $56 per week and, like all antimalarials, isn’t usually covered by insurance.
"The poor man’s alternative is doxycycline," Dr. Miller said. This must be started 1-2 weeks early and continued 2-4 weeks after travel. "This drug can cause photosensitivity, which you need to talk about because these people are going to tropical areas – and it can cause vaginal candidiasis."
Many experts consider primaquine to be a second-line agent. It’s most effective against Plasmodium vivax. A glucose-6-phosphate dehydrogenase deficiency test is necessary for blacks, Asians, and patients of Mediterranean descent, because the drug can cause acute hemolysis in deficient patients. "Since this test takes a while to come back, primaquine is not recommended for patients who want to travel soon," Dr. Miller said.
Immigrants who want to return to their native countries might claim they don’t need malarial prophylaxis because of exposure during childhood. Two studies have come to different conclusions about this idea, Dr. Miller said.
A 2005 study compared 99 Europeans traveling to malaria-endemic regions with 252 Africans returning home to such areas. The immigrants had lived in Europe for an average of 4 or more years. "Of those who contracted malaria, the Africans had lower mean parasite densities, less severe disease, and accelerated parasite clearance," Dr. Miller said (Am. J. Trop. Med. Hyg. 2005;72:21-25).
The second study, conducted in 2004, found no significant clinical differences between 93 immigrants returning to malaria-endemic countries and 167 to nonendemic countries. Of those who contracted the disease, "There was a trend toward less ICU admission in the previously exposed group, but it wasn’t significant (4% vs. 11%). So clearly, these people can get malaria, they can go to the ICU for it, and if they are in the ICU, there is a chance of dying from it. If someone is returning to an endemic area, I would absolutely recommend prophylaxis" (QJM 2004;10:645-9).
Dr. Miller divides his vaccination protocol into three parts, according to destination. "If they’re going to Central America, the Caribbean or the mid-East, they get chloroquine. If they’re going to the area of the Thai-Burma border, they get atovaquone/proguanil or doxycycline. If they’re going anywhere else, they get mefloquine or, if they can’t take that, either doxycycline or atovaquone/proguanil."
The Centers for Disease Control and Prevention’s Yellow Book notes which antimalarials are effective in any given country. For that resource, go to www.cdc.gov/travel.
Dr. Miller reported having financial relationships with Pfizer, Cubist Pharmaceuticals, GlaxoSmithKline, and Merck. He has been a consultant with Theravance.
FROM THE ANNUAL MEETING OF THE AMERICAN COLLEGE OF PHYSICIANS
Malaria Prophylaxis: Destination Designates Drug
SAN DIEGO – A very small insect causes more than 1 million deaths each year, and in some countries, ranks second only to HIV/AIDS in associated mortality rates.
But malaria doesn’t just affect people who live in endemic regions, Dr. Loren Miller said at the annual meeting of the American College of Physicians. Americans who travel to those countries for business or pleasure are also at risk, and should discuss malaria prophylaxis with their health care providers.
Prophylaxis is generally very safe and extremely effective, but choosing the right agent is key, said Dr. Miller, director of the infection control program at Harbor-UCLA Medical Center, Torrance, Calif. Two of the most common agents – chloroquine and mefloquine – are no longer effective in many parts of Mexico, Central and South America, the Middle East, sub-Saharan Africa, India, and Southeast Asia.
In chloroquine-susceptible regions, the drug is a good choice, but still has limitations. "It has to be started 1-2 weeks before travel, so it’s not useful in people who come to the travel clinic and tell you they’re leaving in a few days," Dr. Miller said. The drug also has to be continued for a month after travel. It’s generally well tolerated, although it can exacerbate psoriasis.
Mefloquine can also be an option in countries of susceptible malaria strains. It too, must be started well in advance of travel [1-3 weeks] and continued for 4 weeks afterward. Adverse effects include the rare possibility of psychiatric symptoms, and exacerbation of seizure disorders and cardiac conduction abnormalities. "The lay press really has it in for this drug," he added. "There have been anecdotal reports of patients having hallucinations after getting it, and patients may come to you having read about this in a travel magazine. But in my experience, these are very rare."
The combination of atovaquone/proguanil is another option. "This can be started just 1 day before travel and it has to be continued for 7 days afterward. It’s very well tolerated, although contraindicated in those with a creatinine clearance of less than 30 mL/minute."
