User login
American Academy of Neurology (AAN): Annual Meeting 2017
Extrapolation of AED Efficacy Data From Adults to Pediatric Patients
BOSTON—Across a likely spectrum of syndromes with generalized seizures, the effect of adjunctive antiepileptic drug (AED) treatment on primary generalized tonic-clonic seizures appears similar between adults and children, according to a report presented at the 69th Annual Meeting of the American Academy of Neurology.
The availability of new AEDs for pediatric patients has been delayed due to the challenges of conducting clinical trials in children. In response to this challenge, the feasibility of extrapolation of adjunctive efficacy results for partial-onset seizures has been previously shown by Pellock et al from adult to pediatric populations when the disease course and pharmacokinetics of drug effects are comparable between populations. In response to a request from the Pediatric Committee of the European Medicines Agency, Douglas Nordli Jr, MD, and colleagues explored the feasibility of extrapolating AED efficacy data from adults to pediatric patients with primary generalized tonic-clonic seizures. Dr. Nordli is the Chief of the Division of Pediatric Neurology and Codirector of the Neurosciences Institute at Children's Hospital Los Angeles.
Dr. Nordli and colleagues conducted literature searches in EMBASE, Medline, and the Cochrane Central Register of Controlled Trials for randomized, placebo-controlled clinical trials of adjunctive AED treatment for primary generalized tonic-clonic seizures in adults and children published from 1970 to 2015. Outcome data, expressed as median percent reduction in seizure frequency and greater than or equal to 50% responder rate, were extracted from eligible trials for adult and pediatric patients receiving adjunctive AEDs or placebo and used to determine the relative strength of baseline-subtracted efficacy measures.
Seven published trials of AED adjunctive therapy for primary generalized tonic-clonic seizures were eligible for quantitative analysis. Dr. Nordli and colleagues found that changes in efficacy measures were similar in adults and children with primary generalized tonic-clonic seizures and were not age-dependent. The 95% confidence intervals for the standardized mean difference in median percent reduction of seizure frequency and estimated risk ratios in greater than or equal to 50% responder rate were consistently in favor of the AED treatment and comparable between adult and pediatric groups.
Dr. Nordli's study was supported by Eisai.
Suggested Reading
Pellock JM, Carman WJ, Thyagarajan V, et al. Efficacy of antiepileptic drugs in adults predicts efficacy in children: a systematic review. Neurology. 2012;79(14):1482-1489.
BOSTON—Across a likely spectrum of syndromes with generalized seizures, the effect of adjunctive antiepileptic drug (AED) treatment on primary generalized tonic-clonic seizures appears similar between adults and children, according to a report presented at the 69th Annual Meeting of the American Academy of Neurology.
The availability of new AEDs for pediatric patients has been delayed due to the challenges of conducting clinical trials in children. In response to this challenge, the feasibility of extrapolation of adjunctive efficacy results for partial-onset seizures has been previously shown by Pellock et al from adult to pediatric populations when the disease course and pharmacokinetics of drug effects are comparable between populations. In response to a request from the Pediatric Committee of the European Medicines Agency, Douglas Nordli Jr, MD, and colleagues explored the feasibility of extrapolating AED efficacy data from adults to pediatric patients with primary generalized tonic-clonic seizures. Dr. Nordli is the Chief of the Division of Pediatric Neurology and Codirector of the Neurosciences Institute at Children's Hospital Los Angeles.
Dr. Nordli and colleagues conducted literature searches in EMBASE, Medline, and the Cochrane Central Register of Controlled Trials for randomized, placebo-controlled clinical trials of adjunctive AED treatment for primary generalized tonic-clonic seizures in adults and children published from 1970 to 2015. Outcome data, expressed as median percent reduction in seizure frequency and greater than or equal to 50% responder rate, were extracted from eligible trials for adult and pediatric patients receiving adjunctive AEDs or placebo and used to determine the relative strength of baseline-subtracted efficacy measures.
Seven published trials of AED adjunctive therapy for primary generalized tonic-clonic seizures were eligible for quantitative analysis. Dr. Nordli and colleagues found that changes in efficacy measures were similar in adults and children with primary generalized tonic-clonic seizures and were not age-dependent. The 95% confidence intervals for the standardized mean difference in median percent reduction of seizure frequency and estimated risk ratios in greater than or equal to 50% responder rate were consistently in favor of the AED treatment and comparable between adult and pediatric groups.
Dr. Nordli's study was supported by Eisai.
Suggested Reading
Pellock JM, Carman WJ, Thyagarajan V, et al. Efficacy of antiepileptic drugs in adults predicts efficacy in children: a systematic review. Neurology. 2012;79(14):1482-1489.
BOSTON—Across a likely spectrum of syndromes with generalized seizures, the effect of adjunctive antiepileptic drug (AED) treatment on primary generalized tonic-clonic seizures appears similar between adults and children, according to a report presented at the 69th Annual Meeting of the American Academy of Neurology.
The availability of new AEDs for pediatric patients has been delayed due to the challenges of conducting clinical trials in children. In response to this challenge, the feasibility of extrapolation of adjunctive efficacy results for partial-onset seizures has been previously shown by Pellock et al from adult to pediatric populations when the disease course and pharmacokinetics of drug effects are comparable between populations. In response to a request from the Pediatric Committee of the European Medicines Agency, Douglas Nordli Jr, MD, and colleagues explored the feasibility of extrapolating AED efficacy data from adults to pediatric patients with primary generalized tonic-clonic seizures. Dr. Nordli is the Chief of the Division of Pediatric Neurology and Codirector of the Neurosciences Institute at Children's Hospital Los Angeles.
Dr. Nordli and colleagues conducted literature searches in EMBASE, Medline, and the Cochrane Central Register of Controlled Trials for randomized, placebo-controlled clinical trials of adjunctive AED treatment for primary generalized tonic-clonic seizures in adults and children published from 1970 to 2015. Outcome data, expressed as median percent reduction in seizure frequency and greater than or equal to 50% responder rate, were extracted from eligible trials for adult and pediatric patients receiving adjunctive AEDs or placebo and used to determine the relative strength of baseline-subtracted efficacy measures.
Seven published trials of AED adjunctive therapy for primary generalized tonic-clonic seizures were eligible for quantitative analysis. Dr. Nordli and colleagues found that changes in efficacy measures were similar in adults and children with primary generalized tonic-clonic seizures and were not age-dependent. The 95% confidence intervals for the standardized mean difference in median percent reduction of seizure frequency and estimated risk ratios in greater than or equal to 50% responder rate were consistently in favor of the AED treatment and comparable between adult and pediatric groups.
Dr. Nordli's study was supported by Eisai.
Suggested Reading
Pellock JM, Carman WJ, Thyagarajan V, et al. Efficacy of antiepileptic drugs in adults predicts efficacy in children: a systematic review. Neurology. 2012;79(14):1482-1489.
How Can Neurologists Diagnose Traumatic Encephalopathy Syndrome?
BOSTON—Proposed diagnostic criteria for probable or possible chronic traumatic encephalopathy (CTE), a progressive neurodegenerative disease associated with repetitive brain trauma, include a history of head impacts and various core clinical and supportive features.
The preliminary criteria, which were presented by Andrew Budson, MD, Professor of Neurology at Boston University School of Medicine, at the 69th Annual Meeting of the American Academy of Neurology, primarily were designed for research purposes, but can serve as a guide for neurologists for the diagnosis of traumatic encephalopathy syndrome. CTE is a neuropathologic diagnosis, whereas traumatic encephalopathy syndrome is a clinical diagnosis. In addition to presenting the general criteria, Dr. Budson shared diagnostic subtypes, potential biomarkers, and treatment options.
General Criteria
There are five general criteria that patients must meet to receive a diagnosis of traumatic encephalopathy syndrome, said Dr. Budson. First, there must be a history of impacts to the head based on types of injuries (eg, mild or severe traumatic brain injury, concussions, or subconcussive trauma) and sources of exposure, such as military service or involvement in contact sports for a minimum of six years, including at least two years at the college level or higher.
Second, patients must not have another neurologic disorder that likely accounts for the clinical features. Third, clinical features must be present for at least 12 months. The fourth requirement is that at least one core clinical feature (ie, cognitive, behavioral, or mood features) must be present and considered a change from baseline. Finally, at least two of nine supportive features must be present.
Core Clinical and Supportive Features
Of the core clinical features, difficulties in cognition must be reported by the patient, an informant, or a clinician and substantiated by standardized tests. Core behavioral clinical features include emotionally explosive behavior or physical and verbal abuse. Core mood clinical features include feeling overly sad, depressed, or hopeless.
In addition to core clinical features, two of the following supportive features must be present: impulsivity, anxiety, apathy, paranoia, suicidality, headache, motor signs (eg, dysarthria, dysgraphia, or other features of parkinsonism), documented decline for at least a year, or delayed onset of clinical features after a significant head impact exposure (usually at least two years).
Syndrome Subtypes
Patients may have one of four possible traumatic encephalopathy syndrome diagnostic subtypes. A behavioral/mood variant is more common among younger patients, whereas a cognitive variant is more common in older populations, said Dr. Budson. Patients also may have a mixed variant or dementia. Patients with the dementia subtype must have a progressive course of cognitive core clinical features, with or without behavior or mood features. In addition, patients with dementia must have cognitive impairment that interferes with their ability to function independently during normal daily activities.
Biomarkers and Treatment
Cavum septum pellucidum, cavum vergae, or fenestrations on neuroimaging are potential CTE biomarkers, said Dr. Budson. Normal beta amyloid CSF levels, elevated CSF p-tau/tau ratio, negative amyloid imaging, as well as cortical atrophy beyond that expected for age could also be signs of CTE. Potential experimental biomarkers include positive tau imaging and cortical thinning based on MRI.
Once physicians have made a diagnosis of probable or possible CTE, there are several treatments that may benefit patients, although no medications are approved for the treatment of CTE. Cholinesterase inhibitors may help to treat memory impairment, said Dr. Budson. For patients with depression and anxiety, selective serotonin reuptake inhibitors may be helpful. For patients with violent or explosive behavior, atypical neuroleptics may be efficacious. Memantine may benefit patients with moderate or severe dementia. Finally, to manage agitation, a combination of dextromethorphan and quinidine may be a treatment option.
—Erica Tricarico
Suggested Reading
Montenigro PH, Baugh CM, Daneshvar DH, et al. Clinical subtypes of chronic traumatic encephalopathy: literature review and proposed research diagnostic criteria for traumatic encephalopathy syndrome. Alzheimers Res Ther. 2014;6(5):68.
BOSTON—Proposed diagnostic criteria for probable or possible chronic traumatic encephalopathy (CTE), a progressive neurodegenerative disease associated with repetitive brain trauma, include a history of head impacts and various core clinical and supportive features.
The preliminary criteria, which were presented by Andrew Budson, MD, Professor of Neurology at Boston University School of Medicine, at the 69th Annual Meeting of the American Academy of Neurology, primarily were designed for research purposes, but can serve as a guide for neurologists for the diagnosis of traumatic encephalopathy syndrome. CTE is a neuropathologic diagnosis, whereas traumatic encephalopathy syndrome is a clinical diagnosis. In addition to presenting the general criteria, Dr. Budson shared diagnostic subtypes, potential biomarkers, and treatment options.
