User login
Lymphedema Common After Head & Neck Cancer
SAN FRANCISCO – Lymphedema is highly common and a source of considerable morbidity among patients who undergo treatment for head and neck cancer, finds a cross-sectional study among 103 survivors.
Fully three-fourths had developed some degree of lymphedema, according to results presented at the annual Oncology Congress presented by Reed Medical Education. The more severe it was, the more likely patients were to have symptoms, functional impairments, and poorer quality of life.
Disease and treatment-related factors such as high radiation dose and combined surgery and radiation therapy were risk factors for the development of lymphedema.
"This is the first study that we are aware of in the United States of this depth to systematically examine lymphedema" in this population, noted lead investigator Jie Deng, Ph.D., R.N., O.C.N., a postdoctoral fellow at the Vanderbilt University, Nashville, Tenn.
"Health care professionals should be aware that lymphedema is a frequent late effect in the head and neck cancer population," she advised. "We need to educate patients about the risk of lymphedema prior to treatment, during treatment, and posttreatment, and we need to conduct external and internal examinations to evaluate related signs and symptoms at each clinic visit."
Patients found to have any signs or symptoms should be referred for lymphedema assessment. Furthermore, "it’s very important we have very detailed documentation so we can follow up on patients’ treatment effect and also identify potential issues in this population," Dr. Deng stressed. "An interdisciplinary approach is needed to best manage lymphedema."
She and her colleagues are now evaluating interventions to treat head and neck lymphedema. Manual lymphatic drainage is one possibility. Elevating the head of the bed at night is another, as anecdotal comments suggest that symptoms worsen in the recumbent position.
"Patients will mention in the morning they feel more tightness, more fluid accumulated in the submental area; around noontime or afternoon, they feel it has drained by itself," she explained.
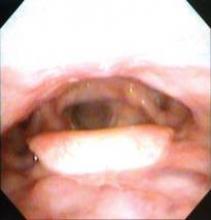
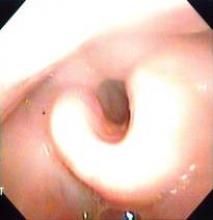
There are more than half a million survivors of head and neck cancer in the United States, according to Dr. Deng. As a result of their disease and its treatment, these patients can develop both external lymphedema, causing symptoms such as facial puffiness, and internal lymphedema, causing issues such as epiglottal swelling.
The investigators studied adult patients treated for head and neck cancer at the Vanderbilt-Ingram Cancer Center who were at least 3 months out from the end of their treatment and had no evidence of cancer. External lymphedema was assessed with a clinical exam, using the Foldi scale. Internal lymphedema was assessed with an endoscopic exam, using the Patterson scale for edema of the larynx and pharynx.
The patients were 60 years old, on average. The majority were male (69%) and white (89%). In terms of health behaviors, 66% had a history of smoking and 38% had a history of alcohol use.
In all, 81% of the patients had had locally advanced cancer, and 90% had received at least two treatment modalities. The median time since end of treatment was 20 months.
Study results, reported at the meeting and also recently published, showed that 75% of the patients overall had lymphedema of the head and neck; of those with lymphedema (61 out of 81), 10% had only the external kind, 39% had only the internal kind, and 51% had both (J. Pain Symptom Manage. 2011 July 29 [doi:10.1016/j.jpainsymman.2011.03.019]).
The type and severity of lymphedema were associated with both physical and psychological symptoms, Dr. Deng reported.
As the severity of lymphedema increased, patients were significantly more likely to report difficulty swallowing, issues with mucus or dry mouth, nutritional problems, pain, and voice problems (P = .001 to P = .047, depending on the type of lymphedema and the specific symptom).
Additionally, increasing severity was associated with poorer body image (P = .028 to P = .049). "However, there was no statistically significant relationship between lymphedema and anxiety and depressive symptoms," she noted.
Lymphedema severity was also associated with hearing deficits, limitation of neck range of motion, and impaired quality of life (P = .004 to P = .045).
Analyses identified certain disease and treatment-related factors to be risk factors for the development of lymphedema, according to Dr. Deng.