The drug is very expensive, running about $56 per week and, like all antimalarials, isn’t usually covered by insurance.
"The poor man’s alternative is doxycycline," Dr. Miller said. This must be started 1-2 weeks early and continued 2-4 weeks after travel. "This drug can cause photosensitivity, which you need to talk about because these people are going to tropical areas – and it can cause vaginal candidiasis."
Many experts consider primaquine to be a second-line agent. It’s most effective against Plasmodium vivax. A glucose-6-phosphate dehydrogenase deficiency test is necessary for blacks, Asians, and patients of Mediterranean descent, because the drug can cause acute hemolysis in deficient patients. "Since this test takes a while to come back, primaquine is not recommended for patients who want to travel soon," Dr. Miller said.
Immigrants who want to return to their native countries might claim they don’t need malarial prophylaxis because of exposure during childhood. Two studies have come to different conclusions about this idea, Dr. Miller said.
A 2005 study compared 99 Europeans traveling to malaria-endemic regions with 252 Africans returning home to such areas. The immigrants had lived in Europe for an average of 4 or more years. "Of those who contracted malaria, the Africans had lower mean parasite densities, less severe disease, and accelerated parasite clearance," Dr. Miller said (Am. J. Trop. Med. Hyg. 2005;72:21-25).
The second study, conducted in 2004, found no significant clinical differences between 93 immigrants returning to malaria-endemic countries and 167 to nonendemic countries. Of those who contracted the disease, "There was a trend toward less ICU admission in the previously exposed group, but it wasn’t significant (4% vs. 11%). So clearly, these people can get malaria, they can go to the ICU for it, and if they are in the ICU, there is a chance of dying from it. If someone is returning to an endemic area, I would absolutely recommend prophylaxis" (QJM 2004;10:645-9).
Dr. Miller divides his vaccination protocol into three parts, according to destination. "If they’re going to Central America, the Caribbean or the mid-East, they get chloroquine. If they’re going to the area of the Thai-Burma border, they get atovaquone/proguanil or doxycycline. If they’re going anywhere else, they get mefloquine or, if they can’t take that, either doxycycline or atovaquone/proguanil."
The Centers for Disease Control and Prevention’s Yellow Book notes which antimalarials are effective in any given country. For that resource, go to www.cdc.gov/travel.
Dr. Miller reported having financial relationships with Pfizer, Cubist Pharmaceuticals, GlaxoSmithKline, and Merck. He has been a consultant with Theravance.
SAN DIEGO – A very small insect causes more than 1 million deaths each year, and in some countries, ranks second only to HIV/AIDS in associated mortality rates.
But malaria doesn’t just affect people who live in endemic regions, Dr. Loren Miller said at the annual meeting of the American College of Physicians. Americans who travel to those countries for business or pleasure are also at risk, and should discuss malaria prophylaxis with their health care providers.
Prophylaxis is generally very safe and extremely effective, but choosing the right agent is key, said Dr. Miller, director of the infection control program at Harbor-UCLA Medical Center, Torrance, Calif. Two of the most common agents – chloroquine and mefloquine – are no longer effective in many parts of Mexico, Central and South America, the Middle East, sub-Saharan Africa, India, and Southeast Asia.
In chloroquine-susceptible regions, the drug is a good choice, but still has limitations. "It has to be started 1-2 weeks before travel, so it’s not useful in people who come to the travel clinic and tell you they’re leaving in a few days," Dr. Miller said. The drug also has to be continued for a month after travel. It’s generally well tolerated, although it can exacerbate psoriasis.
Mefloquine can also be an option in countries of susceptible malaria strains. It too, must be started well in advance of travel [1-3 weeks] and continued for 4 weeks afterward. Adverse effects include the rare possibility of psychiatric symptoms, and exacerbation of seizure disorders and cardiac conduction abnormalities. "The lay press really has it in for this drug," he added. "There have been anecdotal reports of patients having hallucinations after getting it, and patients may come to you having read about this in a travel magazine. But in my experience, these are very rare."
The combination of atovaquone/proguanil is another option. "This can be started just 1 day before travel and it has to be continued for 7 days afterward. It’s very well tolerated, although contraindicated in those with a creatinine clearance of less than 30 mL/minute."
The drug is very expensive, running about $56 per week and, like all antimalarials, isn’t usually covered by insurance.