General Criteria
There are five general criteria that patients must meet to receive a diagnosis of traumatic encephalopathy syndrome, said Dr. Budson. First, there must be a history of impacts to the head based on types of injuries (eg, mild or severe traumatic brain injury, concussions, or subconcussive trauma) and sources of exposure, such as military service or involvement in contact sports for a minimum of six years, including at least two years at the college level or higher.
Second, patients must not have another neurologic disorder that likely accounts for the clinical features. Third, clinical features must be present for at least 12 months. The fourth requirement is that at least one core clinical feature (ie, cognitive, behavioral, or mood features) must be present and considered a change from baseline. Finally, at least two of nine supportive features must be present.
Core Clinical and Supportive Features
Of the core clinical features, difficulties in cognition must be reported by the patient, an informant, or a clinician and substantiated by standardized tests. Core behavioral clinical features include emotionally explosive behavior or physical and verbal abuse. Core mood clinical features include feeling overly sad, depressed, or hopeless.
In addition to core clinical features, two of the following supportive features must be present: impulsivity, anxiety, apathy, paranoia, suicidality, headache, motor signs (eg, dysarthria, dysgraphia, or other features of parkinsonism), documented decline for at least a year, or delayed onset of clinical features after a significant head impact exposure (usually at least two years).
Syndrome Subtypes
Patients may have one of four possible traumatic encephalopathy syndrome diagnostic subtypes. A behavioral/mood variant is more common among younger patients, whereas a cognitive variant is more common in older populations, said Dr. Budson. Patients also may have a mixed variant or dementia. Patients with the dementia subtype must have a progressive course of cognitive core clinical features, with or without behavior or mood features. In addition, patients with dementia must have cognitive impairment that interferes with their ability to function independently during normal daily activities.
Biomarkers and Treatment
Cavum septum pellucidum, cavum vergae, or fenestrations on neuroimaging are potential CTE biomarkers, said Dr. Budson. Normal beta amyloid CSF levels, elevated CSF p-tau/tau ratio, negative amyloid imaging, as well as cortical atrophy beyond that expected for age could also be signs of CTE. Potential experimental biomarkers include positive tau imaging and cortical thinning based on MRI.
Once physicians have made a diagnosis of probable or possible CTE, there are several treatments that may benefit patients, although no medications are approved for the treatment of CTE. Cholinesterase inhibitors may help to treat memory impairment, said Dr. Budson. For patients with depression and anxiety, selective serotonin reuptake inhibitors may be helpful. For patients with violent or explosive behavior, atypical neuroleptics may be efficacious. Memantine may benefit patients with moderate or severe dementia. Finally, to manage agitation, a combination of dextromethorphan and quinidine may be a treatment option.
—Erica Tricarico
Suggested Reading
Montenigro PH, Baugh CM, Daneshvar DH, et al. Clinical subtypes of chronic traumatic encephalopathy: literature review and proposed research diagnostic criteria for traumatic encephalopathy syndrome. Alzheimers Res Ther. 2014;6(5):68.
BOSTON—Proposed diagnostic criteria for probable or possible chronic traumatic encephalopathy (CTE), a progressive neurodegenerative disease associated with repetitive brain trauma, include a history of head impacts and various core clinical and supportive features.
The preliminary criteria, which were presented by Andrew Budson, MD, Professor of Neurology at Boston University School of Medicine, at the 69th Annual Meeting of the American Academy of Neurology, primarily were designed for research purposes, but can serve as a guide for neurologists for the diagnosis of traumatic encephalopathy syndrome. CTE is a neuropathologic diagnosis, whereas traumatic encephalopathy syndrome is a clinical diagnosis. In addition to presenting the general criteria, Dr. Budson shared diagnostic subtypes, potential biomarkers, and treatment options.
General Criteria
There are five general criteria that patients must meet to receive a diagnosis of traumatic encephalopathy syndrome, said Dr. Budson. First, there must be a history of impacts to the head based on types of injuries (eg, mild or severe traumatic brain injury, concussions, or subconcussive trauma) and sources of exposure, such as military service or involvement in contact sports for a minimum of six years, including at least two years at the college level or higher.
Second, patients must not have another neurologic disorder that likely accounts for the clinical features. Third, clinical features must be present for at least 12 months. The fourth requirement is that at least one core clinical feature (ie, cognitive, behavioral, or mood features) must be present and considered a change from baseline. Finally, at least two of nine supportive features must be present.
Core Clinical and Supportive Features
Of the core clinical features, difficulties in cognition must be reported by the patient, an informant, or a clinician and substantiated by standardized tests. Core behavioral clinical features include emotionally explosive behavior or physical and verbal abuse. Core mood clinical features include feeling overly sad, depressed, or hopeless.
In addition to core clinical features, two of the following supportive features must be present: impulsivity, anxiety, apathy, paranoia, suicidality, headache, motor signs (eg, dysarthria, dysgraphia, or other features of parkinsonism), documented decline for at least a year, or delayed onset of clinical features after a significant head impact exposure (usually at least two years).
Syndrome Subtypes
Patients may have one of four possible traumatic encephalopathy syndrome diagnostic subtypes. A behavioral/mood variant is more common among younger patients, whereas a cognitive variant is more common in older populations, said Dr. Budson. Patients also may have a mixed variant or dementia. Patients with the dementia subtype must have a progressive course of cognitive core clinical features, with or without behavior or mood features. In addition, patients with dementia must have cognitive impairment that interferes with their ability to function independently during normal daily activities.
Biomarkers and Treatment
Cavum septum pellucidum, cavum vergae, or fenestrations on neuroimaging are potential CTE biomarkers, said Dr. Budson. Normal beta amyloid CSF levels, elevated CSF p-tau/tau ratio, negative amyloid imaging, as well as cortical atrophy beyond that expected for age could also be signs of CTE. Potential experimental biomarkers include positive tau imaging and cortical thinning based on MRI.
Once physicians have made a diagnosis of probable or possible CTE, there are several treatments that may benefit patients, although no medications are approved for the treatment of CTE. Cholinesterase inhibitors may help to treat memory impairment, said Dr. Budson. For patients with depression and anxiety, selective serotonin reuptake inhibitors may be helpful. For patients with violent or explosive behavior, atypical neuroleptics may be efficacious. Memantine may benefit patients with moderate or severe dementia. Finally, to manage agitation, a combination of dextromethorphan and quinidine may be a treatment option.
—Erica Tricarico
Suggested Reading
Montenigro PH, Baugh CM, Daneshvar DH, et al. Clinical subtypes of chronic traumatic encephalopathy: literature review and proposed research diagnostic criteria for traumatic encephalopathy syndrome. Alzheimers Res Ther. 2014;6(5):68.
ACT2: Noninvasive vagus nerve stimulation benefits episodic cluster headache
BOSTON – Noninvasive vagus nerve stimulation (nVNS) proved safe and effective for the treatment of episodic cluster headaches in the pivotal, multicenter ACT2 study.
The findings confirmed those of the ACT1 study – and together, the results led to the April 18 approval by the Food and Drug Administration of the gammaCore stimulation device for the treatment of episodic cluster headaches (eCH).
In ACT2, nVNS was used to treat 495 cluster headache attacks in 50 patients randomized to the treatment group, and a sham treatment was used for 400 attacks in 52 patients. The proportion of patients who achieved pain freedom within 15 minutes (responders) was 13.5% with stimulation, compared with 11.5% with the sham treatment (odds ratio, 1.22).
Stimulation led to pain freedom within 15 minutes in 47.5% of 101 attacks in 14 patients with eCH, compared with 6.2% of 81 attacks in eCH patients in the sham treatment group (OR, 9.19), said Dr. Goadsby of King’s College, London. Conversely, the proportion of responders was only 4.8% with stimulation for 394 attacks in 34 patients with cCH, compared with 12.9% in 319 attacks in 31 patients in the sham treatment group (OR, 0.41).
Further, the proportion of patients with any type of cluster headache who achieved responder status for at least 50% of attacks was 39.5% with stimulation vs. 13.6% with sham. Among those with eCH, the proportions were 64.3% and 15.4%, respectively, and with cCH they were 29.4% and 12.9%, respectively.
Study patients were adults with eCH or cCH who were enrolled from nine tertiary care centers in four European countries. Patients agreed to not start any new treatment and to not change the dose of any existing treatment during a 1-week run-in period and during a 2-week double-blind period.
Vagus nerve stimulation was self administered using the gammaCore study device at the onset of a cluster headache attack. Three consecutive 120-second stimulations were administered, and if pain freedom was not achieved by 9 minutes, three additional stimulations could be administered.
Patients were asked to refrain from use of rescue treatments for 15 minutes after beginning stimulation (use of rescue treatments constituted a treatment failure), and an initial assessment of pain intensity was conducted at 15 minutes. All patients were eligible for nVNS during a 2-week open-label period after the double-blind period. Patients recorded cluster headache attack data in diaries.
Treatment was safe and well tolerated. A total of 20 and 14 patients in the treatment and sham groups experienced adverse events in the double-blind period (total number of events, 65 and 39, respectively); and 9 and 10 in the groups, respectively, experienced adverse drug events. The adverse events occurring more often in the treatment group included application-site irritation in two patients, application-site paresthesia in two patients, and skin irritation in two patients.
Vagus nerve stimulation is a well-established neuromodulation therapy for epilepsy and medication-resistant depression. The gammaCore device used in the ACT1 and ACT2 studies delivers treatment to the cervical branch of the vagus nerve transcutaneously, whereas prior methods involved surgically implanted devices, which are more prone to cause infection and other complications and costs.
The current study’s findings demonstrate the superiority of nVNS over sham therapy for the acute treatment of attacks in patients with eCH, Dr. Goadsby noted. The lack of benefit in those with cCH likely affected the results for the total population, he added.
The findings indicate the nVNS can be safely and easily incorporated into existing therapeutic regimens, Dr. Goadsby concluded.
The ACT1 and ACT2 studies were sponsored by electroCore, the maker of gammaCore. Dr. Goadsby reported receiving funding from electroCore and numerous other companies. He also reported having a pending patent for magnetic stimulation for headache.
BOSTON – Noninvasive vagus nerve stimulation (nVNS) proved safe and effective for the treatment of episodic cluster headaches in the pivotal, multicenter ACT2 study.
The findings confirmed those of the ACT1 study – and together, the results led to the April 18 approval by the Food and Drug Administration of the gammaCore stimulation device for the treatment of episodic cluster headaches (eCH).
In ACT2, nVNS was used to treat 495 cluster headache attacks in 50 patients randomized to the treatment group, and a sham treatment was used for 400 attacks in 52 patients. The proportion of patients who achieved pain freedom within 15 minutes (responders) was 13.5% with stimulation, compared with 11.5% with the sham treatment (odds ratio, 1.22).
Stimulation led to pain freedom within 15 minutes in 47.5% of 101 attacks in 14 patients with eCH, compared with 6.2% of 81 attacks in eCH patients in the sham treatment group (OR, 9.19), said Dr. Goadsby of King’s College, London. Conversely, the proportion of responders was only 4.8% with stimulation for 394 attacks in 34 patients with cCH, compared with 12.9% in 319 attacks in 31 patients in the sham treatment group (OR, 0.41).
Further, the proportion of patients with any type of cluster headache who achieved responder status for at least 50% of attacks was 39.5% with stimulation vs. 13.6% with sham. Among those with eCH, the proportions were 64.3% and 15.4%, respectively, and with cCH they were 29.4% and 12.9%, respectively.
Study patients were adults with eCH or cCH who were enrolled from nine tertiary care centers in four European countries. Patients agreed to not start any new treatment and to not change the dose of any existing treatment during a 1-week run-in period and during a 2-week double-blind period.
