User login
American Heart Association (AHA): Scientific Sessions 2014
Mechanical mitral valves outshine bioprostheses in long term
CHICAGO – Biologic prostheses in patients with severe ischemic mitral regurgitation undergoing mitral valve replacement were associated with worse valve hemodynamics and decreased functional capacity, compared with mechanical prostheses, long-term follow-up data showed.
Dr. Carlo Fino reported the results of a retrospective study involving 86 consecutive patients who received mitral valve replacement with either biologic (41) or mechanical (45) prostheses for chronic ischemic mitral valve regurgitation. Their mean age was 63 years and 60 were male.
Significantly more patients with mechanical bioprostheses had a history of heart failure (53% vs. 27%), but all other baseline demographic, clinical, and echocardiographic data were similar. Patients with atrial fibrillation were excluded from the study.
Operative variables were also similar between the mechanical and biologic groups including cardiopulmonary bypass time (mean, 85 vs. 82 minutes), aortic cross clamp time (mean, 71 vs. 68 minutes), arterial grafts per patient (1.2 for both groups), anastomoses per patient (2.7 vs. 2.4), and use of intra-aortic balloon pump (2 patients vs. 1 patient).
At 32 months’ follow-up, patients with mechanical versus biologic prostheses had significantly higher left ventricular ejection fraction (49% vs. 42%) and cardiac index (4.8 vs. 4.5 L/min per square meter) during exercise, Dr. Fino reported at the American Heart Association scientific sessions.
Systolic pulmonary arterial pressure increased modestly during exercise with a mechanical valve, but was significantly higher with a biologic valve (41 vs. 59 mm Hg), as was mitral peak gradient (11.26 vs. 16 mm Hg).
An interesting finding in the study was that effective orifice area and indexed effective orifice area both increased significantly during exercise with a mechanical prosthesis, but did not increase at all with a biologic prosthesis (2.2 vs. 1.8 cm2; 1.57 vs. 1.18 cm2/m2, respectively), said Dr. Fino of Azienda Ospedaliera Papa Giovanni XXIII, in Bergamo, Italy.
“We can’t explain why this effective area does not increase with the bioprosthesis,” he said.
The findings have not changed the center’s clinical approach, but warrant bigger, longer confirmatory studies, he said.
Dr. Fino and his coauthors reported having no relevant financial disclosures.
CHICAGO – Biologic prostheses in patients with severe ischemic mitral regurgitation undergoing mitral valve replacement were associated with worse valve hemodynamics and decreased functional capacity, compared with mechanical prostheses, long-term follow-up data showed.
Dr. Carlo Fino reported the results of a retrospective study involving 86 consecutive patients who received mitral valve replacement with either biologic (41) or mechanical (45) prostheses for chronic ischemic mitral valve regurgitation. Their mean age was 63 years and 60 were male.
Significantly more patients with mechanical bioprostheses had a history of heart failure (53% vs. 27%), but all other baseline demographic, clinical, and echocardiographic data were similar. Patients with atrial fibrillation were excluded from the study.
Operative variables were also similar between the mechanical and biologic groups including cardiopulmonary bypass time (mean, 85 vs. 82 minutes), aortic cross clamp time (mean, 71 vs. 68 minutes), arterial grafts per patient (1.2 for both groups), anastomoses per patient (2.7 vs. 2.4), and use of intra-aortic balloon pump (2 patients vs. 1 patient).
At 32 months’ follow-up, patients with mechanical versus biologic prostheses had significantly higher left ventricular ejection fraction (49% vs. 42%) and cardiac index (4.8 vs. 4.5 L/min per square meter) during exercise, Dr. Fino reported at the American Heart Association scientific sessions.
Systolic pulmonary arterial pressure increased modestly during exercise with a mechanical valve, but was significantly higher with a biologic valve (41 vs. 59 mm Hg), as was mitral peak gradient (11.26 vs. 16 mm Hg).
An interesting finding in the study was that effective orifice area and indexed effective orifice area both increased significantly during exercise with a mechanical prosthesis, but did not increase at all with a biologic prosthesis (2.2 vs. 1.8 cm2; 1.57 vs. 1.18 cm2/m2, respectively), said Dr. Fino of Azienda Ospedaliera Papa Giovanni XXIII, in Bergamo, Italy.
“We can’t explain why this effective area does not increase with the bioprosthesis,” he said.
The findings have not changed the center’s clinical approach, but warrant bigger, longer confirmatory studies, he said.
Dr. Fino and his coauthors reported having no relevant financial disclosures.
CHICAGO – Biologic prostheses in patients with severe ischemic mitral regurgitation undergoing mitral valve replacement were associated with worse valve hemodynamics and decreased functional capacity, compared with mechanical prostheses, long-term follow-up data showed.
Dr. Carlo Fino reported the results of a retrospective study involving 86 consecutive patients who received mitral valve replacement with either biologic (41) or mechanical (45) prostheses for chronic ischemic mitral valve regurgitation. Their mean age was 63 years and 60 were male.
Significantly more patients with mechanical bioprostheses had a history of heart failure (53% vs. 27%), but all other baseline demographic, clinical, and echocardiographic data were similar. Patients with atrial fibrillation were excluded from the study.
Operative variables were also similar between the mechanical and biologic groups including cardiopulmonary bypass time (mean, 85 vs. 82 minutes), aortic cross clamp time (mean, 71 vs. 68 minutes), arterial grafts per patient (1.2 for both groups), anastomoses per patient (2.7 vs. 2.4), and use of intra-aortic balloon pump (2 patients vs. 1 patient).
At 32 months’ follow-up, patients with mechanical versus biologic prostheses had significantly higher left ventricular ejection fraction (49% vs. 42%) and cardiac index (4.8 vs. 4.5 L/min per square meter) during exercise, Dr. Fino reported at the American Heart Association scientific sessions.
Systolic pulmonary arterial pressure increased modestly during exercise with a mechanical valve, but was significantly higher with a biologic valve (41 vs. 59 mm Hg), as was mitral peak gradient (11.26 vs. 16 mm Hg).
An interesting finding in the study was that effective orifice area and indexed effective orifice area both increased significantly during exercise with a mechanical prosthesis, but did not increase at all with a biologic prosthesis (2.2 vs. 1.8 cm2; 1.57 vs. 1.18 cm2/m2, respectively), said Dr. Fino of Azienda Ospedaliera Papa Giovanni XXIII, in Bergamo, Italy.
“We can’t explain why this effective area does not increase with the bioprosthesis,” he said.
The findings have not changed the center’s clinical approach, but warrant bigger, longer confirmatory studies, he said.
Dr. Fino and his coauthors reported having no relevant financial disclosures.
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point: Patients with severe mitral valve regurgitation implanted with a mechanical prosthesis appear to have better cardiovascular performance in the long term than those given biologic prostheses.
Major finding: Indexed effective orifice area increased significantly during exercise with a mechanical prosthesis versus a biologic one (1.57 vs. 1.18 cm2/m2).
Data source: A retrospective study in 86 consecutive patients undergoing mitral valve replacement for severe ischemic mitral regurgitation.
Disclosures: Dr. Fino and his coauthors reported having no relevant financial disclosures.
Bioabsorbable-polymer coronary stent achieves noninferiority endpoint
CHICAGO– A drug-eluting coronary stent with a bioabsorbable polymer that dissolves nearly completely by 4 months after placement proved noninferior for safety and efficacy, compared with a conventional drug-eluting stent for 1 year, in a pivotal U.S. study with 1,684 patients.
If this new drug-eluting coronary stent, Synergy – which uses a bioabsorbable polymer to hold and slowly release the stent’s antirestenosis drug everolimus – were to receive Food and Drug Administration approval in 2015 based on these pivotal trial results, it would become the first such stent on the U.S. market. Some experts hailed the Synergy stent as an incremental improvement over prior devices in its combination of excellent deliverability as well as the ability of the stent’s drug-carrying polymer to vanish once it’s no longer needed, a feature that in theory could reduce the risk for late stent thrombosis and thereby reduce the necessary duration of dual-antiplatelet therapy (DAPT).
“We need to continue to evolve [stents] by improving and enhancing them and reducing the period of mandatory DAPT during the first year” following stent placement, commented Dr. Roxana Mehran, a professor of medicine and interventional cardiologist at Mount Sinai Hospital in New York. The Synergy everolimus-eluting stent with a bioabsorbable polymer is “a little step in the right direction,” she said as designated discussant for the study when its results were reported at the American Heart Association Scientific Sessions.
But others questioned the added value of a new stent that merely shows noninferiority but not superiority to comparator stents when it comes to preventing late stent thrombosis. “The major difference we’ve seen in the different bioabsorbable polymer stents is in their deliverability, which keeps getting better,” said Dr. Christoph Kaiser, professor and head of interventional cardiology at the University of Bern, Switzerland.
EVOLVE II
The EVOLVE II trial enrolled patients who required percutaneous coronary intervention (PCI) with at least one stent at 125 centers in 16 countries during November 2012–December 2013. The investigators randomized patients to PCI with either the Synergy stent or the Promus Element Plus everolimus drug-eluting coronary stent that uses a durable polymer to hold and release everolimus. A third of the patients had unstable angina, and more than a quarter had an acute myocardial infarction. Virtually all patients were on DAPT for at least 6 months, and more than 85% were still on DAPT at 12 months.
After 12 months of follow-up, the combined rate of cardiac death, myocardial infarction caused by a lesion in a stented artery, or ischemia-driven target-vessel revascularization – the trial’s primary endpoint – was 6.7% in patients who received the stent with a bioabsorbable polymer and 6.5% in the durable-polymer stent, a nonsignificant difference that met the trials prespecified definition of noninferiority, reported Dr. Dean Kereiakes, lead investigator for the study and medical director of the Christ Hospital Heart and Vascular Center in Cincinnati.
The safety analysis showed a small but statistically nonsignificant reduction in episodes of definite or probably stent thrombosis, with three such events occurring among the patients who received a bioabsorbable-polymer stent and five in patients who received a durable-polymer stent. “We would need a trial with 20,000 patients to prove superiority” of the bioabsorbable-polymer stent for an endpoint of late stent thrombosis, Dr. Kereiakes said.
The only statistically significant difference in outcomes reported in the EVOLVE II trial between the two stent types tested was in immediate procedural success, which occurred in 98.3% of patients treated with the bioabsorbable-polymer stent and in 96.9% of those treated with a durable-polymer stent, a 1.4% absolute difference.
“As a high-volume operator doing complex cases, the [Synergy] stent is more deliverable and user friendly, with a lower profile and more flexibility. It makes complicated cases easier,” Dr. Kereiakes said.
These technical advantages “will resonate with the interventional community,” commented Dr. Robert Harrington, professor and chairman of medicine and an interventional cardiologist at Stanford (Calif.) University.
Results from a second, similar study that used different bioabsorbable- and durable-polymer stents and reported at the same session showed largely similar results.
BASKET-PROVE II
The BASKET-PROVE II (Basel Stent Kosten Effektivitäts Trial-Prospective Validation Examination Part II) trial randomized 2,291 patients at any of eight centers in four European countries. Patients received a biolimus-eluting stent with a bioabsorbable-polymer (Nobori), an everolimus-eluting stent with a durable polymer (Xience Prime), or a bare-metal stent (PRO-Kinetic), and were followed for 24 months.
The study’s primary efficacy outcome was the same as in EVOLVE II, a combination of cardiac death, myocardial infarction, or need for target-vessel revascularization, but in this study, it was tallied after 24 months. The rates were 7.6% in patients who received stents with a bioabsorbable polymer, and 6.8% in those who got stents with a durable polymer, a difference that was not statistically significant, and which also met the study’s prespecified definition of noninferiority in the intention-to-treat analysis.
The study’s safety endpoint compared patients who received bioabsorbable-polymer stents with those who received bare-metal stents for rates of cardiac death, myocardial infarction, or definite or probable stent thrombosis, which occurred in 5.0% of patients who received bare-metal stents and in 3.7% of those who received stents with a bioabsorbable polymer, a difference that was not statistically significant. Notably, the rates of stent thrombosis during months 13-24 of follow-up were completely identical in patients who received the drug-eluting stent with a bioabsorbable polymer and in those who received bare-metal stents, reported Dr. Kaiser, a coprincipal investigator on the study. Concurrent with his report at the meeting, an article with the results appeared online (Circulation 2014 [doi:10.1161/CIRCULATIONAHA.114.013520/-/DC1]).
These findings “challenge the concept” that polymers are a key issue for any difference in the rate of late stent thrombosis, said Dr. Kaiser.
But another interventionalist, who cochaired the session where the results from both studies were reported, disagreed. “I think it is a step forward to get rid of the polymers. There is no use to having them” once the antirestenosis drug a stent carries is fully released, “so it is good to get rid of them,” said Dr. Stefan James, a cardiologist at Uppsala University in Sweden.
“In our registry [Swedish Coronary Angiography and Angioplasty Registry] we see that the [Synergy] stent has excellent results, with a very low stent thrombosis rate similar to what was shown” on the EVOLVE II trial. “So I think that the [bioabsorbable-polymer] stents are a step forward. After 20 years with drug-eluting stents, we are improving these devices step by step,” Dr. James said.
EVOLVE II was sponsored by Boston Scientific. Dr. Kereiakes is a consultant to and speaker for Boston Scientific, and a consultant to Abbott Vascular and Reva Medical. Dr. Mehran has received honoraria from and has been a consultant to Boston Scientific as well as to several other device and drug companies. Dr. Harrington had been an investigator for several trials that tested drugs patients take following stenting. The BASKET-PROVE II trial had no commercial sponsorship, but the study organizers received the prasugrel that patients took in the study following stenting for free from the manufacturers. Dr. Kaiser has received remuneration for being the advisory boards for Eli Lilly and Daiichi Sankyo. Dr. James had served on an advisory board for Medtronic and has received institutional research grants from Medtronic, Terumo, and Vascular Solutions.
On Twitter @mitchelzoler
CHICAGO– A drug-eluting coronary stent with a bioabsorbable polymer that dissolves nearly completely by 4 months after placement proved noninferior for safety and efficacy, compared with a conventional drug-eluting stent for 1 year, in a pivotal U.S. study with 1,684 patients.
If this new drug-eluting coronary stent, Synergy – which uses a bioabsorbable polymer to hold and slowly release the stent’s antirestenosis drug everolimus – were to receive Food and Drug Administration approval in 2015 based on these pivotal trial results, it would become the first such stent on the U.S. market. Some experts hailed the Synergy stent as an incremental improvement over prior devices in its combination of excellent deliverability as well as the ability of the stent’s drug-carrying polymer to vanish once it’s no longer needed, a feature that in theory could reduce the risk for late stent thrombosis and thereby reduce the necessary duration of dual-antiplatelet therapy (DAPT).
“We need to continue to evolve [stents] by improving and enhancing them and reducing the period of mandatory DAPT during the first year” following stent placement, commented Dr. Roxana Mehran, a professor of medicine and interventional cardiologist at Mount Sinai Hospital in New York. The Synergy everolimus-eluting stent with a bioabsorbable polymer is “a little step in the right direction,” she said as designated discussant for the study when its results were reported at the American Heart Association Scientific Sessions.
But others questioned the added value of a new stent that merely shows noninferiority but not superiority to comparator stents when it comes to preventing late stent thrombosis. “The major difference we’ve seen in the different bioabsorbable polymer stents is in their deliverability, which keeps getting better,” said Dr. Christoph Kaiser, professor and head of interventional cardiology at the University of Bern, Switzerland.
EVOLVE II
The EVOLVE II trial enrolled patients who required percutaneous coronary intervention (PCI) with at least one stent at 125 centers in 16 countries during November 2012–December 2013. The investigators randomized patients to PCI with either the Synergy stent or the Promus Element Plus everolimus drug-eluting coronary stent that uses a durable polymer to hold and release everolimus. A third of the patients had unstable angina, and more than a quarter had an acute myocardial infarction. Virtually all patients were on DAPT for at least 6 months, and more than 85% were still on DAPT at 12 months.
After 12 months of follow-up, the combined rate of cardiac death, myocardial infarction caused by a lesion in a stented artery, or ischemia-driven target-vessel revascularization – the trial’s primary endpoint – was 6.7% in patients who received the stent with a bioabsorbable polymer and 6.5% in the durable-polymer stent, a nonsignificant difference that met the trials prespecified definition of noninferiority, reported Dr. Dean Kereiakes, lead investigator for the study and medical director of the Christ Hospital Heart and Vascular Center in Cincinnati.
The safety analysis showed a small but statistically nonsignificant reduction in episodes of definite or probably stent thrombosis, with three such events occurring among the patients who received a bioabsorbable-polymer stent and five in patients who received a durable-polymer stent. “We would need a trial with 20,000 patients to prove superiority” of the bioabsorbable-polymer stent for an endpoint of late stent thrombosis, Dr. Kereiakes said.
The only statistically significant difference in outcomes reported in the EVOLVE II trial between the two stent types tested was in immediate procedural success, which occurred in 98.3% of patients treated with the bioabsorbable-polymer stent and in 96.9% of those treated with a durable-polymer stent, a 1.4% absolute difference.
“As a high-volume operator doing complex cases, the [Synergy] stent is more deliverable and user friendly, with a lower profile and more flexibility. It makes complicated cases easier,” Dr. Kereiakes said.
These technical advantages “will resonate with the interventional community,” commented Dr. Robert Harrington, professor and chairman of medicine and an interventional cardiologist at Stanford (Calif.) University.
Results from a second, similar study that used different bioabsorbable- and durable-polymer stents and reported at the same session showed largely similar results.
BASKET-PROVE II
The BASKET-PROVE II (Basel Stent Kosten Effektivitäts Trial-Prospective Validation Examination Part II) trial randomized 2,291 patients at any of eight centers in four European countries. Patients received a biolimus-eluting stent with a bioabsorbable-polymer (Nobori), an everolimus-eluting stent with a durable polymer (Xience Prime), or a bare-metal stent (PRO-Kinetic), and were followed for 24 months.
The study’s primary efficacy outcome was the same as in EVOLVE II, a combination of cardiac death, myocardial infarction, or need for target-vessel revascularization, but in this study, it was tallied after 24 months. The rates were 7.6% in patients who received stents with a bioabsorbable polymer, and 6.8% in those who got stents with a durable polymer, a difference that was not statistically significant, and which also met the study’s prespecified definition of noninferiority in the intention-to-treat analysis.
The study’s safety endpoint compared patients who received bioabsorbable-polymer stents with those who received bare-metal stents for rates of cardiac death, myocardial infarction, or definite or probable stent thrombosis, which occurred in 5.0% of patients who received bare-metal stents and in 3.7% of those who received stents with a bioabsorbable polymer, a difference that was not statistically significant. Notably, the rates of stent thrombosis during months 13-24 of follow-up were completely identical in patients who received the drug-eluting stent with a bioabsorbable polymer and in those who received bare-metal stents, reported Dr. Kaiser, a coprincipal investigator on the study. Concurrent with his report at the meeting, an article with the results appeared online (Circulation 2014 [doi:10.1161/CIRCULATIONAHA.114.013520/-/DC1]).