"The poor man’s alternative is doxycycline," Dr. Miller said. This must be started 1-2 weeks early and continued 2-4 weeks after travel. "This drug can cause photosensitivity, which you need to talk about because these people are going to tropical areas – and it can cause vaginal candidiasis."
Many experts consider primaquine to be a second-line agent. It’s most effective against Plasmodium vivax. A glucose-6-phosphate dehydrogenase deficiency test is necessary for blacks, Asians, and patients of Mediterranean descent, because the drug can cause acute hemolysis in deficient patients. "Since this test takes a while to come back, primaquine is not recommended for patients who want to travel soon," Dr. Miller said.
Immigrants who want to return to their native countries might claim they don’t need malarial prophylaxis because of exposure during childhood. Two studies have come to different conclusions about this idea, Dr. Miller said.
A 2005 study compared 99 Europeans traveling to malaria-endemic regions with 252 Africans returning home to such areas. The immigrants had lived in Europe for an average of 4 or more years. "Of those who contracted malaria, the Africans had lower mean parasite densities, less severe disease, and accelerated parasite clearance," Dr. Miller said (Am. J. Trop. Med. Hyg. 2005;72:21-25).
The second study, conducted in 2004, found no significant clinical differences between 93 immigrants returning to malaria-endemic countries and 167 to nonendemic countries. Of those who contracted the disease, "There was a trend toward less ICU admission in the previously exposed group, but it wasn’t significant (4% vs. 11%). So clearly, these people can get malaria, they can go to the ICU for it, and if they are in the ICU, there is a chance of dying from it. If someone is returning to an endemic area, I would absolutely recommend prophylaxis" (QJM 2004;10:645-9).
Dr. Miller divides his vaccination protocol into three parts, according to destination. "If they’re going to Central America, the Caribbean or the mid-East, they get chloroquine. If they’re going to the area of the Thai-Burma border, they get atovaquone/proguanil or doxycycline. If they’re going anywhere else, they get mefloquine or, if they can’t take that, either doxycycline or atovaquone/proguanil."
The Centers for Disease Control and Prevention’s Yellow Book notes which antimalarials are effective in any given country. For that resource, go to www.cdc.gov/travel.
Dr. Miller reported having financial relationships with Pfizer, Cubist Pharmaceuticals, GlaxoSmithKline, and Merck. He has been a consultant with Theravance.
SAN DIEGO – A very small insect causes more than 1 million deaths each year, and in some countries, ranks second only to HIV/AIDS in associated mortality rates.
But malaria doesn’t just affect people who live in endemic regions, Dr. Loren Miller said at the annual meeting of the American College of Physicians. Americans who travel to those countries for business or pleasure are also at risk, and should discuss malaria prophylaxis with their health care providers.
Prophylaxis is generally very safe and extremely effective, but choosing the right agent is key, said Dr. Miller, director of the infection control program at Harbor-UCLA Medical Center, Torrance, Calif. Two of the most common agents – chloroquine and mefloquine – are no longer effective in many parts of Mexico, Central and South America, the Middle East, sub-Saharan Africa, India, and Southeast Asia.
In chloroquine-susceptible regions, the drug is a good choice, but still has limitations. "It has to be started 1-2 weeks before travel, so it’s not useful in people who come to the travel clinic and tell you they’re leaving in a few days," Dr. Miller said. The drug also has to be continued for a month after travel. It’s generally well tolerated, although it can exacerbate psoriasis.
Mefloquine can also be an option in countries of susceptible malaria strains. It too, must be started well in advance of travel [1-3 weeks] and continued for 4 weeks afterward. Adverse effects include the rare possibility of psychiatric symptoms, and exacerbation of seizure disorders and cardiac conduction abnormalities. "The lay press really has it in for this drug," he added. "There have been anecdotal reports of patients having hallucinations after getting it, and patients may come to you having read about this in a travel magazine. But in my experience, these are very rare."
The combination of atovaquone/proguanil is another option. "This can be started just 1 day before travel and it has to be continued for 7 days afterward. It’s very well tolerated, although contraindicated in those with a creatinine clearance of less than 30 mL/minute."
The drug is very expensive, running about $56 per week and, like all antimalarials, isn’t usually covered by insurance.
"The poor man’s alternative is doxycycline," Dr. Miller said. This must be started 1-2 weeks early and continued 2-4 weeks after travel. "This drug can cause photosensitivity, which you need to talk about because these people are going to tropical areas – and it can cause vaginal candidiasis."