Vagus nerve stimulation was self administered using the gammaCore study device at the onset of a cluster headache attack. Three consecutive 120-second stimulations were administered, and if pain freedom was not achieved by 9 minutes, three additional stimulations could be administered.
Patients were asked to refrain from use of rescue treatments for 15 minutes after beginning stimulation (use of rescue treatments constituted a treatment failure), and an initial assessment of pain intensity was conducted at 15 minutes. All patients were eligible for nVNS during a 2-week open-label period after the double-blind period. Patients recorded cluster headache attack data in diaries.
Treatment was safe and well tolerated. A total of 20 and 14 patients in the treatment and sham groups experienced adverse events in the double-blind period (total number of events, 65 and 39, respectively); and 9 and 10 in the groups, respectively, experienced adverse drug events. The adverse events occurring more often in the treatment group included application-site irritation in two patients, application-site paresthesia in two patients, and skin irritation in two patients.
Vagus nerve stimulation is a well-established neuromodulation therapy for epilepsy and medication-resistant depression. The gammaCore device used in the ACT1 and ACT2 studies delivers treatment to the cervical branch of the vagus nerve transcutaneously, whereas prior methods involved surgically implanted devices, which are more prone to cause infection and other complications and costs.
The current study’s findings demonstrate the superiority of nVNS over sham therapy for the acute treatment of attacks in patients with eCH, Dr. Goadsby noted. The lack of benefit in those with cCH likely affected the results for the total population, he added.
The findings indicate the nVNS can be safely and easily incorporated into existing therapeutic regimens, Dr. Goadsby concluded.
The ACT1 and ACT2 studies were sponsored by electroCore, the maker of gammaCore. Dr. Goadsby reported receiving funding from electroCore and numerous other companies. He also reported having a pending patent for magnetic stimulation for headache.
BOSTON – Noninvasive vagus nerve stimulation (nVNS) proved safe and effective for the treatment of episodic cluster headaches in the pivotal, multicenter ACT2 study.
The findings confirmed those of the ACT1 study – and together, the results led to the April 18 approval by the Food and Drug Administration of the gammaCore stimulation device for the treatment of episodic cluster headaches (eCH).
In ACT2, nVNS was used to treat 495 cluster headache attacks in 50 patients randomized to the treatment group, and a sham treatment was used for 400 attacks in 52 patients. The proportion of patients who achieved pain freedom within 15 minutes (responders) was 13.5% with stimulation, compared with 11.5% with the sham treatment (odds ratio, 1.22).
Stimulation led to pain freedom within 15 minutes in 47.5% of 101 attacks in 14 patients with eCH, compared with 6.2% of 81 attacks in eCH patients in the sham treatment group (OR, 9.19), said Dr. Goadsby of King’s College, London. Conversely, the proportion of responders was only 4.8% with stimulation for 394 attacks in 34 patients with cCH, compared with 12.9% in 319 attacks in 31 patients in the sham treatment group (OR, 0.41).
Further, the proportion of patients with any type of cluster headache who achieved responder status for at least 50% of attacks was 39.5% with stimulation vs. 13.6% with sham. Among those with eCH, the proportions were 64.3% and 15.4%, respectively, and with cCH they were 29.4% and 12.9%, respectively.
Study patients were adults with eCH or cCH who were enrolled from nine tertiary care centers in four European countries. Patients agreed to not start any new treatment and to not change the dose of any existing treatment during a 1-week run-in period and during a 2-week double-blind period.
Vagus nerve stimulation was self administered using the gammaCore study device at the onset of a cluster headache attack. Three consecutive 120-second stimulations were administered, and if pain freedom was not achieved by 9 minutes, three additional stimulations could be administered.
Patients were asked to refrain from use of rescue treatments for 15 minutes after beginning stimulation (use of rescue treatments constituted a treatment failure), and an initial assessment of pain intensity was conducted at 15 minutes. All patients were eligible for nVNS during a 2-week open-label period after the double-blind period. Patients recorded cluster headache attack data in diaries.
Treatment was safe and well tolerated. A total of 20 and 14 patients in the treatment and sham groups experienced adverse events in the double-blind period (total number of events, 65 and 39, respectively); and 9 and 10 in the groups, respectively, experienced adverse drug events. The adverse events occurring more often in the treatment group included application-site irritation in two patients, application-site paresthesia in two patients, and skin irritation in two patients.
Vagus nerve stimulation is a well-established neuromodulation therapy for epilepsy and medication-resistant depression. The gammaCore device used in the ACT1 and ACT2 studies delivers treatment to the cervical branch of the vagus nerve transcutaneously, whereas prior methods involved surgically implanted devices, which are more prone to cause infection and other complications and costs.
The current study’s findings demonstrate the superiority of nVNS over sham therapy for the acute treatment of attacks in patients with eCH, Dr. Goadsby noted. The lack of benefit in those with cCH likely affected the results for the total population, he added.
The findings indicate the nVNS can be safely and easily incorporated into existing therapeutic regimens, Dr. Goadsby concluded.
The ACT1 and ACT2 studies were sponsored by electroCore, the maker of gammaCore. Dr. Goadsby reported receiving funding from electroCore and numerous other companies. He also reported having a pending patent for magnetic stimulation for headache.
AT AAN 2017
Key clinical point:
Major finding: The proportion of patients who achieved pain freedom within 15 minutes was 13.5% with noninvasive vagus nerve stimulation, compared with 11.5% with the sham treatment (odds ratio, 1.22).
Data source: The randomized, sham-controlled, multicenter ACT2 study of 102 patients.
Disclosures: The ACT1 and ACT2 studies were sponsored by electroCore, the maker of the gammaCore device. Dr. Goadsby reported receiving funding from electroCore and numerous other companies. He also reported having a pending patent for magnetic stimulation for headache.
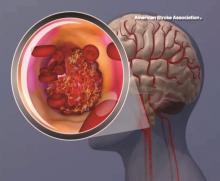
Benefit of rtPA in acute ischemic stroke doesn’t diminish with weight over 100 kg
BOSTON – Body weight over 100 kg in acute ischemic stroke patients does not reduce the clinical benefit derived from a 90-mg fixed dose of intravenous recombinant tissue plasminogen activator, according to a pooled analysis of data from three randomized clinical trials.
Prior small studies have suggested that the magnitude of benefit with intravenous recombinant tissue plasminogen activator (IV rtPA) is reduced in patients with body weight over 100 kg who receive less than 0.9 mg/kg of IV rtPA under current guidelines. However, in the current study, the rate of favorable outcomes at 90 days – defined as modified Rankin scale score of 0-2 – did not differ significantly between 872 patients with weight at or below 100 kg and 105 with body weight over 100 kg (and up to 190 kg) after adjustment for patient demographics, stroke severity, and 90-day modified Rankin scale score (adjusted odds ratio, 0.99), Shahram Majidi, MD, said at the annual meeting of the American Academy of Neurology.
The results were similar when patients with NIHSS score less than 8 were excluded, and when those with weight over 150 kg were compared with those with weight at 100 kg or less, Dr. Majidi said.
There were only eight patients who weighed more than 150 kg, but those patients did very well at 90 days, and had favorable outcomes that were comparable to those in the lower weight group, he noted.
“Body weight more than 100 kg, and receiving less than 0.9 mg/kg of IV rtPA, did not reduce the benefit of IV rtPA in acute ischemic stroke patients, and our results support the current recommendations from the American Stroke Association,” Dr. Majidi concluded.
Dr. Majidi reported having no disclosures.
BOSTON – Body weight over 100 kg in acute ischemic stroke patients does not reduce the clinical benefit derived from a 90-mg fixed dose of intravenous recombinant tissue plasminogen activator, according to a pooled analysis of data from three randomized clinical trials.
Prior small studies have suggested that the magnitude of benefit with intravenous recombinant tissue plasminogen activator (IV rtPA) is reduced in patients with body weight over 100 kg who receive less than 0.9 mg/kg of IV rtPA under current guidelines. However, in the current study, the rate of favorable outcomes at 90 days – defined as modified Rankin scale score of 0-2 – did not differ significantly between 872 patients with weight at or below 100 kg and 105 with body weight over 100 kg (and up to 190 kg) after adjustment for patient demographics, stroke severity, and 90-day modified Rankin scale score (adjusted odds ratio, 0.99), Shahram Majidi, MD, said at the annual meeting of the American Academy of Neurology.
The results were similar when patients with NIHSS score less than 8 were excluded, and when those with weight over 150 kg were compared with those with weight at 100 kg or less, Dr. Majidi said.
There were only eight patients who weighed more than 150 kg, but those patients did very well at 90 days, and had favorable outcomes that were comparable to those in the lower weight group, he noted.
“Body weight more than 100 kg, and receiving less than 0.9 mg/kg of IV rtPA, did not reduce the benefit of IV rtPA in acute ischemic stroke patients, and our results support the current recommendations from the American Stroke Association,” Dr. Majidi concluded.
Dr. Majidi reported having no disclosures.
BOSTON – Body weight over 100 kg in acute ischemic stroke patients does not reduce the clinical benefit derived from a 90-mg fixed dose of intravenous recombinant tissue plasminogen activator, according to a pooled analysis of data from three randomized clinical trials.
Prior small studies have suggested that the magnitude of benefit with intravenous recombinant tissue plasminogen activator (IV rtPA) is reduced in patients with body weight over 100 kg who receive less than 0.9 mg/kg of IV rtPA under current guidelines. However, in the current study, the rate of favorable outcomes at 90 days – defined as modified Rankin scale score of 0-2 – did not differ significantly between 872 patients with weight at or below 100 kg and 105 with body weight over 100 kg (and up to 190 kg) after adjustment for patient demographics, stroke severity, and 90-day modified Rankin scale score (adjusted odds ratio, 0.99), Shahram Majidi, MD, said at the annual meeting of the American Academy of Neurology.
The results were similar when patients with NIHSS score less than 8 were excluded, and when those with weight over 150 kg were compared with those with weight at 100 kg or less, Dr. Majidi said.
There were only eight patients who weighed more than 150 kg, but those patients did very well at 90 days, and had favorable outcomes that were comparable to those in the lower weight group, he noted.
“Body weight more than 100 kg, and receiving less than 0.9 mg/kg of IV rtPA, did not reduce the benefit of IV rtPA in acute ischemic stroke patients, and our results support the current recommendations from the American Stroke Association,” Dr. Majidi concluded.
Dr. Majidi reported having no disclosures.
Key clinical point:
Major finding: Patients with weight at or below 100 kg and those with weight over 100 kg had a similar rate of favorable outcome at 90 days (adjusted OR, 0.99).
Data source: A pooled analysis of data from 977 patients in three randomized trials.
Disclosures: Dr. Majidi reported having no disclosures.
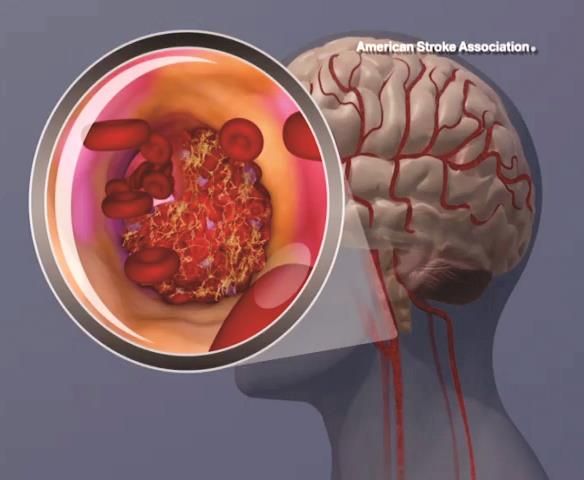
Intravenous tPA ups mortality in children with acute ischemic stroke
BOSTON – Intravenous thrombolysis with tissue plasminogen activator (tPA) is associated with adverse outcomes, including an increased risk of death, in children with acute ischemic stroke, based on a review of cases from the 2006-2010 Nationwide Inpatient Survey.