Namely, patients were more likely to develop lymphedema if they had pharyngeal tumors; were a shorter time out from the end of treatment; received a high total dose of radiation, mirroring what has been seen in breast cancer; received a greater number of treatment modalities, a marker of treatment intensity; or had combined surgery and radiation therapy – specifically, either surgery plus postoperative radiation, or salvage surgery within the irradiated field – as compared with surgery alone.
The higher risk with shorter time since surgery hints that the lymphatics in the area may undergo regeneration over time, she speculated. "This was identified in the murine tail [model of lymphedema]; however, we didn’t know whether or not this phenomenon or similar exists in the head and neck cancer population."
None of the demographic factors or health behaviors assessed were found to be risk factors for the development of lymphedema. But the lack of association between smoking and alcohol consumption and lymphedema may have been related to the fact that patients were asked whether they smoked or drank but not the intensity, or to the cross-sectional nature of the study, Dr. Deng noted. "In the future, longitudinal study is needed to examine whether or not these are risk factors," she said.
Dr. Deng reported that she had no conflicts of interest related to the study. Reed Medical Education and this news organization are owned by Reed Elsevier.
SAN FRANCISCO – Lymphedema is highly common and a source of considerable morbidity among patients who undergo treatment for head and neck cancer, finds a cross-sectional study among 103 survivors.
Fully three-fourths had developed some degree of lymphedema, according to results presented at the annual Oncology Congress presented by Reed Medical Education. The more severe it was, the more likely patients were to have symptoms, functional impairments, and poorer quality of life.
Disease and treatment-related factors such as high radiation dose and combined surgery and radiation therapy were risk factors for the development of lymphedema.
"This is the first study that we are aware of in the United States of this depth to systematically examine lymphedema" in this population, noted lead investigator Jie Deng, Ph.D., R.N., O.C.N., a postdoctoral fellow at the Vanderbilt University, Nashville, Tenn.
"Health care professionals should be aware that lymphedema is a frequent late effect in the head and neck cancer population," she advised. "We need to educate patients about the risk of lymphedema prior to treatment, during treatment, and posttreatment, and we need to conduct external and internal examinations to evaluate related signs and symptoms at each clinic visit."
Patients found to have any signs or symptoms should be referred for lymphedema assessment. Furthermore, "it’s very important we have very detailed documentation so we can follow up on patients’ treatment effect and also identify potential issues in this population," Dr. Deng stressed. "An interdisciplinary approach is needed to best manage lymphedema."
She and her colleagues are now evaluating interventions to treat head and neck lymphedema. Manual lymphatic drainage is one possibility. Elevating the head of the bed at night is another, as anecdotal comments suggest that symptoms worsen in the recumbent position.
"Patients will mention in the morning they feel more tightness, more fluid accumulated in the submental area; around noontime or afternoon, they feel it has drained by itself," she explained.
There are more than half a million survivors of head and neck cancer in the United States, according to Dr. Deng. As a result of their disease and its treatment, these patients can develop both external lymphedema, causing symptoms such as facial puffiness, and internal lymphedema, causing issues such as epiglottal swelling.
The investigators studied adult patients treated for head and neck cancer at the Vanderbilt-Ingram Cancer Center who were at least 3 months out from the end of their treatment and had no evidence of cancer. External lymphedema was assessed with a clinical exam, using the Foldi scale. Internal lymphedema was assessed with an endoscopic exam, using the Patterson scale for edema of the larynx and pharynx.
The patients were 60 years old, on average. The majority were male (69%) and white (89%). In terms of health behaviors, 66% had a history of smoking and 38% had a history of alcohol use.
In all, 81% of the patients had had locally advanced cancer, and 90% had received at least two treatment modalities. The median time since end of treatment was 20 months.
Study results, reported at the meeting and also recently published, showed that 75% of the patients overall had lymphedema of the head and neck; of those with lymphedema (61 out of 81), 10% had only the external kind, 39% had only the internal kind, and 51% had both (J. Pain Symptom Manage. 2011 July 29 [doi:10.1016/j.jpainsymman.2011.03.019]).