These findings “challenge the concept” that polymers are a key issue for any difference in the rate of late stent thrombosis, said Dr. Kaiser.
But another interventionalist, who cochaired the session where the results from both studies were reported, disagreed. “I think it is a step forward to get rid of the polymers. There is no use to having them” once the antirestenosis drug a stent carries is fully released, “so it is good to get rid of them,” said Dr. Stefan James, a cardiologist at Uppsala University in Sweden.
“In our registry [Swedish Coronary Angiography and Angioplasty Registry] we see that the [Synergy] stent has excellent results, with a very low stent thrombosis rate similar to what was shown” on the EVOLVE II trial. “So I think that the [bioabsorbable-polymer] stents are a step forward. After 20 years with drug-eluting stents, we are improving these devices step by step,” Dr. James said.
EVOLVE II was sponsored by Boston Scientific. Dr. Kereiakes is a consultant to and speaker for Boston Scientific, and a consultant to Abbott Vascular and Reva Medical. Dr. Mehran has received honoraria from and has been a consultant to Boston Scientific as well as to several other device and drug companies. Dr. Harrington had been an investigator for several trials that tested drugs patients take following stenting. The BASKET-PROVE II trial had no commercial sponsorship, but the study organizers received the prasugrel that patients took in the study following stenting for free from the manufacturers. Dr. Kaiser has received remuneration for being the advisory boards for Eli Lilly and Daiichi Sankyo. Dr. James had served on an advisory board for Medtronic and has received institutional research grants from Medtronic, Terumo, and Vascular Solutions.
On Twitter @mitchelzoler
CHICAGO– A drug-eluting coronary stent with a bioabsorbable polymer that dissolves nearly completely by 4 months after placement proved noninferior for safety and efficacy, compared with a conventional drug-eluting stent for 1 year, in a pivotal U.S. study with 1,684 patients.
If this new drug-eluting coronary stent, Synergy – which uses a bioabsorbable polymer to hold and slowly release the stent’s antirestenosis drug everolimus – were to receive Food and Drug Administration approval in 2015 based on these pivotal trial results, it would become the first such stent on the U.S. market. Some experts hailed the Synergy stent as an incremental improvement over prior devices in its combination of excellent deliverability as well as the ability of the stent’s drug-carrying polymer to vanish once it’s no longer needed, a feature that in theory could reduce the risk for late stent thrombosis and thereby reduce the necessary duration of dual-antiplatelet therapy (DAPT).
“We need to continue to evolve [stents] by improving and enhancing them and reducing the period of mandatory DAPT during the first year” following stent placement, commented Dr. Roxana Mehran, a professor of medicine and interventional cardiologist at Mount Sinai Hospital in New York. The Synergy everolimus-eluting stent with a bioabsorbable polymer is “a little step in the right direction,” she said as designated discussant for the study when its results were reported at the American Heart Association Scientific Sessions.
But others questioned the added value of a new stent that merely shows noninferiority but not superiority to comparator stents when it comes to preventing late stent thrombosis. “The major difference we’ve seen in the different bioabsorbable polymer stents is in their deliverability, which keeps getting better,” said Dr. Christoph Kaiser, professor and head of interventional cardiology at the University of Bern, Switzerland.
EVOLVE II
The EVOLVE II trial enrolled patients who required percutaneous coronary intervention (PCI) with at least one stent at 125 centers in 16 countries during November 2012–December 2013. The investigators randomized patients to PCI with either the Synergy stent or the Promus Element Plus everolimus drug-eluting coronary stent that uses a durable polymer to hold and release everolimus. A third of the patients had unstable angina, and more than a quarter had an acute myocardial infarction. Virtually all patients were on DAPT for at least 6 months, and more than 85% were still on DAPT at 12 months.
After 12 months of follow-up, the combined rate of cardiac death, myocardial infarction caused by a lesion in a stented artery, or ischemia-driven target-vessel revascularization – the trial’s primary endpoint – was 6.7% in patients who received the stent with a bioabsorbable polymer and 6.5% in the durable-polymer stent, a nonsignificant difference that met the trials prespecified definition of noninferiority, reported Dr. Dean Kereiakes, lead investigator for the study and medical director of the Christ Hospital Heart and Vascular Center in Cincinnati.
The safety analysis showed a small but statistically nonsignificant reduction in episodes of definite or probably stent thrombosis, with three such events occurring among the patients who received a bioabsorbable-polymer stent and five in patients who received a durable-polymer stent. “We would need a trial with 20,000 patients to prove superiority” of the bioabsorbable-polymer stent for an endpoint of late stent thrombosis, Dr. Kereiakes said.
The only statistically significant difference in outcomes reported in the EVOLVE II trial between the two stent types tested was in immediate procedural success, which occurred in 98.3% of patients treated with the bioabsorbable-polymer stent and in 96.9% of those treated with a durable-polymer stent, a 1.4% absolute difference.
“As a high-volume operator doing complex cases, the [Synergy] stent is more deliverable and user friendly, with a lower profile and more flexibility. It makes complicated cases easier,” Dr. Kereiakes said.
These technical advantages “will resonate with the interventional community,” commented Dr. Robert Harrington, professor and chairman of medicine and an interventional cardiologist at Stanford (Calif.) University.
Results from a second, similar study that used different bioabsorbable- and durable-polymer stents and reported at the same session showed largely similar results.
BASKET-PROVE II
The BASKET-PROVE II (Basel Stent Kosten Effektivitäts Trial-Prospective Validation Examination Part II) trial randomized 2,291 patients at any of eight centers in four European countries. Patients received a biolimus-eluting stent with a bioabsorbable-polymer (Nobori), an everolimus-eluting stent with a durable polymer (Xience Prime), or a bare-metal stent (PRO-Kinetic), and were followed for 24 months.
The study’s primary efficacy outcome was the same as in EVOLVE II, a combination of cardiac death, myocardial infarction, or need for target-vessel revascularization, but in this study, it was tallied after 24 months. The rates were 7.6% in patients who received stents with a bioabsorbable polymer, and 6.8% in those who got stents with a durable polymer, a difference that was not statistically significant, and which also met the study’s prespecified definition of noninferiority in the intention-to-treat analysis.
The study’s safety endpoint compared patients who received bioabsorbable-polymer stents with those who received bare-metal stents for rates of cardiac death, myocardial infarction, or definite or probable stent thrombosis, which occurred in 5.0% of patients who received bare-metal stents and in 3.7% of those who received stents with a bioabsorbable polymer, a difference that was not statistically significant. Notably, the rates of stent thrombosis during months 13-24 of follow-up were completely identical in patients who received the drug-eluting stent with a bioabsorbable polymer and in those who received bare-metal stents, reported Dr. Kaiser, a coprincipal investigator on the study. Concurrent with his report at the meeting, an article with the results appeared online (Circulation 2014 [doi:10.1161/CIRCULATIONAHA.114.013520/-/DC1]).
These findings “challenge the concept” that polymers are a key issue for any difference in the rate of late stent thrombosis, said Dr. Kaiser.
But another interventionalist, who cochaired the session where the results from both studies were reported, disagreed. “I think it is a step forward to get rid of the polymers. There is no use to having them” once the antirestenosis drug a stent carries is fully released, “so it is good to get rid of them,” said Dr. Stefan James, a cardiologist at Uppsala University in Sweden.
“In our registry [Swedish Coronary Angiography and Angioplasty Registry] we see that the [Synergy] stent has excellent results, with a very low stent thrombosis rate similar to what was shown” on the EVOLVE II trial. “So I think that the [bioabsorbable-polymer] stents are a step forward. After 20 years with drug-eluting stents, we are improving these devices step by step,” Dr. James said.
EVOLVE II was sponsored by Boston Scientific. Dr. Kereiakes is a consultant to and speaker for Boston Scientific, and a consultant to Abbott Vascular and Reva Medical. Dr. Mehran has received honoraria from and has been a consultant to Boston Scientific as well as to several other device and drug companies. Dr. Harrington had been an investigator for several trials that tested drugs patients take following stenting. The BASKET-PROVE II trial had no commercial sponsorship, but the study organizers received the prasugrel that patients took in the study following stenting for free from the manufacturers. Dr. Kaiser has received remuneration for being the advisory boards for Eli Lilly and Daiichi Sankyo. Dr. James had served on an advisory board for Medtronic and has received institutional research grants from Medtronic, Terumo, and Vascular Solutions.
On Twitter @mitchelzoler
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point: A drug-eluting coronary stent with a bioabsorbable polymer proved noninferior to a conventional drug-eluting stent with a durable polymer in its pivotal U.S. trial, paving the way for marketing approval.
Major finding: The Synergy coronary stent with a bioabsorbable polymer met its prespecified noninferiority endpoints, compared with a standard drug-eluting stent.
Data source: EVOLVE II, a randomized, multicenter trial with 1,684 patients.
Disclosures: EVOLVE II was sponsored by Boston Scientific. Dr. Kereiakes is a consultant to and speaker for Boston Scientific, and a consultant to Abbott Vascular and Reva Medical.
Coordinated regional STEMI care delivers dividends
CHICAGO – Regional coordination of emergency care provided modest but significant improvements in timely reperfusion of patients with ST-elevation myocardial infarction.
In the massive Mission Lifeline: STEMI Accelerator project, in-hospital mortality was 3.6% for patients who spent 30 minutes or less between the hospital door and cardiac catheter lab door, compared with 7% for those with times spent in the emergency department of 30-45 minutes and 10.8% for patients with ED times of more than 45 minutes (P < .001), after adjustment for clinical risk factors such as cardiac arrest and cardiogenic shock among patients presenting directly to a percutaneous coronary intervention–capable hospital via emergency medical services.
“While this relationship may have residual confounding, we believe that it shows ED time is an important indicator of the coordination of care,” Dr. Matthew Sherwood said at the American Heart Association scientific sessions.
The American College of Cardiology Foundation/AHA guidelines recommend the use of regional systems of care for STEMI patients and set a system goal for first medical contact to device time of 90 minutes or less for patients presenting directly to a percutaneous intervention–capable hospital and 120 minutes or less for those transferred from a non-PCI hospital to a PCI facility.
Only half of patients are treated within guideline goals, largely because of delays related to fragmentation of the health care system and lack of coordination between EMS agencies and acute care hospitals, said Dr. Sherwood, an interventional cardiology fellow with Duke University in Durham, N.C. For example, only 17% of EMS agencies can preactivate cardiac catheter labs in all receiving hospitals.
The STEMI Accelerator study sought to improve STEMI care between 484 hospitals and 1,253 EMS agencies in 16 cities, including New York, St. Louis, Pittsburgh, and Houston, representing roughly 10% of the U.S. population.
Leaders of emergency medical services, ED physicians, and interventional cardiologists were brought together in each region to identify areas for improvement and establish a regional coordinator who would implement a feedback system. They also developed a uniform protocol for preactivation for STEMI by EMS providers and coordinated a plan for rapid transfer of all STEMI patients to appropriate facilities.
At least 70% of PCI centers were also required to enroll in the National Cardiovascular Data Registry–Action GWTG registry for data collection, Dr. Sherwood said.
The Mission Lifeline: STEMI Systems Accelerator operations manual was also distributed at the national level to assist regions in developing their own, more individualized protocols. In New York City, that involved 16 PCI hospitals in downtown Manhattan developing a plan for STEMI patients to bypass the ED and go directly to the catheter lab – a move that has paid off in improved time to device delivery, he said.
The analysis included 23,809 patients with STEMI treated from the third quarter of 2012 to the first quarter of 2014. Of these, 11,765 presented directly to a PCI center via EMS, 6,502 presented to a PCI center via their own transportation, and 5,542 transferred from a non–PCI capable facility for further care. Their median age was 60 years, 29% were female, and 16% had no insurance.
For all three patient groups, there was a modest, but significant improvement from baseline to Q1 2014 in the percentage of patients meeting first medical contact to device time goals, including: direct-presenting patients (62% vs. 65%; P = .025), direct-presenting patients transported by EMS (54% vs. 59%; P = .0046), and patients requiring hospital transfer (50% vs. 53%; P = .007), Dr. Sherwood reported.
While the overall study showed modest improvements, there was a significant amount of variation in regional time and performance improvements. For example, among patients presenting directly to a PCI hospital, some regions showed up to 20% increases in the proportion of patients treated within guideline goals, he said.
Unadjusted in-hospital mortality also declined over the study period for participating regions, compared with national rates, suggesting that regional STEMI programs can provide important improvements to public health, Dr. Sherwood concluded.
Speaking as a practitioner in one of the contributing hospitals, panelist Dr. Roxana Mehran of Mount Sinai Hospital in New York, said that the STEMI program was invaluable in expanding their focus beyond door to balloon time.
“This particular program was extremely helpful for us in not just the timing of getting the patient, but also on the post discharge, which is also a part of this program,” she said. “I must tell you it had a huge impact, and we saw our numbers change dramatically just over the few quarters we saw patients.”
The Medicines Company, AstraZeneca, Philips Healthcare, and Abiomed sponsored the study. Dr. Sherwood reported no relevant financial relationships.
CHICAGO – Regional coordination of emergency care provided modest but significant improvements in timely reperfusion of patients with ST-elevation myocardial infarction.
In the massive Mission Lifeline: STEMI Accelerator project, in-hospital mortality was 3.6% for patients who spent 30 minutes or less between the hospital door and cardiac catheter lab door, compared with 7% for those with times spent in the emergency department of 30-45 minutes and 10.8% for patients with ED times of more than 45 minutes (P < .001), after adjustment for clinical risk factors such as cardiac arrest and cardiogenic shock among patients presenting directly to a percutaneous coronary intervention–capable hospital via emergency medical services.
“While this relationship may have residual confounding, we believe that it shows ED time is an important indicator of the coordination of care,” Dr. Matthew Sherwood said at the American Heart Association scientific sessions.
The American College of Cardiology Foundation/AHA guidelines recommend the use of regional systems of care for STEMI patients and set a system goal for first medical contact to device time of 90 minutes or less for patients presenting directly to a percutaneous intervention–capable hospital and 120 minutes or less for those transferred from a non-PCI hospital to a PCI facility.
Only half of patients are treated within guideline goals, largely because of delays related to fragmentation of the health care system and lack of coordination between EMS agencies and acute care hospitals, said Dr. Sherwood, an interventional cardiology fellow with Duke University in Durham, N.C. For example, only 17% of EMS agencies can preactivate cardiac catheter labs in all receiving hospitals.
The STEMI Accelerator study sought to improve STEMI care between 484 hospitals and 1,253 EMS agencies in 16 cities, including New York, St. Louis, Pittsburgh, and Houston, representing roughly 10% of the U.S. population.
Leaders of emergency medical services, ED physicians, and interventional cardiologists were brought together in each region to identify areas for improvement and establish a regional coordinator who would implement a feedback system. They also developed a uniform protocol for preactivation for STEMI by EMS providers and coordinated a plan for rapid transfer of all STEMI patients to appropriate facilities.
At least 70% of PCI centers were also required to enroll in the National Cardiovascular Data Registry–Action GWTG registry for data collection, Dr. Sherwood said.
The Mission Lifeline: STEMI Systems Accelerator operations manual was also distributed at the national level to assist regions in developing their own, more individualized protocols. In New York City, that involved 16 PCI hospitals in downtown Manhattan developing a plan for STEMI patients to bypass the ED and go directly to the catheter lab – a move that has paid off in improved time to device delivery, he said.
The analysis included 23,809 patients with STEMI treated from the third quarter of 2012 to the first quarter of 2014. Of these, 11,765 presented directly to a PCI center via EMS, 6,502 presented to a PCI center via their own transportation, and 5,542 transferred from a non–PCI capable facility for further care. Their median age was 60 years, 29% were female, and 16% had no insurance.
For all three patient groups, there was a modest, but significant improvement from baseline to Q1 2014 in the percentage of patients meeting first medical contact to device time goals, including: direct-presenting patients (62% vs. 65%; P = .025), direct-presenting patients transported by EMS (54% vs. 59%; P = .0046), and patients requiring hospital transfer (50% vs. 53%; P = .007), Dr. Sherwood reported.
While the overall study showed modest improvements, there was a significant amount of variation in regional time and performance improvements. For example, among patients presenting directly to a PCI hospital, some regions showed up to 20% increases in the proportion of patients treated within guideline goals, he said.
Unadjusted in-hospital mortality also declined over the study period for participating regions, compared with national rates, suggesting that regional STEMI programs can provide important improvements to public health, Dr. Sherwood concluded.
Speaking as a practitioner in one of the contributing hospitals, panelist Dr. Roxana Mehran of Mount Sinai Hospital in New York, said that the STEMI program was invaluable in expanding their focus beyond door to balloon time.
“This particular program was extremely helpful for us in not just the timing of getting the patient, but also on the post discharge, which is also a part of this program,” she said. “I must tell you it had a huge impact, and we saw our numbers change dramatically just over the few quarters we saw patients.”
The Medicines Company, AstraZeneca, Philips Healthcare, and Abiomed sponsored the study. Dr. Sherwood reported no relevant financial relationships.
CHICAGO – Regional coordination of emergency care provided modest but significant improvements in timely reperfusion of patients with ST-elevation myocardial infarction.
In the massive Mission Lifeline: STEMI Accelerator project, in-hospital mortality was 3.6% for patients who spent 30 minutes or less between the hospital door and cardiac catheter lab door, compared with 7% for those with times spent in the emergency department of 30-45 minutes and 10.8% for patients with ED times of more than 45 minutes (P < .001), after adjustment for clinical risk factors such as cardiac arrest and cardiogenic shock among patients presenting directly to a percutaneous coronary intervention–capable hospital via emergency medical services.
“While this relationship may have residual confounding, we believe that it shows ED time is an important indicator of the coordination of care,” Dr. Matthew Sherwood said at the American Heart Association scientific sessions.
The American College of Cardiology Foundation/AHA guidelines recommend the use of regional systems of care for STEMI patients and set a system goal for first medical contact to device time of 90 minutes or less for patients presenting directly to a percutaneous intervention–capable hospital and 120 minutes or less for those transferred from a non-PCI hospital to a PCI facility.
Only half of patients are treated within guideline goals, largely because of delays related to fragmentation of the health care system and lack of coordination between EMS agencies and acute care hospitals, said Dr. Sherwood, an interventional cardiology fellow with Duke University in Durham, N.C. For example, only 17% of EMS agencies can preactivate cardiac catheter labs in all receiving hospitals.
The STEMI Accelerator study sought to improve STEMI care between 484 hospitals and 1,253 EMS agencies in 16 cities, including New York, St. Louis, Pittsburgh, and Houston, representing roughly 10% of the U.S. population.