Of 20,587 patients aged 0-17 years who were included in the survey, 198 received an intervention, including tPA in 169 patients, intra-arterial thrombectomy (IAT) in 5 patients, and both tPA and IAT in 24 patients. The overall mortality was 7.8%, but in those who received tPA it was 13.8%, compared with 7.7% in those who did not, Kathryn Ess, MD, reported at the annual meeting of the American Academy of Neurology.
Other outcomes were also worse in those who received tPA. For example, untreated patients were more likely to be discharged home than were tPA-treated patients (67.8% vs. 47.5%), and intracerebral hemorrhage was more common in treated vs. untreated patients (10.1% vs. 3.8%). Costs for treated patients averaged $200,346 vs. $123,015 for untreated patients.
Children included in the review had a mean age of 6 years, 43.9% were girls, and 47.7% were white. Treated patients were older (10 years vs. 5.9 years), and comorbidities included Moyamoya disease in 12.4% of patients, cardiac valvular disease in 6.6%, and sickle cell disease in 6.5%. Those who received tPA had a higher prevalence of procoagulable conditions (15.2% vs. 2%). Of note, the higher prevalence of intracerebral hemorrhage in treated patients was not explained by Moyamoya or sickle cell disease, as patients with those comorbidities were less likely than those without those conditions to receive treatment, Dr. Ess said.
Though limited by the retrospective study design, small numbers of treated patients, a lack of data on stroke severity or functional outcomes, and the inclusion of data from years before newer thrombectomy devices became available, the findings highlight concerns about the safety and efficacy of tPA in children with ischemic stroke, she said, noting that few studies have looked at the utility of tPA with or without IAT in the pediatric population.
“Studies of the efficacy of ischemic stroke treatment in adults can’t necessarily be extrapolated to children,” she said, adding that this is especially true given the difference in etiologies of pediatric acute ischemic stroke.
Indeed, the findings underscore “the age-old adage that children are not just little adults,” said Andrew Southerland, MD, of the University of Virginia, Charlottesville, who was the discussant for the session.
“We need prospective clinical trials in children,” he said.
Dr. Ess and Dr. Southerland reported having no relevant financial disclosures.
BOSTON – Intravenous thrombolysis with tissue plasminogen activator (tPA) is associated with adverse outcomes, including an increased risk of death, in children with acute ischemic stroke, based on a review of cases from the 2006-2010 Nationwide Inpatient Survey.
Of 20,587 patients aged 0-17 years who were included in the survey, 198 received an intervention, including tPA in 169 patients, intra-arterial thrombectomy (IAT) in 5 patients, and both tPA and IAT in 24 patients. The overall mortality was 7.8%, but in those who received tPA it was 13.8%, compared with 7.7% in those who did not, Kathryn Ess, MD, reported at the annual meeting of the American Academy of Neurology.
Other outcomes were also worse in those who received tPA. For example, untreated patients were more likely to be discharged home than were tPA-treated patients (67.8% vs. 47.5%), and intracerebral hemorrhage was more common in treated vs. untreated patients (10.1% vs. 3.8%). Costs for treated patients averaged $200,346 vs. $123,015 for untreated patients.
Children included in the review had a mean age of 6 years, 43.9% were girls, and 47.7% were white. Treated patients were older (10 years vs. 5.9 years), and comorbidities included Moyamoya disease in 12.4% of patients, cardiac valvular disease in 6.6%, and sickle cell disease in 6.5%. Those who received tPA had a higher prevalence of procoagulable conditions (15.2% vs. 2%). Of note, the higher prevalence of intracerebral hemorrhage in treated patients was not explained by Moyamoya or sickle cell disease, as patients with those comorbidities were less likely than those without those conditions to receive treatment, Dr. Ess said.
Though limited by the retrospective study design, small numbers of treated patients, a lack of data on stroke severity or functional outcomes, and the inclusion of data from years before newer thrombectomy devices became available, the findings highlight concerns about the safety and efficacy of tPA in children with ischemic stroke, she said, noting that few studies have looked at the utility of tPA with or without IAT in the pediatric population.
“Studies of the efficacy of ischemic stroke treatment in adults can’t necessarily be extrapolated to children,” she said, adding that this is especially true given the difference in etiologies of pediatric acute ischemic stroke.
Indeed, the findings underscore “the age-old adage that children are not just little adults,” said Andrew Southerland, MD, of the University of Virginia, Charlottesville, who was the discussant for the session.
“We need prospective clinical trials in children,” he said.
Dr. Ess and Dr. Southerland reported having no relevant financial disclosures.
BOSTON – Intravenous thrombolysis with tissue plasminogen activator (tPA) is associated with adverse outcomes, including an increased risk of death, in children with acute ischemic stroke, based on a review of cases from the 2006-2010 Nationwide Inpatient Survey.
Of 20,587 patients aged 0-17 years who were included in the survey, 198 received an intervention, including tPA in 169 patients, intra-arterial thrombectomy (IAT) in 5 patients, and both tPA and IAT in 24 patients. The overall mortality was 7.8%, but in those who received tPA it was 13.8%, compared with 7.7% in those who did not, Kathryn Ess, MD, reported at the annual meeting of the American Academy of Neurology.
Other outcomes were also worse in those who received tPA. For example, untreated patients were more likely to be discharged home than were tPA-treated patients (67.8% vs. 47.5%), and intracerebral hemorrhage was more common in treated vs. untreated patients (10.1% vs. 3.8%). Costs for treated patients averaged $200,346 vs. $123,015 for untreated patients.
Children included in the review had a mean age of 6 years, 43.9% were girls, and 47.7% were white. Treated patients were older (10 years vs. 5.9 years), and comorbidities included Moyamoya disease in 12.4% of patients, cardiac valvular disease in 6.6%, and sickle cell disease in 6.5%. Those who received tPA had a higher prevalence of procoagulable conditions (15.2% vs. 2%). Of note, the higher prevalence of intracerebral hemorrhage in treated patients was not explained by Moyamoya or sickle cell disease, as patients with those comorbidities were less likely than those without those conditions to receive treatment, Dr. Ess said.
Though limited by the retrospective study design, small numbers of treated patients, a lack of data on stroke severity or functional outcomes, and the inclusion of data from years before newer thrombectomy devices became available, the findings highlight concerns about the safety and efficacy of tPA in children with ischemic stroke, she said, noting that few studies have looked at the utility of tPA with or without IAT in the pediatric population.
“Studies of the efficacy of ischemic stroke treatment in adults can’t necessarily be extrapolated to children,” she said, adding that this is especially true given the difference in etiologies of pediatric acute ischemic stroke.
Indeed, the findings underscore “the age-old adage that children are not just little adults,” said Andrew Southerland, MD, of the University of Virginia, Charlottesville, who was the discussant for the session.
“We need prospective clinical trials in children,” he said.
Dr. Ess and Dr. Southerland reported having no relevant financial disclosures.
AT AAN 2017
Key clinical point:
Major finding: Mortality for pediatric acute ischemic stroke was 7.8% overall, 7.7% in those who did not receive tPA, and 13.8% in those who did receive tPA.
Data source: A retrospective review of cases from the 2006-2010 Nationwide Inpatient Sample.
Disclosures: Dr. Ess and Dr. Southerland reported having no relevant financial disclosures.
Study supports link between pediatric MS and remote viral infections
BOSTON – Prior Epstein-Barr virus (EBV) infection and prior herpes simplex virus (HSV) infection each appear to be associated with development of pediatric-onset multiple sclerosis (MS), according to findings from a large national case-control study.
Samples from 360 children with MS or clinically isolated syndrome and 496 frequency-matched controls recruited from 16 pediatric MS centers across the United States were tested for EBV, cytomegalovirus (CMV), and HSV antibodies and for 25-(OH)-vitamin D levels. After adjusting for age, sex, and race/ethnicity, evidence of a remote infection with EBV was strongly associated with higher risk of pediatric-onset MS (odds ratio, 3.6), Bardia Nourbakhsh, MD, reported at the annual meeting of the American Academy of Neurology.
“We didn’t see an association between CMV and the risk of developing pediatric MS,” he said, noting that prior studies had shown a protective effect of prior CMV.
There was a trend toward an association between lower serum vitamin D levels and the risk of developing pediatric MS, but the findings are questionable because of vitamin D supplementation started after diagnosis in most patients, he noted.
Further, analysis showed that race also played a role in the relationships between prior infections and MS.
The association between HSV-1 and -2 infection was significant only among white patients, the association between prior EBV and MS was much stronger in whites than non-whites, and the association between EBV and MS was stronger in non-Hispanics than in Hispanics, he said.
The MS risk variant HLA DRB1*1501 also played a role in the associations. The association between prior HSV-1 and -2 infection and MS risk was apparent only in DRB1-negative individuals, and, conversely, the association between prior EBV and MS risk was much stronger in those who were DRB1-positive, he said.
Patients included in the study had a mean age of 15.2 years, 64% were girls, and the mean disease duration was 354 days. Controls had a mean age of 14.3 years.
“Remote viral infections have been known as one of the most commonly cited risk factors for adult and pediatric MS,” Dr. Nourbakhsh said, noting that a prior case-control study showed these associations and that other studies suggested associations with vitamin D deficiency.
The current study was conducted in an attempt to replicate those prior findings, he said.
The results of this large study support an association between prior EBV and HSV infections and MS risk and a possible association between vitamin D deficiency and MS risk but are limited by lack of testing before disease development and by vitamin D supplementation in almost all patients after diagnosis, he said.
“In the future, hopefully, we can look further at the interaction of genes and environment and the heterogeneity of the effect of risk factors in different subpopulations,” he concluded.
Dr. Nourbakhsh reported having no disclosures.
BOSTON – Prior Epstein-Barr virus (EBV) infection and prior herpes simplex virus (HSV) infection each appear to be associated with development of pediatric-onset multiple sclerosis (MS), according to findings from a large national case-control study.
Samples from 360 children with MS or clinically isolated syndrome and 496 frequency-matched controls recruited from 16 pediatric MS centers across the United States were tested for EBV, cytomegalovirus (CMV), and HSV antibodies and for 25-(OH)-vitamin D levels. After adjusting for age, sex, and race/ethnicity, evidence of a remote infection with EBV was strongly associated with higher risk of pediatric-onset MS (odds ratio, 3.6), Bardia Nourbakhsh, MD, reported at the annual meeting of the American Academy of Neurology.
“We didn’t see an association between CMV and the risk of developing pediatric MS,” he said, noting that prior studies had shown a protective effect of prior CMV.
There was a trend toward an association between lower serum vitamin D levels and the risk of developing pediatric MS, but the findings are questionable because of vitamin D supplementation started after diagnosis in most patients, he noted.
Further, analysis showed that race also played a role in the relationships between prior infections and MS.
The association between HSV-1 and -2 infection was significant only among white patients, the association between prior EBV and MS was much stronger in whites than non-whites, and the association between EBV and MS was stronger in non-Hispanics than in Hispanics, he said.