Many experts consider primaquine to be a second-line agent. It’s most effective against Plasmodium vivax. A glucose-6-phosphate dehydrogenase deficiency test is necessary for blacks, Asians, and patients of Mediterranean descent, because the drug can cause acute hemolysis in deficient patients. "Since this test takes a while to come back, primaquine is not recommended for patients who want to travel soon," Dr. Miller said.
Immigrants who want to return to their native countries might claim they don’t need malarial prophylaxis because of exposure during childhood. Two studies have come to different conclusions about this idea, Dr. Miller said.
A 2005 study compared 99 Europeans traveling to malaria-endemic regions with 252 Africans returning home to such areas. The immigrants had lived in Europe for an average of 4 or more years. "Of those who contracted malaria, the Africans had lower mean parasite densities, less severe disease, and accelerated parasite clearance," Dr. Miller said (Am. J. Trop. Med. Hyg. 2005;72:21-25).
The second study, conducted in 2004, found no significant clinical differences between 93 immigrants returning to malaria-endemic countries and 167 to nonendemic countries. Of those who contracted the disease, "There was a trend toward less ICU admission in the previously exposed group, but it wasn’t significant (4% vs. 11%). So clearly, these people can get malaria, they can go to the ICU for it, and if they are in the ICU, there is a chance of dying from it. If someone is returning to an endemic area, I would absolutely recommend prophylaxis" (QJM 2004;10:645-9).
Dr. Miller divides his vaccination protocol into three parts, according to destination. "If they’re going to Central America, the Caribbean or the mid-East, they get chloroquine. If they’re going to the area of the Thai-Burma border, they get atovaquone/proguanil or doxycycline. If they’re going anywhere else, they get mefloquine or, if they can’t take that, either doxycycline or atovaquone/proguanil."
The Centers for Disease Control and Prevention’s Yellow Book notes which antimalarials are effective in any given country. For that resource, go to www.cdc.gov/travel.
Dr. Miller reported having financial relationships with Pfizer, Cubist Pharmaceuticals, GlaxoSmithKline, and Merck. He has been a consultant with Theravance.
FROM THE ANNUAL MEETING OF THE AMERICAN COLLEGE OF PHYSICIANS
Antidepressant Discontinuation Syndrome - Start Slow? Stop Fast?
SAN DIEGO – The risk of discontinuation syndrome is small when antidepressant treatment is suddenly stopped, but the symptoms – though somewhat brief – are still unpleasant for patients, according to Dr. Kurt Kroenke.
Unfortunately, he said, there are no firm data that predict who might develop discontinuation syndrome, which drugs are likely to cause it, or whether medication tapering can avoid it.
"If you just immediately stop an antidepressant, you can expect to have a 5%-15% incidence of discontinuation syndrome," Dr. Kroenke said at the annual meeting of the American College of Physicians. "It usually starts within a few days and stops within a few weeks," but it’s no picnic for patients.
FINISH is the acronym that describes this syndrome, said Dr. Kroenke, an internist at Indiana University, Bloomington. Patients experience flulike symptoms, insomnia, nausea, imbalance, strange sensory dysesthesias, and hyperarousal or anxiety.
The syndrome is probably less likely if a patient is switching to another drug rather than ceasing medication altogether, but no studies have determined the best way to implement either change. "It’s not clear if a long taper is better than a short taper, or even if discontinuation syndrome is something that’s dose related," he said. "If you’re on 200 mg of sertraline and you stop, as opposed to 50 mg, we can’t say the higher dose equals an increased risk."
Some studies seem to suggest that the risk is highest with paroxetine. "Fluoxetine probably has the lowest risk because of its long half-life. Venlafaxine is associated with an intermediate risk and all the others fall somewhere below that," Dr. Kroenke said.
The Sequenced Treatment Alternatives to Relieve Depression (STAR*D) trial provides the largest single body of evidence on antidepressant switching (Am. J. Psychiatry 2006;163:1905-17). The methodology in the trial, which involved four levels of treatment and seven antidepressants, was basically simple, Dr. Kroenke said. "You either discontinued one medication and immediately began another or you decreased the first medication while initiating the second at a low dose, doing this taper/titration over 1 week. It doesn’t get much simpler than that." Most investigators in the study chose the first option, he said.
"Having said that, I would probably go for some version of option two, and if the patient is on a higher dose, I might spend a week tapering one while titrating the other."