Leaders of emergency medical services, ED physicians, and interventional cardiologists were brought together in each region to identify areas for improvement and establish a regional coordinator who would implement a feedback system. They also developed a uniform protocol for preactivation for STEMI by EMS providers and coordinated a plan for rapid transfer of all STEMI patients to appropriate facilities.
At least 70% of PCI centers were also required to enroll in the National Cardiovascular Data Registry–Action GWTG registry for data collection, Dr. Sherwood said.
The Mission Lifeline: STEMI Systems Accelerator operations manual was also distributed at the national level to assist regions in developing their own, more individualized protocols. In New York City, that involved 16 PCI hospitals in downtown Manhattan developing a plan for STEMI patients to bypass the ED and go directly to the catheter lab – a move that has paid off in improved time to device delivery, he said.
The analysis included 23,809 patients with STEMI treated from the third quarter of 2012 to the first quarter of 2014. Of these, 11,765 presented directly to a PCI center via EMS, 6,502 presented to a PCI center via their own transportation, and 5,542 transferred from a non–PCI capable facility for further care. Their median age was 60 years, 29% were female, and 16% had no insurance.
For all three patient groups, there was a modest, but significant improvement from baseline to Q1 2014 in the percentage of patients meeting first medical contact to device time goals, including: direct-presenting patients (62% vs. 65%; P = .025), direct-presenting patients transported by EMS (54% vs. 59%; P = .0046), and patients requiring hospital transfer (50% vs. 53%; P = .007), Dr. Sherwood reported.
While the overall study showed modest improvements, there was a significant amount of variation in regional time and performance improvements. For example, among patients presenting directly to a PCI hospital, some regions showed up to 20% increases in the proportion of patients treated within guideline goals, he said.
Unadjusted in-hospital mortality also declined over the study period for participating regions, compared with national rates, suggesting that regional STEMI programs can provide important improvements to public health, Dr. Sherwood concluded.
Speaking as a practitioner in one of the contributing hospitals, panelist Dr. Roxana Mehran of Mount Sinai Hospital in New York, said that the STEMI program was invaluable in expanding their focus beyond door to balloon time.
“This particular program was extremely helpful for us in not just the timing of getting the patient, but also on the post discharge, which is also a part of this program,” she said. “I must tell you it had a huge impact, and we saw our numbers change dramatically just over the few quarters we saw patients.”
The Medicines Company, AstraZeneca, Philips Healthcare, and Abiomed sponsored the study. Dr. Sherwood reported no relevant financial relationships.
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point: Organizing regional STEMI care results in significant improvements in the percentage of patients achieving national reperfusion goals.
Major finding: For all three patient groups, there was a modest but significant improvement from baseline to study end in the percentage of patients meeting first medical contact to device time goals.
Data source: Mission Lifeline: STEMI Accelerator, an observational study in 23,809 patients with STEMI.
Disclosures: The Medicines Company, AstraZeneca, Philips Healthcare, and Abiomed sponsored the study. Dr. Sherwood reported no relevant financial relationships.
Rivaroxaban matches warfarin’s total Afib costs
CHICAGO – Patients with atrial fibrillation who start treatment with a new oral anticoagulant may spend more on their medication than if they were prescribed generic warfarin, but their overall health care costs may wind up being about the same, based on an analysis of health care expense records for more than 4,500 U.S. patients.
“Despite higher anticoagulant costs, total all-cause and atrial fibrillation–related costs remain comparable” between patients prescribed warfarin and those who received the new oral anticoagulant rivaroxaban (Xarelto), said Concetta Crivera, Pharm.D., at the American Heart Association Scientific Sessions. Higher drug costs for daily treatment with rivaroxaban were offset by reduced hospital lengths of stays and hence reduced hospitalization costs, said Dr. Crivera, director of cardiovascular health economics and outcomes research at Janssen in Raritan, N.J., the company that markets rivaroxaban along with Bayer.
“I can believe that hospitalized days would be reduced because patients treated with rivaroxaban or any of the other new oral anticoagulants don’t need to remain in the hospital while you wait for their international normalized ratio to enter the therapeutic range,” which is what happens with patients treated with warfarin, commented Dr. Jeffrey Weitz, professor of medicine and director of the Juravinski Hospital and Cancer Centre of McMaster University in Hamilton, Ont.
The new findings by Dr. Crivera “are not definitive on their own, but they add to a growing body of data that indicate that all the new oral anticoagulants are cost effective,” said Dr. Weitz, who specializes in thrombosis and anticoagulants. “The worst time for a patient on warfarin is when they start treatment. You do a disservice to patients with new-onset atrial fibrillation by using warfarin, because some patients will get into the therapeutic range but others never will. That’s why the new oral anticoagulants are better, because everybody gets into therapeutic range,” he said in an interview.
Dr. Crivera and her associates conducted a retrospective study of health records for 2,253 patients with nonvalvular atrial fibrillation (AF) who started anticoagulation treatment with rivaroxaban during the period of November 2011 (when the drug received U.S. marketing approval) through December 2012. The data came from the patient records of Humana, a U.S. HMO and insurer that covers both commercially insured patients and those covered by Medicare. The researchers matched each of the patients initiating rivaroxaban treatment with a similar patient from the Humana database with nonvalvular AF who started on warfarin during the same period. The average age of patients in the study was about 74 years, and patients in the two groups were closely matched for their demographic and clinical characteristics, including comorbidities. Data on health care use were available for patients for an average of about 4 months following their start of anticoagulant treatment.
The analysis showed that during the first months on treatment, patients prescribed rivaroxaban averaged 2.11 days of hospitalization for an AF-related episode, compared with 3.02 days for those prescribed warfarin, a statistically significant difference. Hospitalizations for any cause averaged a total of 2.71 days in the rivaroxaban group and 3.87 days in the patients on warfarin, also a significant difference, Dr. Crivera reported.
This difference in days hospitalized translated into reduced hospitalization costs, a roughly $2,000 average difference per patient in actual hospitalization costs in favor of the rivaroxaban patients for all-cause hospitalizations, and an average $1,300 difference per patient for hospitalization costs directly related to AF.
Although the rivaroxaban patients spent an average of $2,700 more per patient on pharmaceuticals for all causes, and an average $2,200 more for AF-related drugs, the total average all-cause and AF-related costs for drugs, hospitalizations, outpatient visits, and emergency department visits were similar in the two subgroups: an average total of $17,590 per patient for the rivaroxaban patients and $18,676 for warfarin patients for all causes, and an average of $7,394 for the rivaroxaban patients and $7,319 for those on warfarin for AF-related care. The between-group differences for both sets of total costs were not statistically significant.
The study was sponsored by Janssen, which along with Bayer markets rivaroxaban (Xarelto). Dr. Crivera is a Janssen employee. Dr. Weitz has been an adviser or a consultant to Janssen and Bayer and to Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Johnson & Johnson, Merck, Pfizer, and Portola.
On Twitter @mitchelzoler
CHICAGO – Patients with atrial fibrillation who start treatment with a new oral anticoagulant may spend more on their medication than if they were prescribed generic warfarin, but their overall health care costs may wind up being about the same, based on an analysis of health care expense records for more than 4,500 U.S. patients.
“Despite higher anticoagulant costs, total all-cause and atrial fibrillation–related costs remain comparable” between patients prescribed warfarin and those who received the new oral anticoagulant rivaroxaban (Xarelto), said Concetta Crivera, Pharm.D., at the American Heart Association Scientific Sessions. Higher drug costs for daily treatment with rivaroxaban were offset by reduced hospital lengths of stays and hence reduced hospitalization costs, said Dr. Crivera, director of cardiovascular health economics and outcomes research at Janssen in Raritan, N.J., the company that markets rivaroxaban along with Bayer.
“I can believe that hospitalized days would be reduced because patients treated with rivaroxaban or any of the other new oral anticoagulants don’t need to remain in the hospital while you wait for their international normalized ratio to enter the therapeutic range,” which is what happens with patients treated with warfarin, commented Dr. Jeffrey Weitz, professor of medicine and director of the Juravinski Hospital and Cancer Centre of McMaster University in Hamilton, Ont.
The new findings by Dr. Crivera “are not definitive on their own, but they add to a growing body of data that indicate that all the new oral anticoagulants are cost effective,” said Dr. Weitz, who specializes in thrombosis and anticoagulants. “The worst time for a patient on warfarin is when they start treatment. You do a disservice to patients with new-onset atrial fibrillation by using warfarin, because some patients will get into the therapeutic range but others never will. That’s why the new oral anticoagulants are better, because everybody gets into therapeutic range,” he said in an interview.
Dr. Crivera and her associates conducted a retrospective study of health records for 2,253 patients with nonvalvular atrial fibrillation (AF) who started anticoagulation treatment with rivaroxaban during the period of November 2011 (when the drug received U.S. marketing approval) through December 2012. The data came from the patient records of Humana, a U.S. HMO and insurer that covers both commercially insured patients and those covered by Medicare. The researchers matched each of the patients initiating rivaroxaban treatment with a similar patient from the Humana database with nonvalvular AF who started on warfarin during the same period. The average age of patients in the study was about 74 years, and patients in the two groups were closely matched for their demographic and clinical characteristics, including comorbidities. Data on health care use were available for patients for an average of about 4 months following their start of anticoagulant treatment.
The analysis showed that during the first months on treatment, patients prescribed rivaroxaban averaged 2.11 days of hospitalization for an AF-related episode, compared with 3.02 days for those prescribed warfarin, a statistically significant difference. Hospitalizations for any cause averaged a total of 2.71 days in the rivaroxaban group and 3.87 days in the patients on warfarin, also a significant difference, Dr. Crivera reported.
This difference in days hospitalized translated into reduced hospitalization costs, a roughly $2,000 average difference per patient in actual hospitalization costs in favor of the rivaroxaban patients for all-cause hospitalizations, and an average $1,300 difference per patient for hospitalization costs directly related to AF.
Although the rivaroxaban patients spent an average of $2,700 more per patient on pharmaceuticals for all causes, and an average $2,200 more for AF-related drugs, the total average all-cause and AF-related costs for drugs, hospitalizations, outpatient visits, and emergency department visits were similar in the two subgroups: an average total of $17,590 per patient for the rivaroxaban patients and $18,676 for warfarin patients for all causes, and an average of $7,394 for the rivaroxaban patients and $7,319 for those on warfarin for AF-related care. The between-group differences for both sets of total costs were not statistically significant.
The study was sponsored by Janssen, which along with Bayer markets rivaroxaban (Xarelto). Dr. Crivera is a Janssen employee. Dr. Weitz has been an adviser or a consultant to Janssen and Bayer and to Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Johnson & Johnson, Merck, Pfizer, and Portola.
On Twitter @mitchelzoler
CHICAGO – Patients with atrial fibrillation who start treatment with a new oral anticoagulant may spend more on their medication than if they were prescribed generic warfarin, but their overall health care costs may wind up being about the same, based on an analysis of health care expense records for more than 4,500 U.S. patients.
“Despite higher anticoagulant costs, total all-cause and atrial fibrillation–related costs remain comparable” between patients prescribed warfarin and those who received the new oral anticoagulant rivaroxaban (Xarelto), said Concetta Crivera, Pharm.D., at the American Heart Association Scientific Sessions. Higher drug costs for daily treatment with rivaroxaban were offset by reduced hospital lengths of stays and hence reduced hospitalization costs, said Dr. Crivera, director of cardiovascular health economics and outcomes research at Janssen in Raritan, N.J., the company that markets rivaroxaban along with Bayer.
“I can believe that hospitalized days would be reduced because patients treated with rivaroxaban or any of the other new oral anticoagulants don’t need to remain in the hospital while you wait for their international normalized ratio to enter the therapeutic range,” which is what happens with patients treated with warfarin, commented Dr. Jeffrey Weitz, professor of medicine and director of the Juravinski Hospital and Cancer Centre of McMaster University in Hamilton, Ont.
The new findings by Dr. Crivera “are not definitive on their own, but they add to a growing body of data that indicate that all the new oral anticoagulants are cost effective,” said Dr. Weitz, who specializes in thrombosis and anticoagulants. “The worst time for a patient on warfarin is when they start treatment. You do a disservice to patients with new-onset atrial fibrillation by using warfarin, because some patients will get into the therapeutic range but others never will. That’s why the new oral anticoagulants are better, because everybody gets into therapeutic range,” he said in an interview.
Dr. Crivera and her associates conducted a retrospective study of health records for 2,253 patients with nonvalvular atrial fibrillation (AF) who started anticoagulation treatment with rivaroxaban during the period of November 2011 (when the drug received U.S. marketing approval) through December 2012. The data came from the patient records of Humana, a U.S. HMO and insurer that covers both commercially insured patients and those covered by Medicare. The researchers matched each of the patients initiating rivaroxaban treatment with a similar patient from the Humana database with nonvalvular AF who started on warfarin during the same period. The average age of patients in the study was about 74 years, and patients in the two groups were closely matched for their demographic and clinical characteristics, including comorbidities. Data on health care use were available for patients for an average of about 4 months following their start of anticoagulant treatment.
The analysis showed that during the first months on treatment, patients prescribed rivaroxaban averaged 2.11 days of hospitalization for an AF-related episode, compared with 3.02 days for those prescribed warfarin, a statistically significant difference. Hospitalizations for any cause averaged a total of 2.71 days in the rivaroxaban group and 3.87 days in the patients on warfarin, also a significant difference, Dr. Crivera reported.
This difference in days hospitalized translated into reduced hospitalization costs, a roughly $2,000 average difference per patient in actual hospitalization costs in favor of the rivaroxaban patients for all-cause hospitalizations, and an average $1,300 difference per patient for hospitalization costs directly related to AF.
Although the rivaroxaban patients spent an average of $2,700 more per patient on pharmaceuticals for all causes, and an average $2,200 more for AF-related drugs, the total average all-cause and AF-related costs for drugs, hospitalizations, outpatient visits, and emergency department visits were similar in the two subgroups: an average total of $17,590 per patient for the rivaroxaban patients and $18,676 for warfarin patients for all causes, and an average of $7,394 for the rivaroxaban patients and $7,319 for those on warfarin for AF-related care. The between-group differences for both sets of total costs were not statistically significant.
The study was sponsored by Janssen, which along with Bayer markets rivaroxaban (Xarelto). Dr. Crivera is a Janssen employee. Dr. Weitz has been an adviser or a consultant to Janssen and Bayer and to Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Johnson & Johnson, Merck, Pfizer, and Portola.
On Twitter @mitchelzoler
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point: Patients with atrial fibrillation who started on rivaroxaban treatment had total hospitalization and drug costs that were similar to those of patients begun on warfarin.
Major finding: Rivaroxaban-treated patients averaged $17,590 in total all-cause hospitalization and drug costs, compared with $18,676 for warfarin-treated patients.
Data source: A retrospective, matched-cohort study of 4,506 U.S. patients with atrial fibrillation and newly initiated anticoagulant treatment using health records maintained by Humana.
Disclosures: The study was sponsored by Janssen, which along with Bayer markets rivaroxaban (Xarelto). Dr. Crivera is a Janssen employee. Dr. Weitz has been an adviser or a consultant to Janssen and Bayer and to Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Johnson & Johnson, Merck, Pfizer, and Portola.
Losartan fails in hypertrophic cardiomyopathy
CHICAGO – A year of losartan had no effect on left ventricular mass in patients with overt hypertrophic cardiomyopathy, compared with placebo, in the INHERIT trial.
The angiotensin receptor blocker did not alter any secondary endpoint, including maximal wall thickness, left ventricular fibrosis, diastolic function, and exercise capacity.
There also was no significant change in symptoms or resting left ventricular outflow tract (LVOT) gradient, Dr. Anna Axelsson reported at the American Heart Association scientific sessions.
Current therapies for hypertrophic cardiomyopathy (HCM) are palliative for symptoms only.
Losartan is known to reduce heart wall thickening in patients with hypertension, but is only used with caution in patients with HCM because of a suspicion of deleterious side effects.
INHERIT was initiated based on promising effects with angiotensin receptor blockade in animal and human studies, said Dr. Axelsson of Rigshospitalet, University of Copenhagen, and Harvard Medical School, Boston.
Losartan (Cozaar) has been shown to prevent the development of hypertrophy and fibrosis in HCM mice when started in the prehypertrophic phase. Candesartan (Atacand) use in humans was associated with regression of LV hypertrophy and improvement in LV function and exercise capacity in a small pilot study (J. Mol. Diagn. 2009;11:35-41).
INHERIT (Inhibition of the Renin Angiotensin System With Losartan in Patients With Hypertrophic Cardiomyopathy) evenly allocated 133 adults with overt HCM to daily losartan 100 mg or placebo for 12 months. LV mass was 108 g/m2 in the placebo group and 105 g/m2 in the losartan group, and 83% of patients had fibrosis.
Of the 124 patients completing the study, 93% were compliant (> 80%,) as assessed by pill count. Corroborating this was a significant decrease in blood pressure in the losartan group compared with the placebo group (P value = .001), Dr. Axelsson said.
At 12 months, both groups had a small decrease from baseline in LV mass, as assessed by magnetic resonance imaging or computed tomography, but the difference between groups was not statistically significant or clinically relevant (mean difference 1 g/m2), she said.
Adverse events were few, and comparable between the losartan and control groups. Sudden cardiac death occurred in two patients on placebo. One patient in each group had worsening of New York Heart Association class. Two controls and one losartan-treated patient had increases in LVOT gradient of at least 10 mm Hg. One patient discontinued losartan because of angioedema and two discontinued because of unspecified symptoms leading to withdrawal of consent.
“The observed safety suggests that losartan may be used for other indications in patients with obstructive physiology,” Dr. Axelsson said.
When asked whether clinicians can conclude that it’s safe to give patients with obstructive physiology a vasodilator, Dr. Axelsson said, “perhaps it shouldn’t be considered such a contraindication anymore, but it should still be done cautiously.”
Invited discussant Dr. Euan Ashley of Stanford (Calif.) University, said that only 15% of patients in the trial had an LVOT gradient of more than 30 mm Hg at baseline, “so to try and extrapolate that vasodilators are safe in older hypertrophics may be going too far.”
Finally, Dr. Axelsson said the overall results do not “close the door” to treatment with angiotensin receptor blockers in HCM, and that future trials may determine whether ARBs can prevent development of disease in preclinical or earlier stages of HCM.
The ongoing VANISH trial is evaluating valsartan (Diovan) in younger patients with early sarcomeric HCM. Also, the Liberty-HCM study will evaluate the effects of the investigational late sodium channel inhibitor, GS-6615, in patients aged 18-65 years, with symptomatic HCM.
INHERIT was funded by the Danish Heart Foundation and several other Danish research organizations. Dr. Axelsson holds stock with AstraZeneca.
CHICAGO – A year of losartan had no effect on left ventricular mass in patients with overt hypertrophic cardiomyopathy, compared with placebo, in the INHERIT trial.