The MS risk variant HLA DRB1*1501 also played a role in the associations. The association between prior HSV-1 and -2 infection and MS risk was apparent only in DRB1-negative individuals, and, conversely, the association between prior EBV and MS risk was much stronger in those who were DRB1-positive, he said.
Patients included in the study had a mean age of 15.2 years, 64% were girls, and the mean disease duration was 354 days. Controls had a mean age of 14.3 years.
“Remote viral infections have been known as one of the most commonly cited risk factors for adult and pediatric MS,” Dr. Nourbakhsh said, noting that a prior case-control study showed these associations and that other studies suggested associations with vitamin D deficiency.
The current study was conducted in an attempt to replicate those prior findings, he said.
The results of this large study support an association between prior EBV and HSV infections and MS risk and a possible association between vitamin D deficiency and MS risk but are limited by lack of testing before disease development and by vitamin D supplementation in almost all patients after diagnosis, he said.
“In the future, hopefully, we can look further at the interaction of genes and environment and the heterogeneity of the effect of risk factors in different subpopulations,” he concluded.
Dr. Nourbakhsh reported having no disclosures.
BOSTON – Prior Epstein-Barr virus (EBV) infection and prior herpes simplex virus (HSV) infection each appear to be associated with development of pediatric-onset multiple sclerosis (MS), according to findings from a large national case-control study.
Samples from 360 children with MS or clinically isolated syndrome and 496 frequency-matched controls recruited from 16 pediatric MS centers across the United States were tested for EBV, cytomegalovirus (CMV), and HSV antibodies and for 25-(OH)-vitamin D levels. After adjusting for age, sex, and race/ethnicity, evidence of a remote infection with EBV was strongly associated with higher risk of pediatric-onset MS (odds ratio, 3.6), Bardia Nourbakhsh, MD, reported at the annual meeting of the American Academy of Neurology.
“We didn’t see an association between CMV and the risk of developing pediatric MS,” he said, noting that prior studies had shown a protective effect of prior CMV.
There was a trend toward an association between lower serum vitamin D levels and the risk of developing pediatric MS, but the findings are questionable because of vitamin D supplementation started after diagnosis in most patients, he noted.
Further, analysis showed that race also played a role in the relationships between prior infections and MS.
The association between HSV-1 and -2 infection was significant only among white patients, the association between prior EBV and MS was much stronger in whites than non-whites, and the association between EBV and MS was stronger in non-Hispanics than in Hispanics, he said.
The MS risk variant HLA DRB1*1501 also played a role in the associations. The association between prior HSV-1 and -2 infection and MS risk was apparent only in DRB1-negative individuals, and, conversely, the association between prior EBV and MS risk was much stronger in those who were DRB1-positive, he said.
Patients included in the study had a mean age of 15.2 years, 64% were girls, and the mean disease duration was 354 days. Controls had a mean age of 14.3 years.
“Remote viral infections have been known as one of the most commonly cited risk factors for adult and pediatric MS,” Dr. Nourbakhsh said, noting that a prior case-control study showed these associations and that other studies suggested associations with vitamin D deficiency.
The current study was conducted in an attempt to replicate those prior findings, he said.
The results of this large study support an association between prior EBV and HSV infections and MS risk and a possible association between vitamin D deficiency and MS risk but are limited by lack of testing before disease development and by vitamin D supplementation in almost all patients after diagnosis, he said.
“In the future, hopefully, we can look further at the interaction of genes and environment and the heterogeneity of the effect of risk factors in different subpopulations,” he concluded.
Dr. Nourbakhsh reported having no disclosures.
Key clinical point:
Major finding: Remote infections with EBV and HSV were associated with higher risk of pediatric-onset MS (odds ratios, 3.6 and 1.5, respectively).
Data source: A study of 360 pediatric MS patients and 496 controls.
Disclosures: Dr. Nourbakhsh reported having no disclosures.
VIDEO: Care withdrawal becoming more common in ischemic stroke patients
BOSTON – U.S. physicians chose to withdraw life-sustaining care from critically ill ischemic stroke patients at much higher rates in 2011 than in 2006, according to results of a new study, although overall percentages remain low.
The trend was bolstered by big jumps in “withdrawal of care” among patients who underwent thrombolysis or both thrombolysis and endovascular treatment: Over the 5-year period, their likelihood of having care withdrawn increased fivefold.
The study authors don’t know whether the trends have continued over the past 6 years, and it’s not clear why the rates rose so much from 2006 to 2011. The researchers speculate that the increase could be linked to disease severity and the preferences of patients and their families.
Whatever the case, study lead author Malik Muhammad Adil, MD, a neurology resident at the Ochsner Clinic Foundation in New Orleans, cautioned that prematurely withdrawing care can throw off stroke prognostication estimates that are already fuzzy. In an interview, he said this can then lead to “significant consequences, including suboptimal outcomes and higher risk of short-term mortality.”
Dr. Adil defines withdrawal of care as “discontinuation of life-sustaining interventions from a patient who is expected to die without this support.” These interventions include such treatments as intubation, mechanical ventilation, feeding tubes, antibiotics, and brain surgery, he said.
For the new study, researchers examined the Nationwide Inpatient Survey database for the years 2006-2011. They reported their findings at the annual meeting of the American Academy of Neurology.
The study reports the following regarding withdrawal of care among ischemic stroke patients:
- The rate grew in those who received neither thrombolysis nor endovascular treatment from 0.8% in 2006 to 3.0% in 2011 (P less than or equal to .0001).
- In those who received thrombolysis alone, the rate rose from 0.9% to 5.5% (P less than or equal to .0001).
- In those who received endovascular treatment alone, the rate increased from 2.8% to 9.0% (P = .0006).
- In patients who received both thrombolysis and endovascular treatment, the rate grew from 2.0% to 10.3% (P = .0009).
Dr. Adil said several factors can affect rates of withdrawal of care in ischemic stroke patients, such as the level of illness (patients receiving aggressive treatment are sicker), advance directives, and the decisions of family members. Some institutions may be more likely to push for withdrawal of care, too, he said. “At my institution, we are not aggressive with withdrawal of care, and I have seen a few better outcomes than expected,” he said.
Also, the lack of useful data regarding prognosis for these patients may lead to premature decisions regarding withdrawal of care, he said.
“We have few prognostic models/scores that predict mortality, and these models are not very sensitive and specific,” Dr. Adil said. “Often, physicians make these decisions based on their previous experiences. All of this leads to premature withdrawal of care. On the other hand, because of premature withdrawal of care, we do not have the data on long-term outcomes on these patients, leading to errors in prognostication.”
In an interview, Adam G. Kelly, MD, a neurologist at the University of Rochester (N.Y.) and chief of neurology at Highland Hospital in Rochester, said the study is important but lacks crucial information such as the circumstances surrounding the care decisions. He hasn’t noticed trends in withdrawal of care among his patients.
“It’s possible that providers have become better at documenting discussions with patients and families which allowed this information to be better captured,” he said. “As the authors mention, it’s also possible that providers are consciously or unconsciously delivering prognoses that are biased towards negative outcomes, leading patients and families to be more apt to choose a palliative approach.”
Dr. Kelly added that neurologists need to objectively offer prognoses in stroke cases. “When the outcome is in doubt, I recommend time-limited trials of interventions of mechanical ventilation, artificial feeding, and other high-intensity interventions to allow patients and families to make the decision they feel is best,” he said.
What about trends in the years since 2011? Dr. Adil said information regarding the years since 2011 wasn’t available to him, but he hopes to analyze the period from 2012 to 2016.
Dr. Adil discussed his study and its implications in a video interview.
No funding is reported. Dr. Adil reports no relevant disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
cnnews@frontlinemedcom.com
BOSTON – U.S. physicians chose to withdraw life-sustaining care from critically ill ischemic stroke patients at much higher rates in 2011 than in 2006, according to results of a new study, although overall percentages remain low.
The trend was bolstered by big jumps in “withdrawal of care” among patients who underwent thrombolysis or both thrombolysis and endovascular treatment: Over the 5-year period, their likelihood of having care withdrawn increased fivefold.
The study authors don’t know whether the trends have continued over the past 6 years, and it’s not clear why the rates rose so much from 2006 to 2011. The researchers speculate that the increase could be linked to disease severity and the preferences of patients and their families.
Whatever the case, study lead author Malik Muhammad Adil, MD, a neurology resident at the Ochsner Clinic Foundation in New Orleans, cautioned that prematurely withdrawing care can throw off stroke prognostication estimates that are already fuzzy. In an interview, he said this can then lead to “significant consequences, including suboptimal outcomes and higher risk of short-term mortality.”
Dr. Adil defines withdrawal of care as “discontinuation of life-sustaining interventions from a patient who is expected to die without this support.” These interventions include such treatments as intubation, mechanical ventilation, feeding tubes, antibiotics, and brain surgery, he said.
For the new study, researchers examined the Nationwide Inpatient Survey database for the years 2006-2011. They reported their findings at the annual meeting of the American Academy of Neurology.
The study reports the following regarding withdrawal of care among ischemic stroke patients:
- The rate grew in those who received neither thrombolysis nor endovascular treatment from 0.8% in 2006 to 3.0% in 2011 (P less than or equal to .0001).
- In those who received thrombolysis alone, the rate rose from 0.9% to 5.5% (P less than or equal to .0001).
- In those who received endovascular treatment alone, the rate increased from 2.8% to 9.0% (P = .0006).
- In patients who received both thrombolysis and endovascular treatment, the rate grew from 2.0% to 10.3% (P = .0009).
Dr. Adil said several factors can affect rates of withdrawal of care in ischemic stroke patients, such as the level of illness (patients receiving aggressive treatment are sicker), advance directives, and the decisions of family members. Some institutions may be more likely to push for withdrawal of care, too, he said. “At my institution, we are not aggressive with withdrawal of care, and I have seen a few better outcomes than expected,” he said.
Also, the lack of useful data regarding prognosis for these patients may lead to premature decisions regarding withdrawal of care, he said.
“We have few prognostic models/scores that predict mortality, and these models are not very sensitive and specific,” Dr. Adil said. “Often, physicians make these decisions based on their previous experiences. All of this leads to premature withdrawal of care. On the other hand, because of premature withdrawal of care, we do not have the data on long-term outcomes on these patients, leading to errors in prognostication.”
In an interview, Adam G. Kelly, MD, a neurologist at the University of Rochester (N.Y.) and chief of neurology at Highland Hospital in Rochester, said the study is important but lacks crucial information such as the circumstances surrounding the care decisions. He hasn’t noticed trends in withdrawal of care among his patients.
“It’s possible that providers have become better at documenting discussions with patients and families which allowed this information to be better captured,” he said. “As the authors mention, it’s also possible that providers are consciously or unconsciously delivering prognoses that are biased towards negative outcomes, leading patients and families to be more apt to choose a palliative approach.”
Dr. Kelly added that neurologists need to objectively offer prognoses in stroke cases. “When the outcome is in doubt, I recommend time-limited trials of interventions of mechanical ventilation, artificial feeding, and other high-intensity interventions to allow patients and families to make the decision they feel is best,” he said.
What about trends in the years since 2011? Dr. Adil said information regarding the years since 2011 wasn’t available to him, but he hopes to analyze the period from 2012 to 2016.
Dr. Adil discussed his study and its implications in a video interview.