The type and severity of lymphedema were associated with both physical and psychological symptoms, Dr. Deng reported.
As the severity of lymphedema increased, patients were significantly more likely to report difficulty swallowing, issues with mucus or dry mouth, nutritional problems, pain, and voice problems (P = .001 to P = .047, depending on the type of lymphedema and the specific symptom).
Additionally, increasing severity was associated with poorer body image (P = .028 to P = .049). "However, there was no statistically significant relationship between lymphedema and anxiety and depressive symptoms," she noted.
Lymphedema severity was also associated with hearing deficits, limitation of neck range of motion, and impaired quality of life (P = .004 to P = .045).
Analyses identified certain disease and treatment-related factors to be risk factors for the development of lymphedema, according to Dr. Deng.
Namely, patients were more likely to develop lymphedema if they had pharyngeal tumors; were a shorter time out from the end of treatment; received a high total dose of radiation, mirroring what has been seen in breast cancer; received a greater number of treatment modalities, a marker of treatment intensity; or had combined surgery and radiation therapy – specifically, either surgery plus postoperative radiation, or salvage surgery within the irradiated field – as compared with surgery alone.
The higher risk with shorter time since surgery hints that the lymphatics in the area may undergo regeneration over time, she speculated. "This was identified in the murine tail [model of lymphedema]; however, we didn’t know whether or not this phenomenon or similar exists in the head and neck cancer population."
None of the demographic factors or health behaviors assessed were found to be risk factors for the development of lymphedema. But the lack of association between smoking and alcohol consumption and lymphedema may have been related to the fact that patients were asked whether they smoked or drank but not the intensity, or to the cross-sectional nature of the study, Dr. Deng noted. "In the future, longitudinal study is needed to examine whether or not these are risk factors," she said.
Dr. Deng reported that she had no conflicts of interest related to the study. Reed Medical Education and this news organization are owned by Reed Elsevier.
SAN FRANCISCO – Lymphedema is highly common and a source of considerable morbidity among patients who undergo treatment for head and neck cancer, finds a cross-sectional study among 103 survivors.
Fully three-fourths had developed some degree of lymphedema, according to results presented at the annual Oncology Congress presented by Reed Medical Education. The more severe it was, the more likely patients were to have symptoms, functional impairments, and poorer quality of life.
Disease and treatment-related factors such as high radiation dose and combined surgery and radiation therapy were risk factors for the development of lymphedema.
"This is the first study that we are aware of in the United States of this depth to systematically examine lymphedema" in this population, noted lead investigator Jie Deng, Ph.D., R.N., O.C.N., a postdoctoral fellow at the Vanderbilt University, Nashville, Tenn.
"Health care professionals should be aware that lymphedema is a frequent late effect in the head and neck cancer population," she advised. "We need to educate patients about the risk of lymphedema prior to treatment, during treatment, and posttreatment, and we need to conduct external and internal examinations to evaluate related signs and symptoms at each clinic visit."
Patients found to have any signs or symptoms should be referred for lymphedema assessment. Furthermore, "it’s very important we have very detailed documentation so we can follow up on patients’ treatment effect and also identify potential issues in this population," Dr. Deng stressed. "An interdisciplinary approach is needed to best manage lymphedema."
She and her colleagues are now evaluating interventions to treat head and neck lymphedema. Manual lymphatic drainage is one possibility. Elevating the head of the bed at night is another, as anecdotal comments suggest that symptoms worsen in the recumbent position.
"Patients will mention in the morning they feel more tightness, more fluid accumulated in the submental area; around noontime or afternoon, they feel it has drained by itself," she explained.
There are more than half a million survivors of head and neck cancer in the United States, according to Dr. Deng. As a result of their disease and its treatment, these patients can develop both external lymphedema, causing symptoms such as facial puffiness, and internal lymphedema, causing issues such as epiglottal swelling.