The angiotensin receptor blocker did not alter any secondary endpoint, including maximal wall thickness, left ventricular fibrosis, diastolic function, and exercise capacity.
There also was no significant change in symptoms or resting left ventricular outflow tract (LVOT) gradient, Dr. Anna Axelsson reported at the American Heart Association scientific sessions.
Current therapies for hypertrophic cardiomyopathy (HCM) are palliative for symptoms only.
Losartan is known to reduce heart wall thickening in patients with hypertension, but is only used with caution in patients with HCM because of a suspicion of deleterious side effects.
INHERIT was initiated based on promising effects with angiotensin receptor blockade in animal and human studies, said Dr. Axelsson of Rigshospitalet, University of Copenhagen, and Harvard Medical School, Boston.
Losartan (Cozaar) has been shown to prevent the development of hypertrophy and fibrosis in HCM mice when started in the prehypertrophic phase. Candesartan (Atacand) use in humans was associated with regression of LV hypertrophy and improvement in LV function and exercise capacity in a small pilot study (J. Mol. Diagn. 2009;11:35-41).
INHERIT (Inhibition of the Renin Angiotensin System With Losartan in Patients With Hypertrophic Cardiomyopathy) evenly allocated 133 adults with overt HCM to daily losartan 100 mg or placebo for 12 months. LV mass was 108 g/m2 in the placebo group and 105 g/m2 in the losartan group, and 83% of patients had fibrosis.
Of the 124 patients completing the study, 93% were compliant (> 80%,) as assessed by pill count. Corroborating this was a significant decrease in blood pressure in the losartan group compared with the placebo group (P value = .001), Dr. Axelsson said.
At 12 months, both groups had a small decrease from baseline in LV mass, as assessed by magnetic resonance imaging or computed tomography, but the difference between groups was not statistically significant or clinically relevant (mean difference 1 g/m2), she said.
Adverse events were few, and comparable between the losartan and control groups. Sudden cardiac death occurred in two patients on placebo. One patient in each group had worsening of New York Heart Association class. Two controls and one losartan-treated patient had increases in LVOT gradient of at least 10 mm Hg. One patient discontinued losartan because of angioedema and two discontinued because of unspecified symptoms leading to withdrawal of consent.
“The observed safety suggests that losartan may be used for other indications in patients with obstructive physiology,” Dr. Axelsson said.
When asked whether clinicians can conclude that it’s safe to give patients with obstructive physiology a vasodilator, Dr. Axelsson said, “perhaps it shouldn’t be considered such a contraindication anymore, but it should still be done cautiously.”
Invited discussant Dr. Euan Ashley of Stanford (Calif.) University, said that only 15% of patients in the trial had an LVOT gradient of more than 30 mm Hg at baseline, “so to try and extrapolate that vasodilators are safe in older hypertrophics may be going too far.”
Finally, Dr. Axelsson said the overall results do not “close the door” to treatment with angiotensin receptor blockers in HCM, and that future trials may determine whether ARBs can prevent development of disease in preclinical or earlier stages of HCM.
The ongoing VANISH trial is evaluating valsartan (Diovan) in younger patients with early sarcomeric HCM. Also, the Liberty-HCM study will evaluate the effects of the investigational late sodium channel inhibitor, GS-6615, in patients aged 18-65 years, with symptomatic HCM.
INHERIT was funded by the Danish Heart Foundation and several other Danish research organizations. Dr. Axelsson holds stock with AstraZeneca.
CHICAGO – A year of losartan had no effect on left ventricular mass in patients with overt hypertrophic cardiomyopathy, compared with placebo, in the INHERIT trial.
The angiotensin receptor blocker did not alter any secondary endpoint, including maximal wall thickness, left ventricular fibrosis, diastolic function, and exercise capacity.
There also was no significant change in symptoms or resting left ventricular outflow tract (LVOT) gradient, Dr. Anna Axelsson reported at the American Heart Association scientific sessions.
Current therapies for hypertrophic cardiomyopathy (HCM) are palliative for symptoms only.
Losartan is known to reduce heart wall thickening in patients with hypertension, but is only used with caution in patients with HCM because of a suspicion of deleterious side effects.
INHERIT was initiated based on promising effects with angiotensin receptor blockade in animal and human studies, said Dr. Axelsson of Rigshospitalet, University of Copenhagen, and Harvard Medical School, Boston.
Losartan (Cozaar) has been shown to prevent the development of hypertrophy and fibrosis in HCM mice when started in the prehypertrophic phase. Candesartan (Atacand) use in humans was associated with regression of LV hypertrophy and improvement in LV function and exercise capacity in a small pilot study (J. Mol. Diagn. 2009;11:35-41).
INHERIT (Inhibition of the Renin Angiotensin System With Losartan in Patients With Hypertrophic Cardiomyopathy) evenly allocated 133 adults with overt HCM to daily losartan 100 mg or placebo for 12 months. LV mass was 108 g/m2 in the placebo group and 105 g/m2 in the losartan group, and 83% of patients had fibrosis.
Of the 124 patients completing the study, 93% were compliant (> 80%,) as assessed by pill count. Corroborating this was a significant decrease in blood pressure in the losartan group compared with the placebo group (P value = .001), Dr. Axelsson said.
At 12 months, both groups had a small decrease from baseline in LV mass, as assessed by magnetic resonance imaging or computed tomography, but the difference between groups was not statistically significant or clinically relevant (mean difference 1 g/m2), she said.
Adverse events were few, and comparable between the losartan and control groups. Sudden cardiac death occurred in two patients on placebo. One patient in each group had worsening of New York Heart Association class. Two controls and one losartan-treated patient had increases in LVOT gradient of at least 10 mm Hg. One patient discontinued losartan because of angioedema and two discontinued because of unspecified symptoms leading to withdrawal of consent.
“The observed safety suggests that losartan may be used for other indications in patients with obstructive physiology,” Dr. Axelsson said.
When asked whether clinicians can conclude that it’s safe to give patients with obstructive physiology a vasodilator, Dr. Axelsson said, “perhaps it shouldn’t be considered such a contraindication anymore, but it should still be done cautiously.”
Invited discussant Dr. Euan Ashley of Stanford (Calif.) University, said that only 15% of patients in the trial had an LVOT gradient of more than 30 mm Hg at baseline, “so to try and extrapolate that vasodilators are safe in older hypertrophics may be going too far.”
Finally, Dr. Axelsson said the overall results do not “close the door” to treatment with angiotensin receptor blockers in HCM, and that future trials may determine whether ARBs can prevent development of disease in preclinical or earlier stages of HCM.
The ongoing VANISH trial is evaluating valsartan (Diovan) in younger patients with early sarcomeric HCM. Also, the Liberty-HCM study will evaluate the effects of the investigational late sodium channel inhibitor, GS-6615, in patients aged 18-65 years, with symptomatic HCM.
INHERIT was funded by the Danish Heart Foundation and several other Danish research organizations. Dr. Axelsson holds stock with AstraZeneca.
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point: Losartan 100 mg daily did not alter LV mass or any secondary endpoint in patients with hypertrophic cardiomyopathy.
Major finding: At 12 months, the change in LV mass from baseline was similar between patients receiving losartan and those on placebo.
Data source: INHERIT, a double-blind, randomized, placebo controlled trial of 133 patients with hypertrophic cardiomyopathy.
Disclosures: The Danish Heart Foundation and several other Danish research organizations funded the study. Dr. Axelsson holds stock with AstraZeneca.
Polypharmacy linked to increased AFib bleeding
CHICAGO – Atrial fibrillation patients treated with an anticoagulant plus four or more other medications had significantly more bleeding complications than did those on fewer medications in a retrospective study.
“This is the first study examining polypharmacy in patients with nonvalvular atrial fibrillation,” Dr. Jonathan P. Piccini said at the American Heart Association Scientific Sessions. The main consequence is, “We should do everything we can to reduce the number of medications a patient receives,” he said.
His exploratory, retrospective analysis of data from more than 14,000 patients enrolled in an anticoagulant treatment trial showed that atrial fibrillation patients on an oral anticoagulant and taking a total of 10 or more medications concurrently had a 46% increased rate of a major bleed or clinically relevant nonmajor bleed. Patients taking five to nine medications had a 17% increased rate for this primary bleeding endpoint. Both increases were statistically significant, reported Dr. Piccini, a cardiologist at Duke University in Durham, N.C. Major bleeds alone were 90% higher in patients on at least 10 drugs and 47% higher in patients on 5-9 total drugs, compared with those on 4 or fewer, also statistically significant differences.
“Increasing medication use was associated with an increased risk of bleeding but not stroke,” he said. In addition, because the data came from a large trial that had compared the new oral anticoagulant rivaroxaban (Xarelto) with warfarin, the analysis was able to show that this polypharmacy effect was similar regardless of which anticoagulant patients took.
Polypharmacy was common among the 14,264 patients (average age, 73 years) enrolled in the trial, as 51% were on 5-9 drugs and 13% were on 10 or more drugs, with a minority of 36% forming the comparator group taking 4 or fewer medications.
The analysis used data collected from 14,264 patients enrolled in the ROCKET AF (Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared With Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation) trial, which was designed as a pivotal trial comparing the safety and efficacy of the new oral anticoagulant rivaroxaban with warfarin for stroke prevention in patients with nonvalvular atrial fibrillation (N. Engl. J. Med. 2011;365:883-91). Dr. Piccini and his associates assessed various measures of bleeding as well as strokes, vascular deaths, and all-cause deaths in the patients by their number of concomitant medications based on data collected at baseline when patients entered the trial. The relative hazard rates the investigators calculated were adjusted for all “clinically important covariates,” but Dr. Piccini acknowledged that residual confounding accounted for by the adjustments likely remained.
All-cause death was also significantly increased in patients taking at least 10 (a 43% increase) or 5-9 drugs (a 25% increase), compared with patients on 4 or fewer drugs. But the rate of stroke or nonstroke embolism was not significantly increased among patients on five or more drugs. The combined rate of stroke, nonstroke embolism, and vascular death was a statistically significant 25% higher in patients on at least 10 drugs, compared with those on 4 or fewer drugs, but there was no significant increase in patients on 5-9 drugs.
The link between higher numbers of concomitant medications and an increased bleeding rate likely has two explanations, Dr. Piccini said. It could happen because the drugs themselves promote bleeding, and because the need to prescribe multiple drugs is a marker for patients who are sicker and have more comorbidities and a higher underlying risk of bleeding.
ROCKET AF was sponsored by Janssen and Bayer. Dr. Piccini has been a consultant to and has received research funding from Janssen, Bayer, and several other companies.
On Twitter @mitchelzoler
The message to physicians should be to assess patients at regular intervals to make sure they really still need all their medications, and try to reduce the number whenever possible.
One of the dangers in using a new oral anticoagulant is that patients are often also taking drugs that inhibit CYP3A4 enzymes, P-glycoprotein, or both. Among patients in Dr. Piccini’s study, 19% of those on 5-9 drugs and 26% of those on 10 or more were on at least one drug that inhibited P-glycoprotein and on at least one drug that was a CYP3A4 inhibitor. The list Dr. Piccini presented of the drugs patients received that fell in this category included amiodarone, diltiazem, verapamil, and erythromycin.
Also, these patients can be on other antithrombotic or antiplatelet drugs. We regularly assess patients’ coagulation status when they are on warfarin, but not when they are on rivaroxaban or another new oral anticoagulant. If you have patients on 10 or more drugs you need to be careful, and must also determine whether they can come off any of the drugs. You need to ask yourself, “What can I stop?” This is particularly true for patients also on aspirin, a thienopyridine, or a nonsteroidal anti-inflammatory drug, which all increase bleeding risk.
Dr. Jeffrey Weitz is professor of medicine and director of the Juravinski Hospital and Cancer Centre of McMaster University in Hamilton, Ont. He has been an adviser or consultant to Janssen, Bayer, and several drug manufacturers. He made these comments in an interview.
The message to physicians should be to assess patients at regular intervals to make sure they really still need all their medications, and try to reduce the number whenever possible.
One of the dangers in using a new oral anticoagulant is that patients are often also taking drugs that inhibit CYP3A4 enzymes, P-glycoprotein, or both. Among patients in Dr. Piccini’s study, 19% of those on 5-9 drugs and 26% of those on 10 or more were on at least one drug that inhibited P-glycoprotein and on at least one drug that was a CYP3A4 inhibitor. The list Dr. Piccini presented of the drugs patients received that fell in this category included amiodarone, diltiazem, verapamil, and erythromycin.
Also, these patients can be on other antithrombotic or antiplatelet drugs. We regularly assess patients’ coagulation status when they are on warfarin, but not when they are on rivaroxaban or another new oral anticoagulant. If you have patients on 10 or more drugs you need to be careful, and must also determine whether they can come off any of the drugs. You need to ask yourself, “What can I stop?” This is particularly true for patients also on aspirin, a thienopyridine, or a nonsteroidal anti-inflammatory drug, which all increase bleeding risk.
Dr. Jeffrey Weitz is professor of medicine and director of the Juravinski Hospital and Cancer Centre of McMaster University in Hamilton, Ont. He has been an adviser or consultant to Janssen, Bayer, and several drug manufacturers. He made these comments in an interview.
The message to physicians should be to assess patients at regular intervals to make sure they really still need all their medications, and try to reduce the number whenever possible.
One of the dangers in using a new oral anticoagulant is that patients are often also taking drugs that inhibit CYP3A4 enzymes, P-glycoprotein, or both. Among patients in Dr. Piccini’s study, 19% of those on 5-9 drugs and 26% of those on 10 or more were on at least one drug that inhibited P-glycoprotein and on at least one drug that was a CYP3A4 inhibitor. The list Dr. Piccini presented of the drugs patients received that fell in this category included amiodarone, diltiazem, verapamil, and erythromycin.
Also, these patients can be on other antithrombotic or antiplatelet drugs. We regularly assess patients’ coagulation status when they are on warfarin, but not when they are on rivaroxaban or another new oral anticoagulant. If you have patients on 10 or more drugs you need to be careful, and must also determine whether they can come off any of the drugs. You need to ask yourself, “What can I stop?” This is particularly true for patients also on aspirin, a thienopyridine, or a nonsteroidal anti-inflammatory drug, which all increase bleeding risk.
Dr. Jeffrey Weitz is professor of medicine and director of the Juravinski Hospital and Cancer Centre of McMaster University in Hamilton, Ont. He has been an adviser or consultant to Janssen, Bayer, and several drug manufacturers. He made these comments in an interview.
CHICAGO – Atrial fibrillation patients treated with an anticoagulant plus four or more other medications had significantly more bleeding complications than did those on fewer medications in a retrospective study.
“This is the first study examining polypharmacy in patients with nonvalvular atrial fibrillation,” Dr. Jonathan P. Piccini said at the American Heart Association Scientific Sessions. The main consequence is, “We should do everything we can to reduce the number of medications a patient receives,” he said.
His exploratory, retrospective analysis of data from more than 14,000 patients enrolled in an anticoagulant treatment trial showed that atrial fibrillation patients on an oral anticoagulant and taking a total of 10 or more medications concurrently had a 46% increased rate of a major bleed or clinically relevant nonmajor bleed. Patients taking five to nine medications had a 17% increased rate for this primary bleeding endpoint. Both increases were statistically significant, reported Dr. Piccini, a cardiologist at Duke University in Durham, N.C. Major bleeds alone were 90% higher in patients on at least 10 drugs and 47% higher in patients on 5-9 total drugs, compared with those on 4 or fewer, also statistically significant differences.
“Increasing medication use was associated with an increased risk of bleeding but not stroke,” he said. In addition, because the data came from a large trial that had compared the new oral anticoagulant rivaroxaban (Xarelto) with warfarin, the analysis was able to show that this polypharmacy effect was similar regardless of which anticoagulant patients took.
Polypharmacy was common among the 14,264 patients (average age, 73 years) enrolled in the trial, as 51% were on 5-9 drugs and 13% were on 10 or more drugs, with a minority of 36% forming the comparator group taking 4 or fewer medications.
The analysis used data collected from 14,264 patients enrolled in the ROCKET AF (Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared With Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation) trial, which was designed as a pivotal trial comparing the safety and efficacy of the new oral anticoagulant rivaroxaban with warfarin for stroke prevention in patients with nonvalvular atrial fibrillation (N. Engl. J. Med. 2011;365:883-91). Dr. Piccini and his associates assessed various measures of bleeding as well as strokes, vascular deaths, and all-cause deaths in the patients by their number of concomitant medications based on data collected at baseline when patients entered the trial. The relative hazard rates the investigators calculated were adjusted for all “clinically important covariates,” but Dr. Piccini acknowledged that residual confounding accounted for by the adjustments likely remained.
All-cause death was also significantly increased in patients taking at least 10 (a 43% increase) or 5-9 drugs (a 25% increase), compared with patients on 4 or fewer drugs. But the rate of stroke or nonstroke embolism was not significantly increased among patients on five or more drugs. The combined rate of stroke, nonstroke embolism, and vascular death was a statistically significant 25% higher in patients on at least 10 drugs, compared with those on 4 or fewer drugs, but there was no significant increase in patients on 5-9 drugs.
The link between higher numbers of concomitant medications and an increased bleeding rate likely has two explanations, Dr. Piccini said. It could happen because the drugs themselves promote bleeding, and because the need to prescribe multiple drugs is a marker for patients who are sicker and have more comorbidities and a higher underlying risk of bleeding.
ROCKET AF was sponsored by Janssen and Bayer. Dr. Piccini has been a consultant to and has received research funding from Janssen, Bayer, and several other companies.
On Twitter @mitchelzoler
CHICAGO – Atrial fibrillation patients treated with an anticoagulant plus four or more other medications had significantly more bleeding complications than did those on fewer medications in a retrospective study.
“This is the first study examining polypharmacy in patients with nonvalvular atrial fibrillation,” Dr. Jonathan P. Piccini said at the American Heart Association Scientific Sessions. The main consequence is, “We should do everything we can to reduce the number of medications a patient receives,” he said.
His exploratory, retrospective analysis of data from more than 14,000 patients enrolled in an anticoagulant treatment trial showed that atrial fibrillation patients on an oral anticoagulant and taking a total of 10 or more medications concurrently had a 46% increased rate of a major bleed or clinically relevant nonmajor bleed. Patients taking five to nine medications had a 17% increased rate for this primary bleeding endpoint. Both increases were statistically significant, reported Dr. Piccini, a cardiologist at Duke University in Durham, N.C. Major bleeds alone were 90% higher in patients on at least 10 drugs and 47% higher in patients on 5-9 total drugs, compared with those on 4 or fewer, also statistically significant differences.
“Increasing medication use was associated with an increased risk of bleeding but not stroke,” he said. In addition, because the data came from a large trial that had compared the new oral anticoagulant rivaroxaban (Xarelto) with warfarin, the analysis was able to show that this polypharmacy effect was similar regardless of which anticoagulant patients took.