No funding is reported. Dr. Adil reports no relevant disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
cnnews@frontlinemedcom.com
BOSTON – U.S. physicians chose to withdraw life-sustaining care from critically ill ischemic stroke patients at much higher rates in 2011 than in 2006, according to results of a new study, although overall percentages remain low.
The trend was bolstered by big jumps in “withdrawal of care” among patients who underwent thrombolysis or both thrombolysis and endovascular treatment: Over the 5-year period, their likelihood of having care withdrawn increased fivefold.
The study authors don’t know whether the trends have continued over the past 6 years, and it’s not clear why the rates rose so much from 2006 to 2011. The researchers speculate that the increase could be linked to disease severity and the preferences of patients and their families.
Whatever the case, study lead author Malik Muhammad Adil, MD, a neurology resident at the Ochsner Clinic Foundation in New Orleans, cautioned that prematurely withdrawing care can throw off stroke prognostication estimates that are already fuzzy. In an interview, he said this can then lead to “significant consequences, including suboptimal outcomes and higher risk of short-term mortality.”
Dr. Adil defines withdrawal of care as “discontinuation of life-sustaining interventions from a patient who is expected to die without this support.” These interventions include such treatments as intubation, mechanical ventilation, feeding tubes, antibiotics, and brain surgery, he said.
For the new study, researchers examined the Nationwide Inpatient Survey database for the years 2006-2011. They reported their findings at the annual meeting of the American Academy of Neurology.
The study reports the following regarding withdrawal of care among ischemic stroke patients:
- The rate grew in those who received neither thrombolysis nor endovascular treatment from 0.8% in 2006 to 3.0% in 2011 (P less than or equal to .0001).
- In those who received thrombolysis alone, the rate rose from 0.9% to 5.5% (P less than or equal to .0001).
- In those who received endovascular treatment alone, the rate increased from 2.8% to 9.0% (P = .0006).
- In patients who received both thrombolysis and endovascular treatment, the rate grew from 2.0% to 10.3% (P = .0009).
Dr. Adil said several factors can affect rates of withdrawal of care in ischemic stroke patients, such as the level of illness (patients receiving aggressive treatment are sicker), advance directives, and the decisions of family members. Some institutions may be more likely to push for withdrawal of care, too, he said. “At my institution, we are not aggressive with withdrawal of care, and I have seen a few better outcomes than expected,” he said.
Also, the lack of useful data regarding prognosis for these patients may lead to premature decisions regarding withdrawal of care, he said.
“We have few prognostic models/scores that predict mortality, and these models are not very sensitive and specific,” Dr. Adil said. “Often, physicians make these decisions based on their previous experiences. All of this leads to premature withdrawal of care. On the other hand, because of premature withdrawal of care, we do not have the data on long-term outcomes on these patients, leading to errors in prognostication.”
In an interview, Adam G. Kelly, MD, a neurologist at the University of Rochester (N.Y.) and chief of neurology at Highland Hospital in Rochester, said the study is important but lacks crucial information such as the circumstances surrounding the care decisions. He hasn’t noticed trends in withdrawal of care among his patients.
“It’s possible that providers have become better at documenting discussions with patients and families which allowed this information to be better captured,” he said. “As the authors mention, it’s also possible that providers are consciously or unconsciously delivering prognoses that are biased towards negative outcomes, leading patients and families to be more apt to choose a palliative approach.”
Dr. Kelly added that neurologists need to objectively offer prognoses in stroke cases. “When the outcome is in doubt, I recommend time-limited trials of interventions of mechanical ventilation, artificial feeding, and other high-intensity interventions to allow patients and families to make the decision they feel is best,” he said.
What about trends in the years since 2011? Dr. Adil said information regarding the years since 2011 wasn’t available to him, but he hopes to analyze the period from 2012 to 2016.
Dr. Adil discussed his study and its implications in a video interview.
No funding is reported. Dr. Adil reports no relevant disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
cnnews@frontlinemedcom.com
Key clinical point:
Major finding: The percentages of ischemic stroke patients whose care was withdrawn grew from 0.8%-2.8% in 2006 to 3.0%-10.3% in 2011, depending on the kind of treatment they received (thrombolysis, endovascular treatment, both, or neither).
Data source: Nationwide Inpatient Survey database.
Disclosures: No funding is reported. Dr. Adil reports no relevant disclosures.
Intensive BP lowering may reduce larger hematoma expansion in ICH
BOSTON – Intensive systolic blood pressure reduction did not significantly reduce hematoma expansion, compared with standard systolic blood pressure reduction in the Antihypertensive Treatment of Acute Cerebral Hemorrhage (ATACH) II trial, but, in a post hoc analysis, it did show a strong trend toward reducing hematoma expansion in those with a larger initial hematoma volume.
In 450 patients randomized to receive intensive treatment and 426 randomized to receive standard treatment in the large, international, phase III randomized trial, the proportion of patients with any hematoma expansion was 46.4% and 52.3% , respectively (relative risk, 0.89; 95% confidence interval, 0.73-1.07). The proportion with hematoma expansion with an increase of greater than 33% was 18.9% and 24.4%, respectively (RR, 0.77; 95% CI, 0.58-1.03), Joshua N. Goldstein, MD, reported at the annual meeting of the American Academy of Neurology.
The confidence intervals suggested only a trend toward improvement, as the difference between the groups did not reach statistical significance, he said.
To determine if intensive systolic blood pressure reduction might have more of an impact among higher-risk patients, the investigators conducted a post hoc, secondary analysis in those with relatively larger hematomas. Among patients with an initial hematoma volume of at least 10 mL (at least half of the study population had smaller hematomas), the proportion with any hematoma expansion was 53.8% in the intensive treatment group and 61.3% in the standard treatment group (RR, 0.88; 95% CI, 0.67-1.13), and the proportion of patients with hematoma expansion of greater than 33% was 18% and 27.6%, respectively (RR, 0.67; 95% CI, 0.45-1.00), said Dr. Goldstein of Massachusetts General Hospital, Boston.
In those with an expansion greater than 33% and a hematoma volume of at least 6 cc, the finding was similar.
“The trend looks a little bit more aggressive,” he said, but the 95% confidence interval in those with larger hematomas reached 1.
Study subjects had elevated blood pressure at arrival, a Glasgow coma scale score of at least 5, and a hematoma volume of less than 60 cc. They were enrolled and randomized within 4.5 hours of symptom onset. Those in the intensive treatment group were treated with a goal of achieving between 110 and 139 mm Hg within 24 hours, and those in the standard treatment group were treated with a goal of achieving between 140 mm Hg and 179 mm Hg within 24 hours. They underwent baseline and 24-hour computed tomography scans, which were analyzed centrally by blinded investigators who recorded any increase of 0.5 mL or more, an increase of more than 33% , an increase of more than 33% or more than 6 mL, and an intraventricular hemorrhage volume greater than 2 mL.
These measures were correlated with death and disability, and hematoma enlargement was shown to be significantly associated with those outcomes at 3 months after randomization (RR, 1.59; 95% CI, 1.25-2.02), Dr. Goldstein said.
Previous studies have suggested that intensive lowering of systolic BP in patients with intracerebral hemorrhage can reduce the rate of hematoma expansion. As such, the hypothesis of the current study was that the intensive treatment of elevated blood pressures – arriving systolic blood pressure of greater than 180 mm Hg – would reduce the likelihood of death and disability at 3 months, Dr. Goldstein said,
The question is whether the biomarker – hematoma expansion – is really linked to clinical outcome, he said, “because a lot of our attempts to treat hematoma expansion are based on the assumption that, if we reduce hematoma expansion, we’re going to improve clinical outcomes.”
“In this trial ... the expanders had more death and disability than nonexpanders, and this was statistically significant, so hematoma expansion does seem to be a statistically significant predictor of poor outcome,” he said.
Thus, the strong trend toward a reduced risk of hematoma expansion with intensive blood pressure lowering in patients with larger hematoma volumes at baseline in this analysis is noteworthy, he said.
“It appears that the treatment is affecting the biomarker, that the treatment is affecting hematoma expansion ... and hematoma expansion was a significant predictor of death and disability ... so why didn’t we get the result we wanted from the trial?” he asked.
It may be that a greater magnitude of reduction in the risk of hematoma expansion was necessary, he said.
“In other words, even if our treatment is having an effect, it’s just not having a big enough effect to change outcomes,” he said, adding that future trials probably need to involve a much bigger impact on the risk of expansion to translate to a change in clinical outcomes.”
This study was sponsored by the National Institute of Neurological Disorders and Stroke. Dr. Goldstein reported having no disclosures.
BOSTON – Intensive systolic blood pressure reduction did not significantly reduce hematoma expansion, compared with standard systolic blood pressure reduction in the Antihypertensive Treatment of Acute Cerebral Hemorrhage (ATACH) II trial, but, in a post hoc analysis, it did show a strong trend toward reducing hematoma expansion in those with a larger initial hematoma volume.
In 450 patients randomized to receive intensive treatment and 426 randomized to receive standard treatment in the large, international, phase III randomized trial, the proportion of patients with any hematoma expansion was 46.4% and 52.3% , respectively (relative risk, 0.89; 95% confidence interval, 0.73-1.07). The proportion with hematoma expansion with an increase of greater than 33% was 18.9% and 24.4%, respectively (RR, 0.77; 95% CI, 0.58-1.03), Joshua N. Goldstein, MD, reported at the annual meeting of the American Academy of Neurology.
The confidence intervals suggested only a trend toward improvement, as the difference between the groups did not reach statistical significance, he said.
To determine if intensive systolic blood pressure reduction might have more of an impact among higher-risk patients, the investigators conducted a post hoc, secondary analysis in those with relatively larger hematomas. Among patients with an initial hematoma volume of at least 10 mL (at least half of the study population had smaller hematomas), the proportion with any hematoma expansion was 53.8% in the intensive treatment group and 61.3% in the standard treatment group (RR, 0.88; 95% CI, 0.67-1.13), and the proportion of patients with hematoma expansion of greater than 33% was 18% and 27.6%, respectively (RR, 0.67; 95% CI, 0.45-1.00), said Dr. Goldstein of Massachusetts General Hospital, Boston.
In those with an expansion greater than 33% and a hematoma volume of at least 6 cc, the finding was similar.
“The trend looks a little bit more aggressive,” he said, but the 95% confidence interval in those with larger hematomas reached 1.
Study subjects had elevated blood pressure at arrival, a Glasgow coma scale score of at least 5, and a hematoma volume of less than 60 cc. They were enrolled and randomized within 4.5 hours of symptom onset. Those in the intensive treatment group were treated with a goal of achieving between 110 and 139 mm Hg within 24 hours, and those in the standard treatment group were treated with a goal of achieving between 140 mm Hg and 179 mm Hg within 24 hours. They underwent baseline and 24-hour computed tomography scans, which were analyzed centrally by blinded investigators who recorded any increase of 0.5 mL or more, an increase of more than 33% , an increase of more than 33% or more than 6 mL, and an intraventricular hemorrhage volume greater than 2 mL.
These measures were correlated with death and disability, and hematoma enlargement was shown to be significantly associated with those outcomes at 3 months after randomization (RR, 1.59; 95% CI, 1.25-2.02), Dr. Goldstein said.
Previous studies have suggested that intensive lowering of systolic BP in patients with intracerebral hemorrhage can reduce the rate of hematoma expansion. As such, the hypothesis of the current study was that the intensive treatment of elevated blood pressures – arriving systolic blood pressure of greater than 180 mm Hg – would reduce the likelihood of death and disability at 3 months, Dr. Goldstein said,
The question is whether the biomarker – hematoma expansion – is really linked to clinical outcome, he said, “because a lot of our attempts to treat hematoma expansion are based on the assumption that, if we reduce hematoma expansion, we’re going to improve clinical outcomes.”