In a few specific situations, Dr. Kroenke said he strongly favors the taper/titrating method. "I always consider tapering if I’m working with paroxetine or venlafaxine, especially if it’s at a higher dose and has been taken for a long duration," he said. "And definitely if the patient has experienced discontinuation syndrome in the past."
Dr. Kroenke said he has consulted for, and received honoraria from, Eli Lilly and Forest.
SAN DIEGO – The risk of discontinuation syndrome is small when antidepressant treatment is suddenly stopped, but the symptoms – though somewhat brief – are still unpleasant for patients, according to Dr. Kurt Kroenke.
Unfortunately, he said, there are no firm data that predict who might develop discontinuation syndrome, which drugs are likely to cause it, or whether medication tapering can avoid it.
"If you just immediately stop an antidepressant, you can expect to have a 5%-15% incidence of discontinuation syndrome," Dr. Kroenke said at the annual meeting of the American College of Physicians. "It usually starts within a few days and stops within a few weeks," but it’s no picnic for patients.
FINISH is the acronym that describes this syndrome, said Dr. Kroenke, an internist at Indiana University, Bloomington. Patients experience flulike symptoms, insomnia, nausea, imbalance, strange sensory dysesthesias, and hyperarousal or anxiety.
The syndrome is probably less likely if a patient is switching to another drug rather than ceasing medication altogether, but no studies have determined the best way to implement either change. "It’s not clear if a long taper is better than a short taper, or even if discontinuation syndrome is something that’s dose related," he said. "If you’re on 200 mg of sertraline and you stop, as opposed to 50 mg, we can’t say the higher dose equals an increased risk."
Some studies seem to suggest that the risk is highest with paroxetine. "Fluoxetine probably has the lowest risk because of its long half-life. Venlafaxine is associated with an intermediate risk and all the others fall somewhere below that," Dr. Kroenke said.
The Sequenced Treatment Alternatives to Relieve Depression (STAR*D) trial provides the largest single body of evidence on antidepressant switching (Am. J. Psychiatry 2006;163:1905-17). The methodology in the trial, which involved four levels of treatment and seven antidepressants, was basically simple, Dr. Kroenke said. "You either discontinued one medication and immediately began another or you decreased the first medication while initiating the second at a low dose, doing this taper/titration over 1 week. It doesn’t get much simpler than that." Most investigators in the study chose the first option, he said.
"Having said that, I would probably go for some version of option two, and if the patient is on a higher dose, I might spend a week tapering one while titrating the other."
In a few specific situations, Dr. Kroenke said he strongly favors the taper/titrating method. "I always consider tapering if I’m working with paroxetine or venlafaxine, especially if it’s at a higher dose and has been taken for a long duration," he said. "And definitely if the patient has experienced discontinuation syndrome in the past."
Dr. Kroenke said he has consulted for, and received honoraria from, Eli Lilly and Forest.
SAN DIEGO – The risk of discontinuation syndrome is small when antidepressant treatment is suddenly stopped, but the symptoms – though somewhat brief – are still unpleasant for patients, according to Dr. Kurt Kroenke.
Unfortunately, he said, there are no firm data that predict who might develop discontinuation syndrome, which drugs are likely to cause it, or whether medication tapering can avoid it.
"If you just immediately stop an antidepressant, you can expect to have a 5%-15% incidence of discontinuation syndrome," Dr. Kroenke said at the annual meeting of the American College of Physicians. "It usually starts within a few days and stops within a few weeks," but it’s no picnic for patients.
FINISH is the acronym that describes this syndrome, said Dr. Kroenke, an internist at Indiana University, Bloomington. Patients experience flulike symptoms, insomnia, nausea, imbalance, strange sensory dysesthesias, and hyperarousal or anxiety.
The syndrome is probably less likely if a patient is switching to another drug rather than ceasing medication altogether, but no studies have determined the best way to implement either change. "It’s not clear if a long taper is better than a short taper, or even if discontinuation syndrome is something that’s dose related," he said. "If you’re on 200 mg of sertraline and you stop, as opposed to 50 mg, we can’t say the higher dose equals an increased risk."
Some studies seem to suggest that the risk is highest with paroxetine. "Fluoxetine probably has the lowest risk because of its long half-life. Venlafaxine is associated with an intermediate risk and all the others fall somewhere below that," Dr. Kroenke said.