Polypharmacy was common among the 14,264 patients (average age, 73 years) enrolled in the trial, as 51% were on 5-9 drugs and 13% were on 10 or more drugs, with a minority of 36% forming the comparator group taking 4 or fewer medications.
The analysis used data collected from 14,264 patients enrolled in the ROCKET AF (Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared With Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation) trial, which was designed as a pivotal trial comparing the safety and efficacy of the new oral anticoagulant rivaroxaban with warfarin for stroke prevention in patients with nonvalvular atrial fibrillation (N. Engl. J. Med. 2011;365:883-91). Dr. Piccini and his associates assessed various measures of bleeding as well as strokes, vascular deaths, and all-cause deaths in the patients by their number of concomitant medications based on data collected at baseline when patients entered the trial. The relative hazard rates the investigators calculated were adjusted for all “clinically important covariates,” but Dr. Piccini acknowledged that residual confounding accounted for by the adjustments likely remained.
All-cause death was also significantly increased in patients taking at least 10 (a 43% increase) or 5-9 drugs (a 25% increase), compared with patients on 4 or fewer drugs. But the rate of stroke or nonstroke embolism was not significantly increased among patients on five or more drugs. The combined rate of stroke, nonstroke embolism, and vascular death was a statistically significant 25% higher in patients on at least 10 drugs, compared with those on 4 or fewer drugs, but there was no significant increase in patients on 5-9 drugs.
The link between higher numbers of concomitant medications and an increased bleeding rate likely has two explanations, Dr. Piccini said. It could happen because the drugs themselves promote bleeding, and because the need to prescribe multiple drugs is a marker for patients who are sicker and have more comorbidities and a higher underlying risk of bleeding.
ROCKET AF was sponsored by Janssen and Bayer. Dr. Piccini has been a consultant to and has received research funding from Janssen, Bayer, and several other companies.
On Twitter @mitchelzoler
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point: Among atrial fibrillation patients on an oral anticoagulant, those on at least four other medications concurrently had a significantly increased bleeding rate, compared with those on three or fewer.
Major finding: Patients on 5-9 drugs had 17% more bleeds, and those on 10 or more drugs had 46% more bleeds, than patients on fewer drugs.
Data source: A retrospective analysis of 14,264 patients enrolled in the ROCKET AF trial.
Disclosures: ROCKET AF was sponsored by Janssen and Bayer. Dr. Piccini has been a consultant to and has received research funding from Janssen, Bayer, and several other companies.
Surprising new findings on hypertension in elderly blacks
CHICAGO– Older black Americans are more likely to have hypertension and less likely to have it under control than are their white counterparts, yet paradoxically they are also on more antihypertensive medications, according to a new analysis from the landmark ARIC study.
This finding is at odds with the conventional wisdom, which holds that the higher rate of poorly controlled hypertension in black patients is due to racial disparities in treatment, with blacks receiving less adequate treatment.
“In our study it appeared they were very well treated, yet they still didn’t achieve the same blood pressure as the older white patients,” Dr. Michael D. Miedema said in presenting the ARIC (Atherosclerosis Risk in Communities) findings at the American Heart Association Scientific Sessions.
Indeed, 88% of elderly black hypertensive patients were on at least one antihypertensive medication, compared with 71% of white hypertensives patients. Black hypertensive patients were also more likely to be on three or more antihypertensive drugs, by a margin of 27% to 16%.
“So it’s not for lack of antihypertensive medication use,” according to Dr. Miedema, a cardiologist at the Minneapolis Heart Institute.
The ARIC study is an ongoing longitudinal study of cardiovascular disease in older black and white men and women. The National Heart, Lung, and Blood Institute–funded study began in the late 1980s. Dr. Miedema’s analysis included 6,088 participants in the fifth clinical visit, which took place in 2011-2013. The subjects’ mean age was 75.6 years, and 23% were black. A total of 82% of subjects had hypertension as defined by blood pressures greater than 140/90 mm Hg; 81% of those with hypertension were aware of that fact. Put another way, nearly 20% of elderly hypertensive subjects were unaware they had hypertension, he noted.
One-third of ARIC participants had diabetes, 30% had cardiovascular disease, and 37% had chronic kidney disease. The prevalence of hypertension in subjects with diabetes or chronic kidney disease was 92%; in those without either comorbidity, it was 69%.
Of the total study population, 63% were at the blood pressure goals defined by JNC-7. This figure shot up to 79% using the less aggressive blood pressure goals recommended in the 2014 expert panel report (JAMA 2014;311:502-20), namely, targets of less than 150/90 mm Hg in individuals aged 60 years or older, and 140/90 in those with diabetes or chronic kidney disease. Thus, one in six elderly subjects in ARIC were reclassified from having high blood pressure to normal blood pressure through the use of the 2014 guidelines.
“Despite the more lenient 2014 blood pressure goals and a high rate of antihypertensive medication use, almost 20% of our total sample were not at goal blood pressure as defined by the 2014 expert committee. This high rate of uncontrolled blood pressure may be caused by a lack of awareness, treatment inertia, or medication nonadherence; it’s hard to say. But further efforts aimed at improving detection and control of hypertension in older individuals remain warranted,” Dr. Miedema commented.
Regardless of whether the 2003 JNC-7 guidelines or the 2014 expert panel recommendations were applied as the yardstick, elderly black patients with hypertension were an absolute 10%-15% less likely to be at target blood pressure, compared with their white counterparts.
It’s possible that the worse blood pressure control in black patients was brought about in part to less-appropriate drug prescribing. The most commonly used class of antihypertensive medications in both black and white hypertensives was ACE inhibitors/angiotensin receptor blockers (ARBs), even though both JNC-7 and the 2014 expert panel guidelines recommend diuretics or calcium channel blockers as first-line antihypertensive therapy in black patients. That’s because black patients with hypertension are often in a low renin state, in which ACE inhibitors and ARBs are less effective. Confusing the picture, however, is the fact that diabetes and chronic kidney disease were particularly prevalent among older black hypertensive patients, and ACE inhibitors and ARBs are preferentially guideline-recommended in patients with those diseases.
Dr. Miedema reported having no financial conflicts of interest.
CHICAGO– Older black Americans are more likely to have hypertension and less likely to have it under control than are their white counterparts, yet paradoxically they are also on more antihypertensive medications, according to a new analysis from the landmark ARIC study.
This finding is at odds with the conventional wisdom, which holds that the higher rate of poorly controlled hypertension in black patients is due to racial disparities in treatment, with blacks receiving less adequate treatment.
“In our study it appeared they were very well treated, yet they still didn’t achieve the same blood pressure as the older white patients,” Dr. Michael D. Miedema said in presenting the ARIC (Atherosclerosis Risk in Communities) findings at the American Heart Association Scientific Sessions.
Indeed, 88% of elderly black hypertensive patients were on at least one antihypertensive medication, compared with 71% of white hypertensives patients. Black hypertensive patients were also more likely to be on three or more antihypertensive drugs, by a margin of 27% to 16%.
“So it’s not for lack of antihypertensive medication use,” according to Dr. Miedema, a cardiologist at the Minneapolis Heart Institute.
The ARIC study is an ongoing longitudinal study of cardiovascular disease in older black and white men and women. The National Heart, Lung, and Blood Institute–funded study began in the late 1980s. Dr. Miedema’s analysis included 6,088 participants in the fifth clinical visit, which took place in 2011-2013. The subjects’ mean age was 75.6 years, and 23% were black. A total of 82% of subjects had hypertension as defined by blood pressures greater than 140/90 mm Hg; 81% of those with hypertension were aware of that fact. Put another way, nearly 20% of elderly hypertensive subjects were unaware they had hypertension, he noted.
One-third of ARIC participants had diabetes, 30% had cardiovascular disease, and 37% had chronic kidney disease. The prevalence of hypertension in subjects with diabetes or chronic kidney disease was 92%; in those without either comorbidity, it was 69%.
Of the total study population, 63% were at the blood pressure goals defined by JNC-7. This figure shot up to 79% using the less aggressive blood pressure goals recommended in the 2014 expert panel report (JAMA 2014;311:502-20), namely, targets of less than 150/90 mm Hg in individuals aged 60 years or older, and 140/90 in those with diabetes or chronic kidney disease. Thus, one in six elderly subjects in ARIC were reclassified from having high blood pressure to normal blood pressure through the use of the 2014 guidelines.
“Despite the more lenient 2014 blood pressure goals and a high rate of antihypertensive medication use, almost 20% of our total sample were not at goal blood pressure as defined by the 2014 expert committee. This high rate of uncontrolled blood pressure may be caused by a lack of awareness, treatment inertia, or medication nonadherence; it’s hard to say. But further efforts aimed at improving detection and control of hypertension in older individuals remain warranted,” Dr. Miedema commented.
Regardless of whether the 2003 JNC-7 guidelines or the 2014 expert panel recommendations were applied as the yardstick, elderly black patients with hypertension were an absolute 10%-15% less likely to be at target blood pressure, compared with their white counterparts.
It’s possible that the worse blood pressure control in black patients was brought about in part to less-appropriate drug prescribing. The most commonly used class of antihypertensive medications in both black and white hypertensives was ACE inhibitors/angiotensin receptor blockers (ARBs), even though both JNC-7 and the 2014 expert panel guidelines recommend diuretics or calcium channel blockers as first-line antihypertensive therapy in black patients. That’s because black patients with hypertension are often in a low renin state, in which ACE inhibitors and ARBs are less effective. Confusing the picture, however, is the fact that diabetes and chronic kidney disease were particularly prevalent among older black hypertensive patients, and ACE inhibitors and ARBs are preferentially guideline-recommended in patients with those diseases.
Dr. Miedema reported having no financial conflicts of interest.
CHICAGO– Older black Americans are more likely to have hypertension and less likely to have it under control than are their white counterparts, yet paradoxically they are also on more antihypertensive medications, according to a new analysis from the landmark ARIC study.
This finding is at odds with the conventional wisdom, which holds that the higher rate of poorly controlled hypertension in black patients is due to racial disparities in treatment, with blacks receiving less adequate treatment.
“In our study it appeared they were very well treated, yet they still didn’t achieve the same blood pressure as the older white patients,” Dr. Michael D. Miedema said in presenting the ARIC (Atherosclerosis Risk in Communities) findings at the American Heart Association Scientific Sessions.
Indeed, 88% of elderly black hypertensive patients were on at least one antihypertensive medication, compared with 71% of white hypertensives patients. Black hypertensive patients were also more likely to be on three or more antihypertensive drugs, by a margin of 27% to 16%.
“So it’s not for lack of antihypertensive medication use,” according to Dr. Miedema, a cardiologist at the Minneapolis Heart Institute.
The ARIC study is an ongoing longitudinal study of cardiovascular disease in older black and white men and women. The National Heart, Lung, and Blood Institute–funded study began in the late 1980s. Dr. Miedema’s analysis included 6,088 participants in the fifth clinical visit, which took place in 2011-2013. The subjects’ mean age was 75.6 years, and 23% were black. A total of 82% of subjects had hypertension as defined by blood pressures greater than 140/90 mm Hg; 81% of those with hypertension were aware of that fact. Put another way, nearly 20% of elderly hypertensive subjects were unaware they had hypertension, he noted.
One-third of ARIC participants had diabetes, 30% had cardiovascular disease, and 37% had chronic kidney disease. The prevalence of hypertension in subjects with diabetes or chronic kidney disease was 92%; in those without either comorbidity, it was 69%.
Of the total study population, 63% were at the blood pressure goals defined by JNC-7. This figure shot up to 79% using the less aggressive blood pressure goals recommended in the 2014 expert panel report (JAMA 2014;311:502-20), namely, targets of less than 150/90 mm Hg in individuals aged 60 years or older, and 140/90 in those with diabetes or chronic kidney disease. Thus, one in six elderly subjects in ARIC were reclassified from having high blood pressure to normal blood pressure through the use of the 2014 guidelines.
“Despite the more lenient 2014 blood pressure goals and a high rate of antihypertensive medication use, almost 20% of our total sample were not at goal blood pressure as defined by the 2014 expert committee. This high rate of uncontrolled blood pressure may be caused by a lack of awareness, treatment inertia, or medication nonadherence; it’s hard to say. But further efforts aimed at improving detection and control of hypertension in older individuals remain warranted,” Dr. Miedema commented.
Regardless of whether the 2003 JNC-7 guidelines or the 2014 expert panel recommendations were applied as the yardstick, elderly black patients with hypertension were an absolute 10%-15% less likely to be at target blood pressure, compared with their white counterparts.
It’s possible that the worse blood pressure control in black patients was brought about in part to less-appropriate drug prescribing. The most commonly used class of antihypertensive medications in both black and white hypertensives was ACE inhibitors/angiotensin receptor blockers (ARBs), even though both JNC-7 and the 2014 expert panel guidelines recommend diuretics or calcium channel blockers as first-line antihypertensive therapy in black patients. That’s because black patients with hypertension are often in a low renin state, in which ACE inhibitors and ARBs are less effective. Confusing the picture, however, is the fact that diabetes and chronic kidney disease were particularly prevalent among older black hypertensive patients, and ACE inhibitors and ARBs are preferentially guideline-recommended in patients with those diseases.
Dr. Miedema reported having no financial conflicts of interest.
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point: Older black patients with hypertension are less likely, by an absolute 10%-15%, to be at goal blood pressure than are their white hypertensive counterparts, despite taking more antihypertensive drugs.
Major finding: 88% of elderly black individuals with hypertension were on at least one antihypertensive agent and 27% were on three or more, compared with rates of 71% and 16%, respectively, in older white hypertensive subjects.
Data source: The Atherosclerosis Risk In Communities (ARIC) study is an ongoing longitudinal observational study.
Disclosures: The ARIC study is funded by the National Heart, Lung, and Blood Institute. The presenter reported having no financial conflicts.
TOPCAT reconsidered: Say ‘nyet’ to Russian data
CHICAGO – Something smells fishy about the results from Russia and Georgia in the TOPCAT trial, according to a study reappraisal conducted by the trial’s leaders.
TOPCAT (Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist) was a randomized, double-blind, placebo-controlled trial of spironolactone for the treatment of heart failure with preserved ejection fraction (HFpEF) in 3,445 patients in six countries. The primary outcome was negative, as presented at last year’s AHA scientific sessions and later published (N. Engl. J. Med. 2014;370:1383-92). However, a new post hoc analysis casts doubt on the validity of the results reported from Russia and Georgia, countries that contributed 49% of TOPCAT participants, Dr. Marc A. Pfeffer reported at the American Heart Association scientific sessions.
The patient outcomes reported from Russia and Georgia were spectacularly at odds with those reported from the United States, Canada, Argentina, and Brazil. Upon careful scrutiny, it appears likely that many Russian and Georgian patients either did not actually have HFpEF or were not taking their spironolactone. And if the results from the two Eastern European countries are put aside, then spironolactone markedly reduced the rate of the primary composite outcome – cardiovascular death or hospitalization for management of heart failure – in the 1,767 study participants in the Americas.
Indeed, the primary composite outcome occurred in 27.3% of spironolactone-treated subjects in the Western Hemisphere during a mean 3.3 years of follow-up, for an event rate of 10.4 per 100 patient-years, compared with rates of 31.8% and 12.6 per 100 patient-years in placebo-treated controls. That translates to a highly significant, 18% relative risk reduction (P = .026) in the primary outcome in spironolactone-treated HFpEF patients in the Americas. Cardiovascular mortality was reduced by 26% and heart failure hospitalization was reduced by 18%, according to Dr. Pfeffer, professor of medicine at Harvard University, Boston.
A post hoc analysis such as this would ordinarily be viewed as hypothesis-generating and nondefinitive. But this is a special situation, according to the cardiologist, who noted that guidelines offer no recommendations for the treatment of HFpEF, which now accounts for roughly 50% of all cases of heart failure.
“HFpEF is a growing part of the heart failure syndrome; it’s a frustrating part of the heart failure syndrome. And if we can improve the prognosis of the 40%-50% of people with symptomatic HFpEF by stating that our observation in the Americas was that spironolactone was associated with reduced cardiovascular deaths as well as hospitalizations for heart failure, then this should be taken into account. Since we don’t have other things we can do for these patients, I bring this to your attention,” Dr. Pfeffer said.
Among the major tip-offs that the Russian/Georgian TOPCAT data were dodgy was the post hoc finding that all-cause mortality, irrespective of treatment, was 21.8% in the Americas but a mere 8.4% in Eastern Europe.
“When I look at anyone’s clinical trial, if I want to ask about the severity of illness, I go to all-cause mortality. And here it was markedly different,” he observed.
Indeed, life-table analyses showed that the Russian/Georgian HFpEF subjects had a life expectancy typical of the region’s general population, whereas death rates for HFpEF enrollees from the Americas were several-fold higher than expected for age- and gender-matched controls.
Discussant Dr. Judith S. Hochman issued the usual caveats about the hazards of drawing conclusions from post hoc analyses of overall negative clinical trials, but then went on to agree with Dr. Pfeffer’s conclusions.
“I conclude, as does Dr. Pfeffer, that it’s reasonable to try mineralocorticoid receptor antagonists for symptomatic HFpEF patients with anticipated risks similar to those enrolled in the Americas. ... This is a growing condition and we have no other treatments,” said Dr. Hochman, professor and associate director of cardiology at New York University.
A key factor in her thinking was the mechanistic plausibility of the differential subgroup treatment effects, she added. For example, hyperkalemia – a well-known side effect of spironolactone – was 3.5-fold more common in patients randomized to spironolactone than in placebo patients in the Americas, as would be expected; but hyperkalemia was equally infrequent in both treatment arms in Russia and Georgia. Conversely, hypokalemia was 49% less likely with spironolactone than placebo in the Americas, yet there was no difference in the incidence of this side effect according to treatment status in Eastern Europe.
Moreover, systolic blood pressure fell by a placebo-subtracted 4.2 mm Hg at 1 year in spironolactone-treated patients in the Americas but was unchanged in Russian and Georgian patients. And while doubling of creatinine levels to above normal range was 60% more common with spironolactone than placebo in the Americas, rates were identical in the two treatment arms in Russia and Georgia.
“There was a physiologic response to spironolactone that paralleled an apparent outcome response,” Dr. Hochman concluded.
As for the discordant Eastern European data, she observed that confirming the diagnosis of HFpEF may be difficult: “Dyspnea, orthopnea, fatigue, lower extremity edema – all of these may be caused by other conditions. So it’s very complicated.”
In contrast to heart failure with reduced ejection fraction, which has seen enormous treatment advances in recent years, not much progress has been made in HFpEF. For that to occur, Dr. Hochman said, it will be essential to come up with refined, objective diagnostic criteria for use in clinical trials. Perhaps echocardiographic findings or elevated natriuretic peptide levels will fill that role, she added.
However, Dr. Pfeffer said that, much to his disappointment, he and other investigators have not seen a consistent correlation between higher baseline brain natriuretic peptide levels and greater clinical response to mineralocorticoid receptor antagonist therapy.