“In this trial ... the expanders had more death and disability than nonexpanders, and this was statistically significant, so hematoma expansion does seem to be a statistically significant predictor of poor outcome,” he said.
Thus, the strong trend toward a reduced risk of hematoma expansion with intensive blood pressure lowering in patients with larger hematoma volumes at baseline in this analysis is noteworthy, he said.
“It appears that the treatment is affecting the biomarker, that the treatment is affecting hematoma expansion ... and hematoma expansion was a significant predictor of death and disability ... so why didn’t we get the result we wanted from the trial?” he asked.
It may be that a greater magnitude of reduction in the risk of hematoma expansion was necessary, he said.
“In other words, even if our treatment is having an effect, it’s just not having a big enough effect to change outcomes,” he said, adding that future trials probably need to involve a much bigger impact on the risk of expansion to translate to a change in clinical outcomes.”
This study was sponsored by the National Institute of Neurological Disorders and Stroke. Dr. Goldstein reported having no disclosures.
BOSTON – Intensive systolic blood pressure reduction did not significantly reduce hematoma expansion, compared with standard systolic blood pressure reduction in the Antihypertensive Treatment of Acute Cerebral Hemorrhage (ATACH) II trial, but, in a post hoc analysis, it did show a strong trend toward reducing hematoma expansion in those with a larger initial hematoma volume.
In 450 patients randomized to receive intensive treatment and 426 randomized to receive standard treatment in the large, international, phase III randomized trial, the proportion of patients with any hematoma expansion was 46.4% and 52.3% , respectively (relative risk, 0.89; 95% confidence interval, 0.73-1.07). The proportion with hematoma expansion with an increase of greater than 33% was 18.9% and 24.4%, respectively (RR, 0.77; 95% CI, 0.58-1.03), Joshua N. Goldstein, MD, reported at the annual meeting of the American Academy of Neurology.
The confidence intervals suggested only a trend toward improvement, as the difference between the groups did not reach statistical significance, he said.
To determine if intensive systolic blood pressure reduction might have more of an impact among higher-risk patients, the investigators conducted a post hoc, secondary analysis in those with relatively larger hematomas. Among patients with an initial hematoma volume of at least 10 mL (at least half of the study population had smaller hematomas), the proportion with any hematoma expansion was 53.8% in the intensive treatment group and 61.3% in the standard treatment group (RR, 0.88; 95% CI, 0.67-1.13), and the proportion of patients with hematoma expansion of greater than 33% was 18% and 27.6%, respectively (RR, 0.67; 95% CI, 0.45-1.00), said Dr. Goldstein of Massachusetts General Hospital, Boston.
In those with an expansion greater than 33% and a hematoma volume of at least 6 cc, the finding was similar.
“The trend looks a little bit more aggressive,” he said, but the 95% confidence interval in those with larger hematomas reached 1.
Study subjects had elevated blood pressure at arrival, a Glasgow coma scale score of at least 5, and a hematoma volume of less than 60 cc. They were enrolled and randomized within 4.5 hours of symptom onset. Those in the intensive treatment group were treated with a goal of achieving between 110 and 139 mm Hg within 24 hours, and those in the standard treatment group were treated with a goal of achieving between 140 mm Hg and 179 mm Hg within 24 hours. They underwent baseline and 24-hour computed tomography scans, which were analyzed centrally by blinded investigators who recorded any increase of 0.5 mL or more, an increase of more than 33% , an increase of more than 33% or more than 6 mL, and an intraventricular hemorrhage volume greater than 2 mL.
These measures were correlated with death and disability, and hematoma enlargement was shown to be significantly associated with those outcomes at 3 months after randomization (RR, 1.59; 95% CI, 1.25-2.02), Dr. Goldstein said.
Previous studies have suggested that intensive lowering of systolic BP in patients with intracerebral hemorrhage can reduce the rate of hematoma expansion. As such, the hypothesis of the current study was that the intensive treatment of elevated blood pressures – arriving systolic blood pressure of greater than 180 mm Hg – would reduce the likelihood of death and disability at 3 months, Dr. Goldstein said,
The question is whether the biomarker – hematoma expansion – is really linked to clinical outcome, he said, “because a lot of our attempts to treat hematoma expansion are based on the assumption that, if we reduce hematoma expansion, we’re going to improve clinical outcomes.”
“In this trial ... the expanders had more death and disability than nonexpanders, and this was statistically significant, so hematoma expansion does seem to be a statistically significant predictor of poor outcome,” he said.
Thus, the strong trend toward a reduced risk of hematoma expansion with intensive blood pressure lowering in patients with larger hematoma volumes at baseline in this analysis is noteworthy, he said.
“It appears that the treatment is affecting the biomarker, that the treatment is affecting hematoma expansion ... and hematoma expansion was a significant predictor of death and disability ... so why didn’t we get the result we wanted from the trial?” he asked.
It may be that a greater magnitude of reduction in the risk of hematoma expansion was necessary, he said.
“In other words, even if our treatment is having an effect, it’s just not having a big enough effect to change outcomes,” he said, adding that future trials probably need to involve a much bigger impact on the risk of expansion to translate to a change in clinical outcomes.”
This study was sponsored by the National Institute of Neurological Disorders and Stroke. Dr. Goldstein reported having no disclosures.
Key clinical point:
Major finding: Among patients with larger initial hematoma volume, expansion greater than 33% occurred in 18% vs. 28% in those with intense vs. standard blood pressure lowering, respectively (RR, 0.67).
Data source: The randomized phase III ATACH II trial involving 876 patients.
Disclosures: This study was sponsored by the National Institute of Neurological Disorders and Stroke. Dr. Goldstein reported having no disclosures.
PATH study: Subcutaneous immunoglobulin safe, effective for CIDP maintenance
BOSTON – Subcutaneously administered immunoglobulin was effective, well tolerated, and preferred over intravenous administration as maintenance treatment for chronic inflammatory demyelinating polyneuropathy in the phase III, randomized, placebo-controlled PATH study.
The 172-patient trial tested a high and low dose of subcutaneous immunoglobulin (SCIg) over the course of 25 weeks to determine their effect on the primary outcome of chronic inflammatory demyelinating polyneuropathy (CIDP) relapse or withdrawal from treatment for any reason. In this evaluation of using SCIg for maintenance of response, relapses or treatment withdrawal occurred in 63% with placebo, 39% with low dose SCIg (0.2 g/kg weekly), and 33% with high dose (0.4 g/kg weekly), Ivo N. van Schaik, MD, reported at the annual meeting of the American Academy of Neurology.
Patients in the trial had received at lease one dose of intravenous immunoglobulin (IVIg) within 8 weeks before screening. They then underwent a screening period first, followed by an IgG dependency period of up to 12 weeks to test for ongoing need for IgG. The patients who experienced CIDP relapse during this test period were administered a standardized IVIG regimen during a 10- to 13-week restabilization period, and those who improved and maintained their Inflammatory Neuropathy Cause and Treatment (INCAT) score continued to the randomized subcutaneous treatment period of the study.
CIDP relapse occurred in 56% of patients in the placebo group, compared with 33% in the low- and 19% in the high-dose SCIg groups, said Dr. van Schaik of the University of Amsterdam (the Netherlands).
“Both [SCIg] doses were effective in preventing relapse. The higher dose performed better than the lower dose, but the difference was not statistically significant,” he said.
Both doses were significantly more effective than placebo.
Study participants were adults with definite or probable CIDP enrolled from 69 neuromuscular centers worldwide between March 2012 and November 2015. Weekly self-administered subcutaneous infusions of SCIg (IgPro20Hizentra) were performed during 1 or 2 consecutive days in two separate sessions using special infusion pumps. Patients reported that learning the self-administration technique was easy, Dr. van Schaik said.
Adverse effects included mainly local reactions, which occurred in 19% of patients, but these were generally mild and rarely resulted in therapy discontinuation, and local reactions decreased considerably over time, he said, noting that systemic effects are reduced with SCIg vs. IVIg.
Subcutaneous administration of immunoglobulin is not new. In fact, it has been used successfully in patients with immunodeficiency syndromes for more than 2 decades and can increase patient autonomy and reduce costs by reducing hospital and infusion center visits, but this is the first study to assess efficacy, safety, and tolerability of this approach in an adequately powered, randomized, clinical trial, he said.
“Subcutaneous immunoglobulin can be used ... for maintenance treatment of patients with CIDP,” he concluded, adding that weekly doses of 0.2-0.4 g/kg are supported by these data, and that maintenance doses should be individualized based on patient factors and previous IVIg dose and frequency.
The PATH study was sponsored by CSL-Behring. Dr. van Schaik chairs a steering committee for CSL-Behring and received departmental honoraria for serving on scientific advisory boards for CSL-Behring, Baxalta, and UCB. He also received speakers fees from CSL-Behring and Kedrion.
BOSTON – Subcutaneously administered immunoglobulin was effective, well tolerated, and preferred over intravenous administration as maintenance treatment for chronic inflammatory demyelinating polyneuropathy in the phase III, randomized, placebo-controlled PATH study.
The 172-patient trial tested a high and low dose of subcutaneous immunoglobulin (SCIg) over the course of 25 weeks to determine their effect on the primary outcome of chronic inflammatory demyelinating polyneuropathy (CIDP) relapse or withdrawal from treatment for any reason. In this evaluation of using SCIg for maintenance of response, relapses or treatment withdrawal occurred in 63% with placebo, 39% with low dose SCIg (0.2 g/kg weekly), and 33% with high dose (0.4 g/kg weekly), Ivo N. van Schaik, MD, reported at the annual meeting of the American Academy of Neurology.
Patients in the trial had received at lease one dose of intravenous immunoglobulin (IVIg) within 8 weeks before screening. They then underwent a screening period first, followed by an IgG dependency period of up to 12 weeks to test for ongoing need for IgG. The patients who experienced CIDP relapse during this test period were administered a standardized IVIG regimen during a 10- to 13-week restabilization period, and those who improved and maintained their Inflammatory Neuropathy Cause and Treatment (INCAT) score continued to the randomized subcutaneous treatment period of the study.
CIDP relapse occurred in 56% of patients in the placebo group, compared with 33% in the low- and 19% in the high-dose SCIg groups, said Dr. van Schaik of the University of Amsterdam (the Netherlands).
“Both [SCIg] doses were effective in preventing relapse. The higher dose performed better than the lower dose, but the difference was not statistically significant,” he said.
Both doses were significantly more effective than placebo.
Study participants were adults with definite or probable CIDP enrolled from 69 neuromuscular centers worldwide between March 2012 and November 2015. Weekly self-administered subcutaneous infusions of SCIg (IgPro20Hizentra) were performed during 1 or 2 consecutive days in two separate sessions using special infusion pumps. Patients reported that learning the self-administration technique was easy, Dr. van Schaik said.
Adverse effects included mainly local reactions, which occurred in 19% of patients, but these were generally mild and rarely resulted in therapy discontinuation, and local reactions decreased considerably over time, he said, noting that systemic effects are reduced with SCIg vs. IVIg.
Subcutaneous administration of immunoglobulin is not new. In fact, it has been used successfully in patients with immunodeficiency syndromes for more than 2 decades and can increase patient autonomy and reduce costs by reducing hospital and infusion center visits, but this is the first study to assess efficacy, safety, and tolerability of this approach in an adequately powered, randomized, clinical trial, he said.