The investigators studied adult patients treated for head and neck cancer at the Vanderbilt-Ingram Cancer Center who were at least 3 months out from the end of their treatment and had no evidence of cancer. External lymphedema was assessed with a clinical exam, using the Foldi scale. Internal lymphedema was assessed with an endoscopic exam, using the Patterson scale for edema of the larynx and pharynx.
The patients were 60 years old, on average. The majority were male (69%) and white (89%). In terms of health behaviors, 66% had a history of smoking and 38% had a history of alcohol use.
In all, 81% of the patients had had locally advanced cancer, and 90% had received at least two treatment modalities. The median time since end of treatment was 20 months.
Study results, reported at the meeting and also recently published, showed that 75% of the patients overall had lymphedema of the head and neck; of those with lymphedema (61 out of 81), 10% had only the external kind, 39% had only the internal kind, and 51% had both (J. Pain Symptom Manage. 2011 July 29 [doi:10.1016/j.jpainsymman.2011.03.019]).
The type and severity of lymphedema were associated with both physical and psychological symptoms, Dr. Deng reported.
As the severity of lymphedema increased, patients were significantly more likely to report difficulty swallowing, issues with mucus or dry mouth, nutritional problems, pain, and voice problems (P = .001 to P = .047, depending on the type of lymphedema and the specific symptom).
Additionally, increasing severity was associated with poorer body image (P = .028 to P = .049). "However, there was no statistically significant relationship between lymphedema and anxiety and depressive symptoms," she noted.
Lymphedema severity was also associated with hearing deficits, limitation of neck range of motion, and impaired quality of life (P = .004 to P = .045).
Analyses identified certain disease and treatment-related factors to be risk factors for the development of lymphedema, according to Dr. Deng.
Namely, patients were more likely to develop lymphedema if they had pharyngeal tumors; were a shorter time out from the end of treatment; received a high total dose of radiation, mirroring what has been seen in breast cancer; received a greater number of treatment modalities, a marker of treatment intensity; or had combined surgery and radiation therapy – specifically, either surgery plus postoperative radiation, or salvage surgery within the irradiated field – as compared with surgery alone.
The higher risk with shorter time since surgery hints that the lymphatics in the area may undergo regeneration over time, she speculated. "This was identified in the murine tail [model of lymphedema]; however, we didn’t know whether or not this phenomenon or similar exists in the head and neck cancer population."
None of the demographic factors or health behaviors assessed were found to be risk factors for the development of lymphedema. But the lack of association between smoking and alcohol consumption and lymphedema may have been related to the fact that patients were asked whether they smoked or drank but not the intensity, or to the cross-sectional nature of the study, Dr. Deng noted. "In the future, longitudinal study is needed to examine whether or not these are risk factors," she said.
Dr. Deng reported that she had no conflicts of interest related to the study. Reed Medical Education and this news organization are owned by Reed Elsevier.
FROM THE ANNUAL ONCOLOGY CONGRESS
Major Finding: Fully 75% of patients had lymphedema. The severity of lymphedema was associated with symptoms, functional impairments, and poorer quality of life.
Data Source: A descriptive cross-sectional study among a convenience sample of 103 patients treated for head and neck cancer.
Disclosures: Dr. Deng reported that she had no conflicts of interest related to the study.
Point/Counterpoint: What is the first-line treatment of choice for metastatic BRAF-mutant melanoma?
Consider immunotherapy as first-line treatment.
Application of immunotherapy is becoming increasingly more sophisticated as we learn to control the many signals that activate and deactivate T cells. We now have two approved immunotherapies for metastatic melanoma – high-dose interleukin-2 (Proleukin) and ipilimumab (Yervoy) – and at least two investigational ones – adoptive T-cell therapy and a novel antibody to PD-1 (MDX-1106; ASCO 2010 annual meeting. Abstract 2506) – that are especially promising.
There is no question that if you give only a single agent and compare agents head to head, ipilimumab will lose to vemurafenib (Zelboraf), a targeted BRAF inhibitor, in terms of response rate, progression-free survival, and probably median survival. But our goal is the best overall outcome in this population, and in that regard, the sequencing of agents comes into play, and there may be advantages to giving immunotherapy first.