Asked what sort of oversight he and the other TOPCAT leaders had over the Russian and Georgian study sites, Dr. Pfeffer replied that the National Institutes of Health–sponsored study was underfunded for such monitoring.
“As one of the leaders of the trial, there are a lot of things that I would have wished to have done differently,” he said. “I have to stand here and say the amount that you get is inadequate to do what happens in industry-sponsored trials if there’s a perceived problem.”
Both Dr. Pfeffer and Dr. Hochman emphasized the critical importance of careful monitoring of serum potassium and creatinine when prescribing spironolactone in patients with HFpEF. But with regular monitoring, Dr. Pfeffer observed, the risk of major elevations is reassuringly low. For example, with monitoring of serum creatinine at every clinic visit and dose change as per TOPCAT protocol, the incidence of a level of 3.0 mg/dL or more was 10% with spironolactone and not significantly different at 9% with placebo in the Americas.
Dr. Pfeffer reported having received consultant fees from 20 pharmaceutical or medical device companies.
CHICAGO – Something smells fishy about the results from Russia and Georgia in the TOPCAT trial, according to a study reappraisal conducted by the trial’s leaders.
TOPCAT (Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist) was a randomized, double-blind, placebo-controlled trial of spironolactone for the treatment of heart failure with preserved ejection fraction (HFpEF) in 3,445 patients in six countries. The primary outcome was negative, as presented at last year’s AHA scientific sessions and later published (N. Engl. J. Med. 2014;370:1383-92). However, a new post hoc analysis casts doubt on the validity of the results reported from Russia and Georgia, countries that contributed 49% of TOPCAT participants, Dr. Marc A. Pfeffer reported at the American Heart Association scientific sessions.
The patient outcomes reported from Russia and Georgia were spectacularly at odds with those reported from the United States, Canada, Argentina, and Brazil. Upon careful scrutiny, it appears likely that many Russian and Georgian patients either did not actually have HFpEF or were not taking their spironolactone. And if the results from the two Eastern European countries are put aside, then spironolactone markedly reduced the rate of the primary composite outcome – cardiovascular death or hospitalization for management of heart failure – in the 1,767 study participants in the Americas.
Indeed, the primary composite outcome occurred in 27.3% of spironolactone-treated subjects in the Western Hemisphere during a mean 3.3 years of follow-up, for an event rate of 10.4 per 100 patient-years, compared with rates of 31.8% and 12.6 per 100 patient-years in placebo-treated controls. That translates to a highly significant, 18% relative risk reduction (P = .026) in the primary outcome in spironolactone-treated HFpEF patients in the Americas. Cardiovascular mortality was reduced by 26% and heart failure hospitalization was reduced by 18%, according to Dr. Pfeffer, professor of medicine at Harvard University, Boston.
A post hoc analysis such as this would ordinarily be viewed as hypothesis-generating and nondefinitive. But this is a special situation, according to the cardiologist, who noted that guidelines offer no recommendations for the treatment of HFpEF, which now accounts for roughly 50% of all cases of heart failure.
“HFpEF is a growing part of the heart failure syndrome; it’s a frustrating part of the heart failure syndrome. And if we can improve the prognosis of the 40%-50% of people with symptomatic HFpEF by stating that our observation in the Americas was that spironolactone was associated with reduced cardiovascular deaths as well as hospitalizations for heart failure, then this should be taken into account. Since we don’t have other things we can do for these patients, I bring this to your attention,” Dr. Pfeffer said.
Among the major tip-offs that the Russian/Georgian TOPCAT data were dodgy was the post hoc finding that all-cause mortality, irrespective of treatment, was 21.8% in the Americas but a mere 8.4% in Eastern Europe.
“When I look at anyone’s clinical trial, if I want to ask about the severity of illness, I go to all-cause mortality. And here it was markedly different,” he observed.
Indeed, life-table analyses showed that the Russian/Georgian HFpEF subjects had a life expectancy typical of the region’s general population, whereas death rates for HFpEF enrollees from the Americas were several-fold higher than expected for age- and gender-matched controls.
Discussant Dr. Judith S. Hochman issued the usual caveats about the hazards of drawing conclusions from post hoc analyses of overall negative clinical trials, but then went on to agree with Dr. Pfeffer’s conclusions.
“I conclude, as does Dr. Pfeffer, that it’s reasonable to try mineralocorticoid receptor antagonists for symptomatic HFpEF patients with anticipated risks similar to those enrolled in the Americas. ... This is a growing condition and we have no other treatments,” said Dr. Hochman, professor and associate director of cardiology at New York University.
A key factor in her thinking was the mechanistic plausibility of the differential subgroup treatment effects, she added. For example, hyperkalemia – a well-known side effect of spironolactone – was 3.5-fold more common in patients randomized to spironolactone than in placebo patients in the Americas, as would be expected; but hyperkalemia was equally infrequent in both treatment arms in Russia and Georgia. Conversely, hypokalemia was 49% less likely with spironolactone than placebo in the Americas, yet there was no difference in the incidence of this side effect according to treatment status in Eastern Europe.
Moreover, systolic blood pressure fell by a placebo-subtracted 4.2 mm Hg at 1 year in spironolactone-treated patients in the Americas but was unchanged in Russian and Georgian patients. And while doubling of creatinine levels to above normal range was 60% more common with spironolactone than placebo in the Americas, rates were identical in the two treatment arms in Russia and Georgia.
“There was a physiologic response to spironolactone that paralleled an apparent outcome response,” Dr. Hochman concluded.
As for the discordant Eastern European data, she observed that confirming the diagnosis of HFpEF may be difficult: “Dyspnea, orthopnea, fatigue, lower extremity edema – all of these may be caused by other conditions. So it’s very complicated.”
In contrast to heart failure with reduced ejection fraction, which has seen enormous treatment advances in recent years, not much progress has been made in HFpEF. For that to occur, Dr. Hochman said, it will be essential to come up with refined, objective diagnostic criteria for use in clinical trials. Perhaps echocardiographic findings or elevated natriuretic peptide levels will fill that role, she added.
However, Dr. Pfeffer said that, much to his disappointment, he and other investigators have not seen a consistent correlation between higher baseline brain natriuretic peptide levels and greater clinical response to mineralocorticoid receptor antagonist therapy.
Asked what sort of oversight he and the other TOPCAT leaders had over the Russian and Georgian study sites, Dr. Pfeffer replied that the National Institutes of Health–sponsored study was underfunded for such monitoring.
“As one of the leaders of the trial, there are a lot of things that I would have wished to have done differently,” he said. “I have to stand here and say the amount that you get is inadequate to do what happens in industry-sponsored trials if there’s a perceived problem.”
Both Dr. Pfeffer and Dr. Hochman emphasized the critical importance of careful monitoring of serum potassium and creatinine when prescribing spironolactone in patients with HFpEF. But with regular monitoring, Dr. Pfeffer observed, the risk of major elevations is reassuringly low. For example, with monitoring of serum creatinine at every clinic visit and dose change as per TOPCAT protocol, the incidence of a level of 3.0 mg/dL or more was 10% with spironolactone and not significantly different at 9% with placebo in the Americas.
Dr. Pfeffer reported having received consultant fees from 20 pharmaceutical or medical device companies.
CHICAGO – Something smells fishy about the results from Russia and Georgia in the TOPCAT trial, according to a study reappraisal conducted by the trial’s leaders.
TOPCAT (Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist) was a randomized, double-blind, placebo-controlled trial of spironolactone for the treatment of heart failure with preserved ejection fraction (HFpEF) in 3,445 patients in six countries. The primary outcome was negative, as presented at last year’s AHA scientific sessions and later published (N. Engl. J. Med. 2014;370:1383-92). However, a new post hoc analysis casts doubt on the validity of the results reported from Russia and Georgia, countries that contributed 49% of TOPCAT participants, Dr. Marc A. Pfeffer reported at the American Heart Association scientific sessions.
The patient outcomes reported from Russia and Georgia were spectacularly at odds with those reported from the United States, Canada, Argentina, and Brazil. Upon careful scrutiny, it appears likely that many Russian and Georgian patients either did not actually have HFpEF or were not taking their spironolactone. And if the results from the two Eastern European countries are put aside, then spironolactone markedly reduced the rate of the primary composite outcome – cardiovascular death or hospitalization for management of heart failure – in the 1,767 study participants in the Americas.
Indeed, the primary composite outcome occurred in 27.3% of spironolactone-treated subjects in the Western Hemisphere during a mean 3.3 years of follow-up, for an event rate of 10.4 per 100 patient-years, compared with rates of 31.8% and 12.6 per 100 patient-years in placebo-treated controls. That translates to a highly significant, 18% relative risk reduction (P = .026) in the primary outcome in spironolactone-treated HFpEF patients in the Americas. Cardiovascular mortality was reduced by 26% and heart failure hospitalization was reduced by 18%, according to Dr. Pfeffer, professor of medicine at Harvard University, Boston.
A post hoc analysis such as this would ordinarily be viewed as hypothesis-generating and nondefinitive. But this is a special situation, according to the cardiologist, who noted that guidelines offer no recommendations for the treatment of HFpEF, which now accounts for roughly 50% of all cases of heart failure.
“HFpEF is a growing part of the heart failure syndrome; it’s a frustrating part of the heart failure syndrome. And if we can improve the prognosis of the 40%-50% of people with symptomatic HFpEF by stating that our observation in the Americas was that spironolactone was associated with reduced cardiovascular deaths as well as hospitalizations for heart failure, then this should be taken into account. Since we don’t have other things we can do for these patients, I bring this to your attention,” Dr. Pfeffer said.
Among the major tip-offs that the Russian/Georgian TOPCAT data were dodgy was the post hoc finding that all-cause mortality, irrespective of treatment, was 21.8% in the Americas but a mere 8.4% in Eastern Europe.
“When I look at anyone’s clinical trial, if I want to ask about the severity of illness, I go to all-cause mortality. And here it was markedly different,” he observed.
Indeed, life-table analyses showed that the Russian/Georgian HFpEF subjects had a life expectancy typical of the region’s general population, whereas death rates for HFpEF enrollees from the Americas were several-fold higher than expected for age- and gender-matched controls.
Discussant Dr. Judith S. Hochman issued the usual caveats about the hazards of drawing conclusions from post hoc analyses of overall negative clinical trials, but then went on to agree with Dr. Pfeffer’s conclusions.
“I conclude, as does Dr. Pfeffer, that it’s reasonable to try mineralocorticoid receptor antagonists for symptomatic HFpEF patients with anticipated risks similar to those enrolled in the Americas. ... This is a growing condition and we have no other treatments,” said Dr. Hochman, professor and associate director of cardiology at New York University.
A key factor in her thinking was the mechanistic plausibility of the differential subgroup treatment effects, she added. For example, hyperkalemia – a well-known side effect of spironolactone – was 3.5-fold more common in patients randomized to spironolactone than in placebo patients in the Americas, as would be expected; but hyperkalemia was equally infrequent in both treatment arms in Russia and Georgia. Conversely, hypokalemia was 49% less likely with spironolactone than placebo in the Americas, yet there was no difference in the incidence of this side effect according to treatment status in Eastern Europe.
Moreover, systolic blood pressure fell by a placebo-subtracted 4.2 mm Hg at 1 year in spironolactone-treated patients in the Americas but was unchanged in Russian and Georgian patients. And while doubling of creatinine levels to above normal range was 60% more common with spironolactone than placebo in the Americas, rates were identical in the two treatment arms in Russia and Georgia.
“There was a physiologic response to spironolactone that paralleled an apparent outcome response,” Dr. Hochman concluded.
As for the discordant Eastern European data, she observed that confirming the diagnosis of HFpEF may be difficult: “Dyspnea, orthopnea, fatigue, lower extremity edema – all of these may be caused by other conditions. So it’s very complicated.”
In contrast to heart failure with reduced ejection fraction, which has seen enormous treatment advances in recent years, not much progress has been made in HFpEF. For that to occur, Dr. Hochman said, it will be essential to come up with refined, objective diagnostic criteria for use in clinical trials. Perhaps echocardiographic findings or elevated natriuretic peptide levels will fill that role, she added.
However, Dr. Pfeffer said that, much to his disappointment, he and other investigators have not seen a consistent correlation between higher baseline brain natriuretic peptide levels and greater clinical response to mineralocorticoid receptor antagonist therapy.
Asked what sort of oversight he and the other TOPCAT leaders had over the Russian and Georgian study sites, Dr. Pfeffer replied that the National Institutes of Health–sponsored study was underfunded for such monitoring.
“As one of the leaders of the trial, there are a lot of things that I would have wished to have done differently,” he said. “I have to stand here and say the amount that you get is inadequate to do what happens in industry-sponsored trials if there’s a perceived problem.”
Both Dr. Pfeffer and Dr. Hochman emphasized the critical importance of careful monitoring of serum potassium and creatinine when prescribing spironolactone in patients with HFpEF. But with regular monitoring, Dr. Pfeffer observed, the risk of major elevations is reassuringly low. For example, with monitoring of serum creatinine at every clinic visit and dose change as per TOPCAT protocol, the incidence of a level of 3.0 mg/dL or more was 10% with spironolactone and not significantly different at 9% with placebo in the Americas.
Dr. Pfeffer reported having received consultant fees from 20 pharmaceutical or medical device companies.
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point: Prescribing spironolactone for patients with symptomatic heart failure with preserved ejection fraction may reduce their risks of cardiovascular death and hospitalizations for heart failure.
Major finding: After exclusion of highly suspect Russian and Georgian data on 1,678 patients, the primary composite outcome of cardiovascular death or heart failure hospitalization in the remaining 1,767 patients with heart failure with preserved ejection fraction in four Western Hemisphere countries occurred in 27.3% of patients on spironolactone, for a significant 18% relative risk reduction compared with the 31.8% rate in placebo-treated controls.
Data source: TOPCAT, a randomized, double-blind, placebo-controlled, six-nation study involving 3,445 patients with heart failure with preserved ejection fraction treated for a mean of 3.3 years.
Disclosures: TOPCAT was sponsored by the National Heart, Lung, and Blood Institute. The presenter has received consulting fees from 20 pharmaceutical or medical device companies.
New evidence suggests 2014 hypertension guidelines could backfire
CHICAGO – Nearly one in seven patients in U.S. ambulatory cardiology practices who would have been recommended for initiation or intensification of antihypertensive drug therapy under the 2003 Seventh Joint National Committee guidelines are no longer treatment candidates under the 2014 expert panel recommendations.
These patients who no longer qualify for antihypertensive therapy under the 2014 guidelines turn out to have a disturbingly high average estimated 10-year risk of cardiovascular events. As a result, widespread adoption of the 2014 expert panel recommendations could have major adverse consequences for cardiovascular health, Dr. William B. Borden cautioned at the American Heart Association scientific sessions.
“Given the size and underlying cardiovascular risk of the population affected by the changes in the 2014 panel recommendations, close monitoring will be required to assess changes in practice patterns, blood pressure control, and – importantly – any changes in cardiovascular morbidity and mortality,” said Dr. Borden, a cardiologist at George Washington University in Washington.
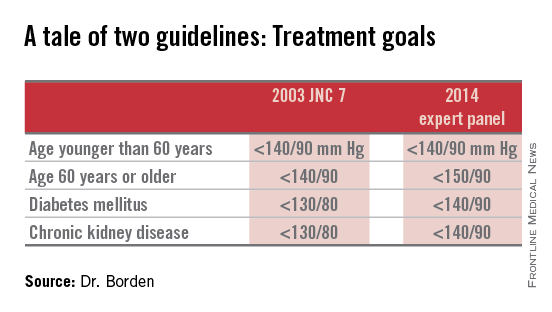
Because the 2014 expert panel guidelines represent a major shift in hypertension management, Dr. Borden and coinvestigators sought to quantify the potential cardiovascular health impact of this more lenient treatment approach. For this purpose they turned to the National Cardiovascular Data Registry Practice Innovation and Clinical Excellence (NCDR PINNACLE) Registry, a voluntary quality improvement project involving outpatient cardiology practices.
Of 1,185,253 patients with hypertension as identified in their chart by a recorded diagnosis or notation of blood pressure greater than 140/90 mm Hg, 60% met the 2003 JNC 7 goals (JAMA 2003;289:2560-72), meaning the other 40% were candidates for initiation or intensification of antihypertensive therapy in order to achieve those goals (see chart). In contrast, 74% of hypertensive patients in U.S. cardiology practices met the less aggressive targets recommended in the 2014 expert panel report (JAMA 2014;311:502-20).
Thus, fewer than two-thirds of hypertensive patients in outpatient cardiology practices met the 2003 JNC 7 blood pressure targets, while three-quarters met the liberalized 2014 targets.
Dr. Borden and coworkers zeroed in on the 15% of hypertensive patients – that’s fully 173,519 individuals in cardiology practices participating in the PINNACLE Registry – who would have been eligible for treatment under the JNC 7 recommendations but not the 2014 expert panel guidelines. Interestingly, that 15% figure was closely similar to the 17% rate reported by Dr. Michael D. Miedema of the Minneapolis Heart Institute in an analysis of a more primary care population of older patients in the Atherosclerosis Risk in Communities (ARIC) study he presented in the same session.
Dr. Borden and coinvestigators determined from medical records that the PINNACLE Registry group whose antihypertensive therapy treatment status changed between the two guidelines was at substantial baseline cardiovascular risk: Nearly two-thirds had been diagnosed with CAD, 54% had diabetes, 27% had a history of heart failure, 25% had a prior MI, and 23% had a prior transient ischemic attack or stroke.
This large group of patients who fell through the cracks between two conflicting sets of guidelines turned out to have a mean 10-year Framingham Risk Score of 8.5%. Upon incorporating the patients’ stroke risk using the atherosclerotic cardiovascular disease (ASCVD) risk score embedded in the 2013 ACC/AHA cholesterol management guidelines, their 10-year risk shot up to 28%.
The investigators then conducted a modeling exercise aimed at estimating the clinical impact of lowering systolic blood pressure in the elderly from about 150 mm Hg, as recommended in the 2014 expert panel guidelines, to about 140 mm Hg, as was the goal in JNC 7. To do so they extrapolated from the results of two randomized controlled clinical trials: the Systolic Hypertension in the Elderly Program (SHEP) and the Hypertension in the Very Elderly Trial (HYVET).
The result? Extrapolating from SHEP data, the 10-year ASCVD risk in these real-world elderly hypertensive patients caught between two conflicting sets of guidelines would drop from 28% to 19%. Using HYVET data, the average 10-year ASCVD risk would fall to 18.4%.
“This is equivalent to a number-needed-to-treat of 10-11 patients for 10 years in order to prevent one cardiovascular event,” according to Dr. Borden.
For the more than 80,000 patients over age 60 in the study population, that works out to roughly 8,000 cardiovascular events averted over the course of 10 years, he added.