“Subcutaneous immunoglobulin can be used ... for maintenance treatment of patients with CIDP,” he concluded, adding that weekly doses of 0.2-0.4 g/kg are supported by these data, and that maintenance doses should be individualized based on patient factors and previous IVIg dose and frequency.
The PATH study was sponsored by CSL-Behring. Dr. van Schaik chairs a steering committee for CSL-Behring and received departmental honoraria for serving on scientific advisory boards for CSL-Behring, Baxalta, and UCB. He also received speakers fees from CSL-Behring and Kedrion.
BOSTON – Subcutaneously administered immunoglobulin was effective, well tolerated, and preferred over intravenous administration as maintenance treatment for chronic inflammatory demyelinating polyneuropathy in the phase III, randomized, placebo-controlled PATH study.
The 172-patient trial tested a high and low dose of subcutaneous immunoglobulin (SCIg) over the course of 25 weeks to determine their effect on the primary outcome of chronic inflammatory demyelinating polyneuropathy (CIDP) relapse or withdrawal from treatment for any reason. In this evaluation of using SCIg for maintenance of response, relapses or treatment withdrawal occurred in 63% with placebo, 39% with low dose SCIg (0.2 g/kg weekly), and 33% with high dose (0.4 g/kg weekly), Ivo N. van Schaik, MD, reported at the annual meeting of the American Academy of Neurology.
Patients in the trial had received at lease one dose of intravenous immunoglobulin (IVIg) within 8 weeks before screening. They then underwent a screening period first, followed by an IgG dependency period of up to 12 weeks to test for ongoing need for IgG. The patients who experienced CIDP relapse during this test period were administered a standardized IVIG regimen during a 10- to 13-week restabilization period, and those who improved and maintained their Inflammatory Neuropathy Cause and Treatment (INCAT) score continued to the randomized subcutaneous treatment period of the study.
CIDP relapse occurred in 56% of patients in the placebo group, compared with 33% in the low- and 19% in the high-dose SCIg groups, said Dr. van Schaik of the University of Amsterdam (the Netherlands).
“Both [SCIg] doses were effective in preventing relapse. The higher dose performed better than the lower dose, but the difference was not statistically significant,” he said.
Both doses were significantly more effective than placebo.
Study participants were adults with definite or probable CIDP enrolled from 69 neuromuscular centers worldwide between March 2012 and November 2015. Weekly self-administered subcutaneous infusions of SCIg (IgPro20Hizentra) were performed during 1 or 2 consecutive days in two separate sessions using special infusion pumps. Patients reported that learning the self-administration technique was easy, Dr. van Schaik said.
Adverse effects included mainly local reactions, which occurred in 19% of patients, but these were generally mild and rarely resulted in therapy discontinuation, and local reactions decreased considerably over time, he said, noting that systemic effects are reduced with SCIg vs. IVIg.
Subcutaneous administration of immunoglobulin is not new. In fact, it has been used successfully in patients with immunodeficiency syndromes for more than 2 decades and can increase patient autonomy and reduce costs by reducing hospital and infusion center visits, but this is the first study to assess efficacy, safety, and tolerability of this approach in an adequately powered, randomized, clinical trial, he said.
“Subcutaneous immunoglobulin can be used ... for maintenance treatment of patients with CIDP,” he concluded, adding that weekly doses of 0.2-0.4 g/kg are supported by these data, and that maintenance doses should be individualized based on patient factors and previous IVIg dose and frequency.
The PATH study was sponsored by CSL-Behring. Dr. van Schaik chairs a steering committee for CSL-Behring and received departmental honoraria for serving on scientific advisory boards for CSL-Behring, Baxalta, and UCB. He also received speakers fees from CSL-Behring and Kedrion.
Key clinical point:
Major finding: CIDP relapse occurred in 56% of patients in the placebo group, compared with 33% in the low- and 19% in the high-dose SCIg groups.
Data source: The randomized, placebo-controlled phase III PATH study of 172 CIDP patients.
Disclosures: The PATH study was sponsored by CSL-Behring. Dr. van Schaik chairs a steering committee for CSL-Behring and received departmental honoraria for serving on scientific advisory boards for CSL-Behring, Baxalta, and UCB. He also received speakers fees from CSL-Behring and Kedrion.
24/7 neurologist coverage improved community hospital stroke outcomes
BOSTON – Around-the-clock availability of a neurologist for acute stroke treatment significantly reduced door-to-needle times at a community-based primary stroke center serving as a regional tertiary referral center for western North Carolina.
The program involving the 24/7 availability of a neurologist in the hospital was implemented in October 2015 at Mission Hospital, a 763-bed hospital in Asheville, N.C., where nighttime emergency stroke coverage had historically been provided by a neurologist on-call from home. The implementation was partly in preparation for an application for Joint Commission–certified comprehensive stroke center status, which was approved in 2016.
A review of 2,022 Code Stroke activations in the emergency department from January 2012 through September 2016 that included only patients treated with intravenous tissue plasminogen activator revealed a significant decrease in door-to-neurologist time from an average of 7.1 minutes before the 24/7 in-hospital availability to 2.5 minutes after implementation. The analysis included 1,524 cases occurring prior to implementation and 498 cases occurring after.
The impact was most significant at night.
“Our nighttime reduction to the bedside went down from 13.6 minutes to 3.4 minutes, and our daytime reduction also improved from 5.2 minutes to 2.2 minutes,” said Dr. Schneider, a vascular neurologist at Mission Hospital.
Door-to-needle times were significantly reduced from 48.3 to 37.8 minutes overall, from 52 minutes to 40 minutes at night, and from 46.6 to 34.5 minutes during the day.
“We think that the trend toward significance at nighttime was mitigated by the fact there were just lesser numbers at night,” he said.
In-hospital mortality was reduced by 31% from 8.94% to 6.13%, he said.
Another variable that improved after the intervention was timing of prenotification by emergency medical services. The overall rate of prenotification did not improve (likely because of a ceiling effect; the rate of notification was already about 90%), but notifications improved from 12.5 minutes before arrival to 10.7 minutes.
There was no significant change in door-to-computed tomography times, which averaged about 15 minutes, Dr. Schneider said.
Though limited by a smaller number of postimplementation cases and lack of 3-month poststroke functional outcome data, the findings suggest that 24/7 in-hospital availability of a neurologist improves door to intravenous tissue plasminogen activator treatment times and in-hospital stroke mortality, Dr. Schneider concluded, noting that ongoing monitoring of outcomes will be necessary to assess for enduring impact.
Dr. Schneider reported having no disclosures.
BOSTON – Around-the-clock availability of a neurologist for acute stroke treatment significantly reduced door-to-needle times at a community-based primary stroke center serving as a regional tertiary referral center for western North Carolina.
The program involving the 24/7 availability of a neurologist in the hospital was implemented in October 2015 at Mission Hospital, a 763-bed hospital in Asheville, N.C., where nighttime emergency stroke coverage had historically been provided by a neurologist on-call from home. The implementation was partly in preparation for an application for Joint Commission–certified comprehensive stroke center status, which was approved in 2016.
A review of 2,022 Code Stroke activations in the emergency department from January 2012 through September 2016 that included only patients treated with intravenous tissue plasminogen activator revealed a significant decrease in door-to-neurologist time from an average of 7.1 minutes before the 24/7 in-hospital availability to 2.5 minutes after implementation. The analysis included 1,524 cases occurring prior to implementation and 498 cases occurring after.
The impact was most significant at night.
“Our nighttime reduction to the bedside went down from 13.6 minutes to 3.4 minutes, and our daytime reduction also improved from 5.2 minutes to 2.2 minutes,” said Dr. Schneider, a vascular neurologist at Mission Hospital.
Door-to-needle times were significantly reduced from 48.3 to 37.8 minutes overall, from 52 minutes to 40 minutes at night, and from 46.6 to 34.5 minutes during the day.
“We think that the trend toward significance at nighttime was mitigated by the fact there were just lesser numbers at night,” he said.
In-hospital mortality was reduced by 31% from 8.94% to 6.13%, he said.
Another variable that improved after the intervention was timing of prenotification by emergency medical services. The overall rate of prenotification did not improve (likely because of a ceiling effect; the rate of notification was already about 90%), but notifications improved from 12.5 minutes before arrival to 10.7 minutes.
There was no significant change in door-to-computed tomography times, which averaged about 15 minutes, Dr. Schneider said.
Though limited by a smaller number of postimplementation cases and lack of 3-month poststroke functional outcome data, the findings suggest that 24/7 in-hospital availability of a neurologist improves door to intravenous tissue plasminogen activator treatment times and in-hospital stroke mortality, Dr. Schneider concluded, noting that ongoing monitoring of outcomes will be necessary to assess for enduring impact.
Dr. Schneider reported having no disclosures.
BOSTON – Around-the-clock availability of a neurologist for acute stroke treatment significantly reduced door-to-needle times at a community-based primary stroke center serving as a regional tertiary referral center for western North Carolina.
The program involving the 24/7 availability of a neurologist in the hospital was implemented in October 2015 at Mission Hospital, a 763-bed hospital in Asheville, N.C., where nighttime emergency stroke coverage had historically been provided by a neurologist on-call from home. The implementation was partly in preparation for an application for Joint Commission–certified comprehensive stroke center status, which was approved in 2016.
A review of 2,022 Code Stroke activations in the emergency department from January 2012 through September 2016 that included only patients treated with intravenous tissue plasminogen activator revealed a significant decrease in door-to-neurologist time from an average of 7.1 minutes before the 24/7 in-hospital availability to 2.5 minutes after implementation. The analysis included 1,524 cases occurring prior to implementation and 498 cases occurring after.
The impact was most significant at night.
“Our nighttime reduction to the bedside went down from 13.6 minutes to 3.4 minutes, and our daytime reduction also improved from 5.2 minutes to 2.2 minutes,” said Dr. Schneider, a vascular neurologist at Mission Hospital.
Door-to-needle times were significantly reduced from 48.3 to 37.8 minutes overall, from 52 minutes to 40 minutes at night, and from 46.6 to 34.5 minutes during the day.
“We think that the trend toward significance at nighttime was mitigated by the fact there were just lesser numbers at night,” he said.
In-hospital mortality was reduced by 31% from 8.94% to 6.13%, he said.
Another variable that improved after the intervention was timing of prenotification by emergency medical services. The overall rate of prenotification did not improve (likely because of a ceiling effect; the rate of notification was already about 90%), but notifications improved from 12.5 minutes before arrival to 10.7 minutes.
There was no significant change in door-to-computed tomography times, which averaged about 15 minutes, Dr. Schneider said.
Though limited by a smaller number of postimplementation cases and lack of 3-month poststroke functional outcome data, the findings suggest that 24/7 in-hospital availability of a neurologist improves door to intravenous tissue plasminogen activator treatment times and in-hospital stroke mortality, Dr. Schneider concluded, noting that ongoing monitoring of outcomes will be necessary to assess for enduring impact.
Dr. Schneider reported having no disclosures.
AT AAN 2017
Key clinical point:
Major finding: Door-to-needle times were significantly reduced from 48.3 to 37.8 minutes overall, from 52 minutes to 40 minutes at night, and from 46.6 to 34.5 minutes during the day.
Data source: A review of 498 preintervention cases and 1,524 postintervention Code Stroke cases.
Disclosures: Dr. Schneider reported having no disclosures.