The Sequenced Treatment Alternatives to Relieve Depression (STAR*D) trial provides the largest single body of evidence on antidepressant switching (Am. J. Psychiatry 2006;163:1905-17). The methodology in the trial, which involved four levels of treatment and seven antidepressants, was basically simple, Dr. Kroenke said. "You either discontinued one medication and immediately began another or you decreased the first medication while initiating the second at a low dose, doing this taper/titration over 1 week. It doesn’t get much simpler than that." Most investigators in the study chose the first option, he said.
"Having said that, I would probably go for some version of option two, and if the patient is on a higher dose, I might spend a week tapering one while titrating the other."
In a few specific situations, Dr. Kroenke said he strongly favors the taper/titrating method. "I always consider tapering if I’m working with paroxetine or venlafaxine, especially if it’s at a higher dose and has been taken for a long duration," he said. "And definitely if the patient has experienced discontinuation syndrome in the past."
Dr. Kroenke said he has consulted for, and received honoraria from, Eli Lilly and Forest.
EXPERT ANALYSIS FROM THE ANNUAL MEETING OF THE AMERICAN COLLEGE OF PHYSICIANS
Antidepressant Discontinuation Syndrome - Start Slow? Stop Fast?
SAN DIEGO – The risk of discontinuation syndrome is small when antidepressant treatment is suddenly stopped, but the symptoms – though somewhat brief – are still unpleasant for patients, according to Dr. Kurt Kroenke.
Unfortunately, he said, there are no firm data that predict who might develop discontinuation syndrome, which drugs are likely to cause it, or whether medication tapering can avoid it.
"If you just immediately stop an antidepressant, you can expect to have a 5%-15% incidence of discontinuation syndrome," Dr. Kroenke said at the annual meeting of the American College of Physicians. "It usually starts within a few days and stops within a few weeks," but it’s no picnic for patients.
FINISH is the acronym that describes this syndrome, said Dr. Kroenke, an internist at Indiana University, Bloomington. Patients experience flulike symptoms, insomnia, nausea, imbalance, strange sensory dysesthesias, and hyperarousal or anxiety.
The syndrome is probably less likely if a patient is switching to another drug rather than ceasing medication altogether, but no studies have determined the best way to implement either change. "It’s not clear if a long taper is better than a short taper, or even if discontinuation syndrome is something that’s dose related," he said. "If you’re on 200 mg of sertraline and you stop, as opposed to 50 mg, we can’t say the higher dose equals an increased risk."
Some studies seem to suggest that the risk is highest with paroxetine. "Fluoxetine probably has the lowest risk because of its long half-life. Venlafaxine is associated with an intermediate risk and all the others fall somewhere below that," Dr. Kroenke said.
The Sequenced Treatment Alternatives to Relieve Depression (STAR*D) trial provides the largest single body of evidence on antidepressant switching (Am. J. Psychiatry 2006;163:1905-17). The methodology in the trial, which involved four levels of treatment and seven antidepressants, was basically simple, Dr. Kroenke said. "You either discontinued one medication and immediately began another or you decreased the first medication while initiating the second at a low dose, doing this taper/titration over 1 week. It doesn’t get much simpler than that." Most investigators in the study chose the first option, he said.
"Having said that, I would probably go for some version of option two, and if the patient is on a higher dose, I might spend a week tapering one while titrating the other."
In a few specific situations, Dr. Kroenke said he strongly favors the taper/titrating method. "I always consider tapering if I’m working with paroxetine or venlafaxine, especially if it’s at a higher dose and has been taken for a long duration," he said. "And definitely if the patient has experienced discontinuation syndrome in the past."
Dr. Kroenke said he has consulted for, and received honoraria from, Eli Lilly and Forest.
SAN DIEGO – The risk of discontinuation syndrome is small when antidepressant treatment is suddenly stopped, but the symptoms – though somewhat brief – are still unpleasant for patients, according to Dr. Kurt Kroenke.
Unfortunately, he said, there are no firm data that predict who might develop discontinuation syndrome, which drugs are likely to cause it, or whether medication tapering can avoid it.
"If you just immediately stop an antidepressant, you can expect to have a 5%-15% incidence of discontinuation syndrome," Dr. Kroenke said at the annual meeting of the American College of Physicians. "It usually starts within a few days and stops within a few weeks," but it’s no picnic for patients.