The 2014 expert panel recommendations were based on a strict evidence-based review of published randomized controlled trials. The guidelines are new enough that it remains unclear if they will be embraced by clinicians or incorporated into performance measures and value-based health care purchasing programs.
The 2014 guidelines are considered highly controversial. The guideline committee comprising some of the nation’s top hypertension researchers was initially convened to come up with what was intended to be the long-awaited JNC 8 report; however, in the midst of the process the sponsoring National Heart, Lung, and Blood Institute declared it was getting out of the guideline-writing business altogether. As a result, the guidelines ultimately published carried the imprimatur of “the 2014 expert panel,” rather than the more prestigious official stamp of JNC 8.
Indeed, five members of the guideline panel felt strongly enough to break away and issued a minority report (Ann. Intern. Med. 2014;160:499-503) in which they argued there is insufficient evidence of harm stemming from the JNC 7 goal of 140/90 mm Hg in patients over age 60 to justify revising the target to 150/90. They warned that this step could reverse the impressive reductions in cardiovascular and cerebrovascular morbidity and mortality realized in recent decades. And they concluded that the burden of proof should be on those who advocate raising the treatment threshold to 150/90 mm Hg to demonstrate that it has benefit in patients over age 60, which they haven’t done.
“I’m very concerned about the [2014 expert panel] guidelines. Older individuals have the highest prevalence of hypertension, they’re the least adequately controlled, and based on the available data I’m concerned that if people follow the new guidelines there’s going to be an increase in cardiovascular events,” said Dr. Wilbert F. Aronow of New York Medical College, Valhalla, who chaired the writing committee for the first-ever ACC/AHA clinical guidelines for controlling high blood pressure in the elderly (J. Am. Coll. Cardiol. 2011;57:2037-114).
The NCDR PINNACLE Registry and this study were supported by the American College of Cardiology Foundation. Dr. Borden and Dr. Aronow reported having no financial conflicts.
CHICAGO – Nearly one in seven patients in U.S. ambulatory cardiology practices who would have been recommended for initiation or intensification of antihypertensive drug therapy under the 2003 Seventh Joint National Committee guidelines are no longer treatment candidates under the 2014 expert panel recommendations.
These patients who no longer qualify for antihypertensive therapy under the 2014 guidelines turn out to have a disturbingly high average estimated 10-year risk of cardiovascular events. As a result, widespread adoption of the 2014 expert panel recommendations could have major adverse consequences for cardiovascular health, Dr. William B. Borden cautioned at the American Heart Association scientific sessions.
“Given the size and underlying cardiovascular risk of the population affected by the changes in the 2014 panel recommendations, close monitoring will be required to assess changes in practice patterns, blood pressure control, and – importantly – any changes in cardiovascular morbidity and mortality,” said Dr. Borden, a cardiologist at George Washington University in Washington.

Because the 2014 expert panel guidelines represent a major shift in hypertension management, Dr. Borden and coinvestigators sought to quantify the potential cardiovascular health impact of this more lenient treatment approach. For this purpose they turned to the National Cardiovascular Data Registry Practice Innovation and Clinical Excellence (NCDR PINNACLE) Registry, a voluntary quality improvement project involving outpatient cardiology practices.
Of 1,185,253 patients with hypertension as identified in their chart by a recorded diagnosis or notation of blood pressure greater than 140/90 mm Hg, 60% met the 2003 JNC 7 goals (JAMA 2003;289:2560-72), meaning the other 40% were candidates for initiation or intensification of antihypertensive therapy in order to achieve those goals (see chart). In contrast, 74% of hypertensive patients in U.S. cardiology practices met the less aggressive targets recommended in the 2014 expert panel report (JAMA 2014;311:502-20).
Thus, fewer than two-thirds of hypertensive patients in outpatient cardiology practices met the 2003 JNC 7 blood pressure targets, while three-quarters met the liberalized 2014 targets.
Dr. Borden and coworkers zeroed in on the 15% of hypertensive patients – that’s fully 173,519 individuals in cardiology practices participating in the PINNACLE Registry – who would have been eligible for treatment under the JNC 7 recommendations but not the 2014 expert panel guidelines. Interestingly, that 15% figure was closely similar to the 17% rate reported by Dr. Michael D. Miedema of the Minneapolis Heart Institute in an analysis of a more primary care population of older patients in the Atherosclerosis Risk in Communities (ARIC) study he presented in the same session.
Dr. Borden and coinvestigators determined from medical records that the PINNACLE Registry group whose antihypertensive therapy treatment status changed between the two guidelines was at substantial baseline cardiovascular risk: Nearly two-thirds had been diagnosed with CAD, 54% had diabetes, 27% had a history of heart failure, 25% had a prior MI, and 23% had a prior transient ischemic attack or stroke.
This large group of patients who fell through the cracks between two conflicting sets of guidelines turned out to have a mean 10-year Framingham Risk Score of 8.5%. Upon incorporating the patients’ stroke risk using the atherosclerotic cardiovascular disease (ASCVD) risk score embedded in the 2013 ACC/AHA cholesterol management guidelines, their 10-year risk shot up to 28%.
The investigators then conducted a modeling exercise aimed at estimating the clinical impact of lowering systolic blood pressure in the elderly from about 150 mm Hg, as recommended in the 2014 expert panel guidelines, to about 140 mm Hg, as was the goal in JNC 7. To do so they extrapolated from the results of two randomized controlled clinical trials: the Systolic Hypertension in the Elderly Program (SHEP) and the Hypertension in the Very Elderly Trial (HYVET).
The result? Extrapolating from SHEP data, the 10-year ASCVD risk in these real-world elderly hypertensive patients caught between two conflicting sets of guidelines would drop from 28% to 19%. Using HYVET data, the average 10-year ASCVD risk would fall to 18.4%.
“This is equivalent to a number-needed-to-treat of 10-11 patients for 10 years in order to prevent one cardiovascular event,” according to Dr. Borden.
For the more than 80,000 patients over age 60 in the study population, that works out to roughly 8,000 cardiovascular events averted over the course of 10 years, he added.
The 2014 expert panel recommendations were based on a strict evidence-based review of published randomized controlled trials. The guidelines are new enough that it remains unclear if they will be embraced by clinicians or incorporated into performance measures and value-based health care purchasing programs.
The 2014 guidelines are considered highly controversial. The guideline committee comprising some of the nation’s top hypertension researchers was initially convened to come up with what was intended to be the long-awaited JNC 8 report; however, in the midst of the process the sponsoring National Heart, Lung, and Blood Institute declared it was getting out of the guideline-writing business altogether. As a result, the guidelines ultimately published carried the imprimatur of “the 2014 expert panel,” rather than the more prestigious official stamp of JNC 8.
Indeed, five members of the guideline panel felt strongly enough to break away and issued a minority report (Ann. Intern. Med. 2014;160:499-503) in which they argued there is insufficient evidence of harm stemming from the JNC 7 goal of 140/90 mm Hg in patients over age 60 to justify revising the target to 150/90. They warned that this step could reverse the impressive reductions in cardiovascular and cerebrovascular morbidity and mortality realized in recent decades. And they concluded that the burden of proof should be on those who advocate raising the treatment threshold to 150/90 mm Hg to demonstrate that it has benefit in patients over age 60, which they haven’t done.
“I’m very concerned about the [2014 expert panel] guidelines. Older individuals have the highest prevalence of hypertension, they’re the least adequately controlled, and based on the available data I’m concerned that if people follow the new guidelines there’s going to be an increase in cardiovascular events,” said Dr. Wilbert F. Aronow of New York Medical College, Valhalla, who chaired the writing committee for the first-ever ACC/AHA clinical guidelines for controlling high blood pressure in the elderly (J. Am. Coll. Cardiol. 2011;57:2037-114).
The NCDR PINNACLE Registry and this study were supported by the American College of Cardiology Foundation. Dr. Borden and Dr. Aronow reported having no financial conflicts.
CHICAGO – Nearly one in seven patients in U.S. ambulatory cardiology practices who would have been recommended for initiation or intensification of antihypertensive drug therapy under the 2003 Seventh Joint National Committee guidelines are no longer treatment candidates under the 2014 expert panel recommendations.
These patients who no longer qualify for antihypertensive therapy under the 2014 guidelines turn out to have a disturbingly high average estimated 10-year risk of cardiovascular events. As a result, widespread adoption of the 2014 expert panel recommendations could have major adverse consequences for cardiovascular health, Dr. William B. Borden cautioned at the American Heart Association scientific sessions.
“Given the size and underlying cardiovascular risk of the population affected by the changes in the 2014 panel recommendations, close monitoring will be required to assess changes in practice patterns, blood pressure control, and – importantly – any changes in cardiovascular morbidity and mortality,” said Dr. Borden, a cardiologist at George Washington University in Washington.

Because the 2014 expert panel guidelines represent a major shift in hypertension management, Dr. Borden and coinvestigators sought to quantify the potential cardiovascular health impact of this more lenient treatment approach. For this purpose they turned to the National Cardiovascular Data Registry Practice Innovation and Clinical Excellence (NCDR PINNACLE) Registry, a voluntary quality improvement project involving outpatient cardiology practices.
Of 1,185,253 patients with hypertension as identified in their chart by a recorded diagnosis or notation of blood pressure greater than 140/90 mm Hg, 60% met the 2003 JNC 7 goals (JAMA 2003;289:2560-72), meaning the other 40% were candidates for initiation or intensification of antihypertensive therapy in order to achieve those goals (see chart). In contrast, 74% of hypertensive patients in U.S. cardiology practices met the less aggressive targets recommended in the 2014 expert panel report (JAMA 2014;311:502-20).
Thus, fewer than two-thirds of hypertensive patients in outpatient cardiology practices met the 2003 JNC 7 blood pressure targets, while three-quarters met the liberalized 2014 targets.
Dr. Borden and coworkers zeroed in on the 15% of hypertensive patients – that’s fully 173,519 individuals in cardiology practices participating in the PINNACLE Registry – who would have been eligible for treatment under the JNC 7 recommendations but not the 2014 expert panel guidelines. Interestingly, that 15% figure was closely similar to the 17% rate reported by Dr. Michael D. Miedema of the Minneapolis Heart Institute in an analysis of a more primary care population of older patients in the Atherosclerosis Risk in Communities (ARIC) study he presented in the same session.
Dr. Borden and coinvestigators determined from medical records that the PINNACLE Registry group whose antihypertensive therapy treatment status changed between the two guidelines was at substantial baseline cardiovascular risk: Nearly two-thirds had been diagnosed with CAD, 54% had diabetes, 27% had a history of heart failure, 25% had a prior MI, and 23% had a prior transient ischemic attack or stroke.
This large group of patients who fell through the cracks between two conflicting sets of guidelines turned out to have a mean 10-year Framingham Risk Score of 8.5%. Upon incorporating the patients’ stroke risk using the atherosclerotic cardiovascular disease (ASCVD) risk score embedded in the 2013 ACC/AHA cholesterol management guidelines, their 10-year risk shot up to 28%.
The investigators then conducted a modeling exercise aimed at estimating the clinical impact of lowering systolic blood pressure in the elderly from about 150 mm Hg, as recommended in the 2014 expert panel guidelines, to about 140 mm Hg, as was the goal in JNC 7. To do so they extrapolated from the results of two randomized controlled clinical trials: the Systolic Hypertension in the Elderly Program (SHEP) and the Hypertension in the Very Elderly Trial (HYVET).
The result? Extrapolating from SHEP data, the 10-year ASCVD risk in these real-world elderly hypertensive patients caught between two conflicting sets of guidelines would drop from 28% to 19%. Using HYVET data, the average 10-year ASCVD risk would fall to 18.4%.
“This is equivalent to a number-needed-to-treat of 10-11 patients for 10 years in order to prevent one cardiovascular event,” according to Dr. Borden.
For the more than 80,000 patients over age 60 in the study population, that works out to roughly 8,000 cardiovascular events averted over the course of 10 years, he added.
The 2014 expert panel recommendations were based on a strict evidence-based review of published randomized controlled trials. The guidelines are new enough that it remains unclear if they will be embraced by clinicians or incorporated into performance measures and value-based health care purchasing programs.
The 2014 guidelines are considered highly controversial. The guideline committee comprising some of the nation’s top hypertension researchers was initially convened to come up with what was intended to be the long-awaited JNC 8 report; however, in the midst of the process the sponsoring National Heart, Lung, and Blood Institute declared it was getting out of the guideline-writing business altogether. As a result, the guidelines ultimately published carried the imprimatur of “the 2014 expert panel,” rather than the more prestigious official stamp of JNC 8.
Indeed, five members of the guideline panel felt strongly enough to break away and issued a minority report (Ann. Intern. Med. 2014;160:499-503) in which they argued there is insufficient evidence of harm stemming from the JNC 7 goal of 140/90 mm Hg in patients over age 60 to justify revising the target to 150/90. They warned that this step could reverse the impressive reductions in cardiovascular and cerebrovascular morbidity and mortality realized in recent decades. And they concluded that the burden of proof should be on those who advocate raising the treatment threshold to 150/90 mm Hg to demonstrate that it has benefit in patients over age 60, which they haven’t done.
“I’m very concerned about the [2014 expert panel] guidelines. Older individuals have the highest prevalence of hypertension, they’re the least adequately controlled, and based on the available data I’m concerned that if people follow the new guidelines there’s going to be an increase in cardiovascular events,” said Dr. Wilbert F. Aronow of New York Medical College, Valhalla, who chaired the writing committee for the first-ever ACC/AHA clinical guidelines for controlling high blood pressure in the elderly (J. Am. Coll. Cardiol. 2011;57:2037-114).
The NCDR PINNACLE Registry and this study were supported by the American College of Cardiology Foundation. Dr. Borden and Dr. Aronow reported having no financial conflicts.
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point: Adoption of the less aggressive blood pressure goal of 150/90 mm Hg for patients age 60 and older as recommended in the 2014 expert panel guidelines could result in significantly more harm than good.
Major finding: Patients in cardiology practices who qualified for antihypertensive therapy under the 2003 JNC 7 guidelines but not under the 2014 expert panel guidelines have a baseline 10-year 28% risk of cardiovascular events or stroke using the risk calculator included in the 2013 ACC/AHA cholesterol management guidelines.
Data source: An analysis of 1,185,253 patients in U.S. ambulatory cardiology practices.
Disclosures: The NCDR PINNACLE Registry is supported by the American College of Cardiology Foundation. The presenter reported having no financial conflicts of interest.
Pushing LDL below 25 mg/dL with alirocumab safe ‘so far’
CHICAGO – Driving LDL cholesterol below 25 mg/dL for a year or more in high-cardiovascular-risk patients using the investigational human monoclonal antibody alirocumab proved well tolerated and displayed a treatment-emergent adverse event profile reassuringly comparable to that of placebo in an interim analysis of the largest double-blind phase III clinical trial to date involving any PCSK9 inhibitor.
While this is encouraging preliminary evidence that it may prove safe to pharmacologically maintain unnaturally low LDL concentrations – levels below those seen in newborns – in high-risk patients, Dr. Jennifer G. Robinson nonetheless urged her cardiology colleagues to curb their enthusiasm as she presented an interim analysis of the 2,341-patient ODYSSEY Long Term trial at the American Heart Association scientific sessions.
“We’re all concerned about the long-term safety of very low LDL levels. The fact that there are no signals so far is good, but it’s only been 52 weeks. In my own mind, we’re treating people with very-high-risk cardiovascular disease who are older and have comorbidities, and I think we just have to establish that there really aren’t any adverse effects of prolonged low levels of LDL,” said Dr. Robinson, professor of medicine at the University of Iowa in Iowa City.
Session cochair Dr. David C. Goff applauded her cautionary stance.
“The 1-year data on safety are promising, although these are still relatively small numbers of patients who have been exposed to the medication for at least a year. So rare adverse events will still be difficult to detect in just a couple thousand people treated for that length of time,” observed Dr. Goff, a cardiovascular epidemiologist and dean of the University of Colorado School of Public Health, Denver.
He was particularly intrigued by a different part of her presentation, a new post hoc pooled analysis of hard cardiovascular outcomes in ODYSSEY Long Term and two other ongoing, phase III, placebo-controlled alirocumab trials totaling 3,459 high-risk patients with follow-up of 52 weeks or more. In this Cox regression analysis, the relative risk of the composite endpoint of adjudicated death from coronary heart disease, nonfatal myocardial infarction, fatal or nonfatal ischemic stroke, or unstable angina requiring hospitalization was 35% lower in alirocumab- than placebo-treated patients. While this hefty-looking difference in risk didn’t achieve statistical significance a mere year into follow-up, the fact that the two event curves diverged from the very beginning of follow-up and the separation continues to expand bodes well indeed.
“Dr. Robinson has shown us a first glimpse of a promise of cardiovascular disease prevention from the pooled data. I think we’re all now looking forward to the results of the ongoing ODYSSEY Outcomes trial, with 18,000 randomized ACS [acute coronary syndrome] patients being followed for 5-6 years, which will provide us with much better evidence with regard to safety and cardiovascular event prevention. But so far so good, from what I’ve seen here today,” Dr. Goff commented.
ODYSSEY Long Term included 2,341 patients with high cardiovascular risk, either because of known coronary heart disease or, in the case of 415 subjects, heterozygous familial hypercholesterolemia (FH). At entry, participants were on maximum tolerated statin therapy, often accompanied by additional lipid-lowering agents, yet their mean baseline LDL was 122 mg/dL. While remaining on their maximum tolerated statin and other LDL-lowering drugs throughout the trial, they were randomized 2:1 to 150 mg of alirocumab by self-administered subcutaneous injection every 2 weeks or placebo. Alirocumab is a human monoclonal antibody designed to inhibit PCSK9 (proprotein convertase subtilisin/kexin type 9), an enzyme that helps govern cholesterol homeostasis.
Seventy-nine percent of alirocumab-treated patients achieved an LDL below 70 mg/dL at week 24. At week 52, mean LDL in the alirocumab group was 53 mg/dL, compared with 123 mg/dL in the control group.
The potential for adverse neurocognitive effects with very low achieved LDL levels has been of particular concern with regard to the PCSK9 inhibitors. The issue is quite relevant because patients on alirocumab commonly develop LDL levels below 25 mg/dL. In ODYSSEY Long Term, for example, 36% of alirocumab-treated patients did so. Lipidologists have been eager to learn more about the impact of maintaining patients in this state of subphysiologic LDL, and Dr. Robinson’s data provided the first meaningful opportunity to do so.
Reassuringly, the rate of treatment-emergent neurocognitive disorders was 1.2% in the total alirocumab-treated population, 0.5% in those with an on-treatment LDL below 25 mg/dL, and 0.5% in placebo-treated controls who, it must be remembered, were on maximum-tolerated therapy with statins and other conventional lipid-lowering agents. Other treatment-emergent adverse events – and more than 30 categories of such events were tracked – followed a similar pattern (see graphic).
There was one event of possible concern that has been reported to the Food and Drug Administration. An alirocumab-treated patient was diagnosed with Miller Fisher syndrome, a rare variant of Guillain-Barre syndrome, at week 27 after a prodromal severe gastroenteritis. At week 24, just 3 weeks earlier, the patient had an LDL of 1.5 mg/dL. The double vision and other symptoms of Miller Fisher syndrome resolved completely after treatment discontinuation.
During a panel discussion of the ODYSSEY clinical trial series, which includes 14 phase III studies, Dr. Henry N. Ginsburg predicted that further experience will eventually show that the dramatic LDL-lowering achieved with alirocumab not only doesn’t promote neurocognitive dysfunction, but actually protects against it.
“I think an important point to make is that, despite our neurology colleagues having tried for about a decade to completely separate the clinical phenotype of Alzheimer’s disease from vascular dementia, we all know that if you have both you’ll do much worse. So if we reduce cerebrovascular atherosclerosis and you have any predisposition to Alzheimer’s disease, you’ll do better. I think there will be studies to show that,” said Dr. Ginsburg, professor of medicine and director of the Irving Institute for Clinical and Translational Research at Columbia University in New York, who earlier in the session presented the findings from the phase III ODYSSEY High FH trial.
Another potential safety concern emerged during Dr. Dean J. Kereiakes’ presentation of the results of the phase III ODYSSEY Combo I study. Thirteen of 199 alirocumab-treated patients (6.6%) developed treatment-emergent, low-titer antialirocumab antibodies. The median time to their detection was 12 weeks. Four of the 13 patients had neutralizing antibodies, and all 4 became antibody negative by 24 weeks.
The presence of these antidrug antibodies had no discernible effect on treatment safety or efficacy. However, Combo I was a 52-week study. Whether the prevalence of these antibodies will increase over time and interfere with what is expected to be lifelong treatment is an unanswered question, noted Dr. Kereiakes, medical director for the Christ Hospital Heart and Vascular Center, Cincinnati.
Discussant Dr. Joshua W. Knowles, medical director of the FH Foundation, commented that “it’s really incredible to see the reductions in LDL that we’re getting with PCSK9 inhibitors in FH patients, the vast majority of whom require more than two standard medications to get to optimal LDL cholesterol levels.”
“The PCSK9 inhibitors are really the poster child for drug discovery in the modern genetic era. It has been a beautiful 10-year journey for them,” declared Dr. Knowles, a cardiologist at Stanford (Calif.) University.
In light of the powerful LDL-lowering effect of these agents, it’s not too soon to start thinking about how to carry out studies of atherosclerotic plaque stabilization and/or regression in treated patients, he added.
Other PCSK9 inhibitors in phase III clinical trials, besides alirocumab, are Amgen’s evolocumab and Pfizer/Renat’s bococizumab. In addition, Eli Lilly’s LY3015014 is in phase II studies, as is Roche/Genentech’s RG7652.
The ODYSSEY studies are funded by Sanofi and Regeneron Pharmaceuticals. Dr. Robinson, Dr. Ginsburg, and Dr. Kereiakes reported serving as consultants to those and other pharmaceutical companies.
CHICAGO – Driving LDL cholesterol below 25 mg/dL for a year or more in high-cardiovascular-risk patients using the investigational human monoclonal antibody alirocumab proved well tolerated and displayed a treatment-emergent adverse event profile reassuringly comparable to that of placebo in an interim analysis of the largest double-blind phase III clinical trial to date involving any PCSK9 inhibitor.
While this is encouraging preliminary evidence that it may prove safe to pharmacologically maintain unnaturally low LDL concentrations – levels below those seen in newborns – in high-risk patients, Dr. Jennifer G. Robinson nonetheless urged her cardiology colleagues to curb their enthusiasm as she presented an interim analysis of the 2,341-patient ODYSSEY Long Term trial at the American Heart Association scientific sessions.
“We’re all concerned about the long-term safety of very low LDL levels. The fact that there are no signals so far is good, but it’s only been 52 weeks. In my own mind, we’re treating people with very-high-risk cardiovascular disease who are older and have comorbidities, and I think we just have to establish that there really aren’t any adverse effects of prolonged low levels of LDL,” said Dr. Robinson, professor of medicine at the University of Iowa in Iowa City.
Session cochair Dr. David C. Goff applauded her cautionary stance.
“The 1-year data on safety are promising, although these are still relatively small numbers of patients who have been exposed to the medication for at least a year. So rare adverse events will still be difficult to detect in just a couple thousand people treated for that length of time,” observed Dr. Goff, a cardiovascular epidemiologist and dean of the University of Colorado School of Public Health, Denver.
He was particularly intrigued by a different part of her presentation, a new post hoc pooled analysis of hard cardiovascular outcomes in ODYSSEY Long Term and two other ongoing, phase III, placebo-controlled alirocumab trials totaling 3,459 high-risk patients with follow-up of 52 weeks or more. In this Cox regression analysis, the relative risk of the composite endpoint of adjudicated death from coronary heart disease, nonfatal myocardial infarction, fatal or nonfatal ischemic stroke, or unstable angina requiring hospitalization was 35% lower in alirocumab- than placebo-treated patients. While this hefty-looking difference in risk didn’t achieve statistical significance a mere year into follow-up, the fact that the two event curves diverged from the very beginning of follow-up and the separation continues to expand bodes well indeed.
“Dr. Robinson has shown us a first glimpse of a promise of cardiovascular disease prevention from the pooled data. I think we’re all now looking forward to the results of the ongoing ODYSSEY Outcomes trial, with 18,000 randomized ACS [acute coronary syndrome] patients being followed for 5-6 years, which will provide us with much better evidence with regard to safety and cardiovascular event prevention. But so far so good, from what I’ve seen here today,” Dr. Goff commented.
ODYSSEY Long Term included 2,341 patients with high cardiovascular risk, either because of known coronary heart disease or, in the case of 415 subjects, heterozygous familial hypercholesterolemia (FH). At entry, participants were on maximum tolerated statin therapy, often accompanied by additional lipid-lowering agents, yet their mean baseline LDL was 122 mg/dL. While remaining on their maximum tolerated statin and other LDL-lowering drugs throughout the trial, they were randomized 2:1 to 150 mg of alirocumab by self-administered subcutaneous injection every 2 weeks or placebo. Alirocumab is a human monoclonal antibody designed to inhibit PCSK9 (proprotein convertase subtilisin/kexin type 9), an enzyme that helps govern cholesterol homeostasis.
Seventy-nine percent of alirocumab-treated patients achieved an LDL below 70 mg/dL at week 24. At week 52, mean LDL in the alirocumab group was 53 mg/dL, compared with 123 mg/dL in the control group.
The potential for adverse neurocognitive effects with very low achieved LDL levels has been of particular concern with regard to the PCSK9 inhibitors. The issue is quite relevant because patients on alirocumab commonly develop LDL levels below 25 mg/dL. In ODYSSEY Long Term, for example, 36% of alirocumab-treated patients did so. Lipidologists have been eager to learn more about the impact of maintaining patients in this state of subphysiologic LDL, and Dr. Robinson’s data provided the first meaningful opportunity to do so.
Reassuringly, the rate of treatment-emergent neurocognitive disorders was 1.2% in the total alirocumab-treated population, 0.5% in those with an on-treatment LDL below 25 mg/dL, and 0.5% in placebo-treated controls who, it must be remembered, were on maximum-tolerated therapy with statins and other conventional lipid-lowering agents. Other treatment-emergent adverse events – and more than 30 categories of such events were tracked – followed a similar pattern (see graphic).
There was one event of possible concern that has been reported to the Food and Drug Administration. An alirocumab-treated patient was diagnosed with Miller Fisher syndrome, a rare variant of Guillain-Barre syndrome, at week 27 after a prodromal severe gastroenteritis. At week 24, just 3 weeks earlier, the patient had an LDL of 1.5 mg/dL. The double vision and other symptoms of Miller Fisher syndrome resolved completely after treatment discontinuation.
During a panel discussion of the ODYSSEY clinical trial series, which includes 14 phase III studies, Dr. Henry N. Ginsburg predicted that further experience will eventually show that the dramatic LDL-lowering achieved with alirocumab not only doesn’t promote neurocognitive dysfunction, but actually protects against it.
“I think an important point to make is that, despite our neurology colleagues having tried for about a decade to completely separate the clinical phenotype of Alzheimer’s disease from vascular dementia, we all know that if you have both you’ll do much worse. So if we reduce cerebrovascular atherosclerosis and you have any predisposition to Alzheimer’s disease, you’ll do better. I think there will be studies to show that,” said Dr. Ginsburg, professor of medicine and director of the Irving Institute for Clinical and Translational Research at Columbia University in New York, who earlier in the session presented the findings from the phase III ODYSSEY High FH trial.
Another potential safety concern emerged during Dr. Dean J. Kereiakes’ presentation of the results of the phase III ODYSSEY Combo I study. Thirteen of 199 alirocumab-treated patients (6.6%) developed treatment-emergent, low-titer antialirocumab antibodies. The median time to their detection was 12 weeks. Four of the 13 patients had neutralizing antibodies, and all 4 became antibody negative by 24 weeks.
The presence of these antidrug antibodies had no discernible effect on treatment safety or efficacy. However, Combo I was a 52-week study. Whether the prevalence of these antibodies will increase over time and interfere with what is expected to be lifelong treatment is an unanswered question, noted Dr. Kereiakes, medical director for the Christ Hospital Heart and Vascular Center, Cincinnati.
Discussant Dr. Joshua W. Knowles, medical director of the FH Foundation, commented that “it’s really incredible to see the reductions in LDL that we’re getting with PCSK9 inhibitors in FH patients, the vast majority of whom require more than two standard medications to get to optimal LDL cholesterol levels.”
“The PCSK9 inhibitors are really the poster child for drug discovery in the modern genetic era. It has been a beautiful 10-year journey for them,” declared Dr. Knowles, a cardiologist at Stanford (Calif.) University.
In light of the powerful LDL-lowering effect of these agents, it’s not too soon to start thinking about how to carry out studies of atherosclerotic plaque stabilization and/or regression in treated patients, he added.
Other PCSK9 inhibitors in phase III clinical trials, besides alirocumab, are Amgen’s evolocumab and Pfizer/Renat’s bococizumab. In addition, Eli Lilly’s LY3015014 is in phase II studies, as is Roche/Genentech’s RG7652.
The ODYSSEY studies are funded by Sanofi and Regeneron Pharmaceuticals. Dr. Robinson, Dr. Ginsburg, and Dr. Kereiakes reported serving as consultants to those and other pharmaceutical companies.
CHICAGO – Driving LDL cholesterol below 25 mg/dL for a year or more in high-cardiovascular-risk patients using the investigational human monoclonal antibody alirocumab proved well tolerated and displayed a treatment-emergent adverse event profile reassuringly comparable to that of placebo in an interim analysis of the largest double-blind phase III clinical trial to date involving any PCSK9 inhibitor.
While this is encouraging preliminary evidence that it may prove safe to pharmacologically maintain unnaturally low LDL concentrations – levels below those seen in newborns – in high-risk patients, Dr. Jennifer G. Robinson nonetheless urged her cardiology colleagues to curb their enthusiasm as she presented an interim analysis of the 2,341-patient ODYSSEY Long Term trial at the American Heart Association scientific sessions.
“We’re all concerned about the long-term safety of very low LDL levels. The fact that there are no signals so far is good, but it’s only been 52 weeks. In my own mind, we’re treating people with very-high-risk cardiovascular disease who are older and have comorbidities, and I think we just have to establish that there really aren’t any adverse effects of prolonged low levels of LDL,” said Dr. Robinson, professor of medicine at the University of Iowa in Iowa City.
Session cochair Dr. David C. Goff applauded her cautionary stance.
“The 1-year data on safety are promising, although these are still relatively small numbers of patients who have been exposed to the medication for at least a year. So rare adverse events will still be difficult to detect in just a couple thousand people treated for that length of time,” observed Dr. Goff, a cardiovascular epidemiologist and dean of the University of Colorado School of Public Health, Denver.
He was particularly intrigued by a different part of her presentation, a new post hoc pooled analysis of hard cardiovascular outcomes in ODYSSEY Long Term and two other ongoing, phase III, placebo-controlled alirocumab trials totaling 3,459 high-risk patients with follow-up of 52 weeks or more. In this Cox regression analysis, the relative risk of the composite endpoint of adjudicated death from coronary heart disease, nonfatal myocardial infarction, fatal or nonfatal ischemic stroke, or unstable angina requiring hospitalization was 35% lower in alirocumab- than placebo-treated patients. While this hefty-looking difference in risk didn’t achieve statistical significance a mere year into follow-up, the fact that the two event curves diverged from the very beginning of follow-up and the separation continues to expand bodes well indeed.
“Dr. Robinson has shown us a first glimpse of a promise of cardiovascular disease prevention from the pooled data. I think we’re all now looking forward to the results of the ongoing ODYSSEY Outcomes trial, with 18,000 randomized ACS [acute coronary syndrome] patients being followed for 5-6 years, which will provide us with much better evidence with regard to safety and cardiovascular event prevention. But so far so good, from what I’ve seen here today,” Dr. Goff commented.
ODYSSEY Long Term included 2,341 patients with high cardiovascular risk, either because of known coronary heart disease or, in the case of 415 subjects, heterozygous familial hypercholesterolemia (FH). At entry, participants were on maximum tolerated statin therapy, often accompanied by additional lipid-lowering agents, yet their mean baseline LDL was 122 mg/dL. While remaining on their maximum tolerated statin and other LDL-lowering drugs throughout the trial, they were randomized 2:1 to 150 mg of alirocumab by self-administered subcutaneous injection every 2 weeks or placebo. Alirocumab is a human monoclonal antibody designed to inhibit PCSK9 (proprotein convertase subtilisin/kexin type 9), an enzyme that helps govern cholesterol homeostasis.
Seventy-nine percent of alirocumab-treated patients achieved an LDL below 70 mg/dL at week 24. At week 52, mean LDL in the alirocumab group was 53 mg/dL, compared with 123 mg/dL in the control group.
The potential for adverse neurocognitive effects with very low achieved LDL levels has been of particular concern with regard to the PCSK9 inhibitors. The issue is quite relevant because patients on alirocumab commonly develop LDL levels below 25 mg/dL. In ODYSSEY Long Term, for example, 36% of alirocumab-treated patients did so. Lipidologists have been eager to learn more about the impact of maintaining patients in this state of subphysiologic LDL, and Dr. Robinson’s data provided the first meaningful opportunity to do so.
Reassuringly, the rate of treatment-emergent neurocognitive disorders was 1.2% in the total alirocumab-treated population, 0.5% in those with an on-treatment LDL below 25 mg/dL, and 0.5% in placebo-treated controls who, it must be remembered, were on maximum-tolerated therapy with statins and other conventional lipid-lowering agents. Other treatment-emergent adverse events – and more than 30 categories of such events were tracked – followed a similar pattern (see graphic).
There was one event of possible concern that has been reported to the Food and Drug Administration. An alirocumab-treated patient was diagnosed with Miller Fisher syndrome, a rare variant of Guillain-Barre syndrome, at week 27 after a prodromal severe gastroenteritis. At week 24, just 3 weeks earlier, the patient had an LDL of 1.5 mg/dL. The double vision and other symptoms of Miller Fisher syndrome resolved completely after treatment discontinuation.
During a panel discussion of the ODYSSEY clinical trial series, which includes 14 phase III studies, Dr. Henry N. Ginsburg predicted that further experience will eventually show that the dramatic LDL-lowering achieved with alirocumab not only doesn’t promote neurocognitive dysfunction, but actually protects against it.
“I think an important point to make is that, despite our neurology colleagues having tried for about a decade to completely separate the clinical phenotype of Alzheimer’s disease from vascular dementia, we all know that if you have both you’ll do much worse. So if we reduce cerebrovascular atherosclerosis and you have any predisposition to Alzheimer’s disease, you’ll do better. I think there will be studies to show that,” said Dr. Ginsburg, professor of medicine and director of the Irving Institute for Clinical and Translational Research at Columbia University in New York, who earlier in the session presented the findings from the phase III ODYSSEY High FH trial.
Another potential safety concern emerged during Dr. Dean J. Kereiakes’ presentation of the results of the phase III ODYSSEY Combo I study. Thirteen of 199 alirocumab-treated patients (6.6%) developed treatment-emergent, low-titer antialirocumab antibodies. The median time to their detection was 12 weeks. Four of the 13 patients had neutralizing antibodies, and all 4 became antibody negative by 24 weeks.
The presence of these antidrug antibodies had no discernible effect on treatment safety or efficacy. However, Combo I was a 52-week study. Whether the prevalence of these antibodies will increase over time and interfere with what is expected to be lifelong treatment is an unanswered question, noted Dr. Kereiakes, medical director for the Christ Hospital Heart and Vascular Center, Cincinnati.
Discussant Dr. Joshua W. Knowles, medical director of the FH Foundation, commented that “it’s really incredible to see the reductions in LDL that we’re getting with PCSK9 inhibitors in FH patients, the vast majority of whom require more than two standard medications to get to optimal LDL cholesterol levels.”
“The PCSK9 inhibitors are really the poster child for drug discovery in the modern genetic era. It has been a beautiful 10-year journey for them,” declared Dr. Knowles, a cardiologist at Stanford (Calif.) University.
In light of the powerful LDL-lowering effect of these agents, it’s not too soon to start thinking about how to carry out studies of atherosclerotic plaque stabilization and/or regression in treated patients, he added.
Other PCSK9 inhibitors in phase III clinical trials, besides alirocumab, are Amgen’s evolocumab and Pfizer/Renat’s bococizumab. In addition, Eli Lilly’s LY3015014 is in phase II studies, as is Roche/Genentech’s RG7652.
The ODYSSEY studies are funded by Sanofi and Regeneron Pharmaceuticals. Dr. Robinson, Dr. Ginsburg, and Dr. Kereiakes reported serving as consultants to those and other pharmaceutical companies.
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point: LDL cholesterol levels below 25 mg/dL induced by a superpotent investigational lipid-lowering agent appear to be safe through year 1 of follow-up.
Major finding: Thirty-six percent of high-cardiovascular-risk patients with a mean baseline LDL of 122 mg/dL despite maximum tolerated statin therapy responded to alirocumab with an LDL below 25 mg/dL, with treatment-emergent adverse events similar to those of placebo.
Data source: A prespecified interim analysis at week 52 of the ongoing double-blind, phase III ODYSSEY Long Term study, involving 2,341 high-cardiovascular-risk patients randomized 2:1 to self-administered subcutaneous injections of alirocumab at 150 mg or placebo every 2 weeks.
Disclosures: The study is sponsored by Sanofi and Regeneron Pharmaceuticals. Dr. Robinson reported serving as a consultant to those and other pharmaceutical companies.