FINISH is the acronym that describes this syndrome, said Dr. Kroenke, an internist at Indiana University, Bloomington. Patients experience flulike symptoms, insomnia, nausea, imbalance, strange sensory dysesthesias, and hyperarousal or anxiety.
The syndrome is probably less likely if a patient is switching to another drug rather than ceasing medication altogether, but no studies have determined the best way to implement either change. "It’s not clear if a long taper is better than a short taper, or even if discontinuation syndrome is something that’s dose related," he said. "If you’re on 200 mg of sertraline and you stop, as opposed to 50 mg, we can’t say the higher dose equals an increased risk."
Some studies seem to suggest that the risk is highest with paroxetine. "Fluoxetine probably has the lowest risk because of its long half-life. Venlafaxine is associated with an intermediate risk and all the others fall somewhere below that," Dr. Kroenke said.
The Sequenced Treatment Alternatives to Relieve Depression (STAR*D) trial provides the largest single body of evidence on antidepressant switching (Am. J. Psychiatry 2006;163:1905-17). The methodology in the trial, which involved four levels of treatment and seven antidepressants, was basically simple, Dr. Kroenke said. "You either discontinued one medication and immediately began another or you decreased the first medication while initiating the second at a low dose, doing this taper/titration over 1 week. It doesn’t get much simpler than that." Most investigators in the study chose the first option, he said.
"Having said that, I would probably go for some version of option two, and if the patient is on a higher dose, I might spend a week tapering one while titrating the other."
In a few specific situations, Dr. Kroenke said he strongly favors the taper/titrating method. "I always consider tapering if I’m working with paroxetine or venlafaxine, especially if it’s at a higher dose and has been taken for a long duration," he said. "And definitely if the patient has experienced discontinuation syndrome in the past."
Dr. Kroenke said he has consulted for, and received honoraria from, Eli Lilly and Forest.
SAN DIEGO – The risk of discontinuation syndrome is small when antidepressant treatment is suddenly stopped, but the symptoms – though somewhat brief – are still unpleasant for patients, according to Dr. Kurt Kroenke.
Unfortunately, he said, there are no firm data that predict who might develop discontinuation syndrome, which drugs are likely to cause it, or whether medication tapering can avoid it.
"If you just immediately stop an antidepressant, you can expect to have a 5%-15% incidence of discontinuation syndrome," Dr. Kroenke said at the annual meeting of the American College of Physicians. "It usually starts within a few days and stops within a few weeks," but it’s no picnic for patients.
FINISH is the acronym that describes this syndrome, said Dr. Kroenke, an internist at Indiana University, Bloomington. Patients experience flulike symptoms, insomnia, nausea, imbalance, strange sensory dysesthesias, and hyperarousal or anxiety.
The syndrome is probably less likely if a patient is switching to another drug rather than ceasing medication altogether, but no studies have determined the best way to implement either change. "It’s not clear if a long taper is better than a short taper, or even if discontinuation syndrome is something that’s dose related," he said. "If you’re on 200 mg of sertraline and you stop, as opposed to 50 mg, we can’t say the higher dose equals an increased risk."
Some studies seem to suggest that the risk is highest with paroxetine. "Fluoxetine probably has the lowest risk because of its long half-life. Venlafaxine is associated with an intermediate risk and all the others fall somewhere below that," Dr. Kroenke said.
The Sequenced Treatment Alternatives to Relieve Depression (STAR*D) trial provides the largest single body of evidence on antidepressant switching (Am. J. Psychiatry 2006;163:1905-17). The methodology in the trial, which involved four levels of treatment and seven antidepressants, was basically simple, Dr. Kroenke said. "You either discontinued one medication and immediately began another or you decreased the first medication while initiating the second at a low dose, doing this taper/titration over 1 week. It doesn’t get much simpler than that." Most investigators in the study chose the first option, he said.
"Having said that, I would probably go for some version of option two, and if the patient is on a higher dose, I might spend a week tapering one while titrating the other."
In a few specific situations, Dr. Kroenke said he strongly favors the taper/titrating method. "I always consider tapering if I’m working with paroxetine or venlafaxine, especially if it’s at a higher dose and has been taken for a long duration," he said. "And definitely if the patient has experienced discontinuation syndrome in the past."
Dr. Kroenke said he has consulted for, and received honoraria from, Eli Lilly and Forest.
EXPERT ANALYSIS FROM THE ANNUAL MEETING OF THE AMERICAN COLLEGE OF PHYSICIANS