The first of several factors to consider when selecting initial therapy is that immunotherapy can produce long-term durable remissions; for example, about 5% of patients given high-dose interleukin-2 have a remission lasting more than 2 years, in some cases more than 10 years, often without maintenance therapy (Clin. Cancer Res. 2006;12:2353s-8s). In contrast, virtually all patients given targeted agents will require some sort of second therapy when they have progression.
Second, the actual treatment duration of immunotherapy is quite limited, and patients having a response may never need a second therapy. But for targeted therapy, as far as we can tell, patients will have to remain on that therapy forever.
A third factor is the speed of response. Reponses to immunotherapy are slow, in some cases taking at least 12 weeks, whereas responses to targeted agents often occur within days. So it may be difficult to give immunotherapy if you wait until a patient has progression on the targeted agent, whereas the targeted agent works so quickly that it may still be an option after failure of immunotherapy.
It is also noteworthy that targeted therapy can be associated with fulminant relapse. Our limited experience has been that a subset of patients has incredibly rapid progression when this therapy begins to fail and the targeted agent is discontinued, so there’s no opportunity to give immunotherapy at that point.
To be sure, patients with high tumor burden and very rapid tumor progression at initial metastatic presentation are generally not good candidates for receiving immunotherapy first. So you really have to look at the overall patient and consider potential predictive markers like low baseline lymphocyte count (which would disfavor immunotherapy) and preexisting autoimmune disease (which would exclude patients from some immunotherapies and favor targeted therapy).
Fourth, it has been proposed that giving immunotherapy first and targeted therapy second may be synergistic. Admittedly, this is purely theoretical. But the idea is that ipilimumab, for instance, remains in the body for up to 3 months, so if a patient starts to develop progression on this agent and a targeted agent is given at that time, you are essentially giving combination therapy.
Also, antitumor immunity may play an important role in the efficacy of targeted therapy. Very interesting preclinical data suggest that some of the activity of targeted agents requires existing immune responses (Cancer Cell. 2010;18:485-98), so inducing or expanding an immune response first may produce a better overall effect from the targeted agent. Clinically, evidence was presented that vemurafenib induces T-cell infiltrates in the tumor.
In conclusion, there are compelling reasons to consider immunotherapy as first-line therapy for melanoma. At present, we sometimes have to make difficult choices between initial therapies based on the individual patient, existing data, and our clinical judgment. But future treatment will likely entail use of these therapies in combination to optimize outcomes. Those trials are being planned. There are all sorts of possible combinations coming down the road that will likely improve outcomes.
Dr. Sznol is professor of medical oncology and codirector of the melanoma program at Yale Cancer Center, New Haven, Conn. He disclosed that he is a consultant to Bristol-Myers Squibb (manufacturer of ipilimumab) and Genesis Biopharma (a developer of adoptive cell therapies).
BRAF-targeted therapy has better overall survival.
About half of melanomas have a mutation of BRAF, providing a target for therapy that at the present time has demonstrated great promise. Vemurafenib achieves rapid, often dramatic responses in most patients. In BRIM-2, a phase II trial among patients who had had a failure of a prior therapy, nearly all had regression of disease on vemurafenib and more than 50% achieved a confirmed RECIST (Response Evaluation Criteria in Solid Tumors) clinical response (N. Engl. J. Med., in press). In BRIM-3, a phase III trial among patients with untreated disease, vemurafenib provided a dramatic improvement in both progression-free survival and overall survival over dacarbazine with hazard ratios of 0.26 and 0.37, respectively (N. Engl. J. Med. 2011;364:2507-16).
One misconception about vemurafenib therapy is that everybody is going to have a relapse in 6-7 months and die. The progression-free survival has a median of 6.8 months, but we are seeing impressive median overall survival, with a 15.9-month median in BRIM-2, probably the longest in any similar trial. The 63% relative reduction in the risk of death in BRIM-3 with vemurafenib benefited nearly all subgroups but was most impressive in patients having high lactate dehydrogenase (LDH) levels or M1c disease. We are unlikely to get good long-term survival data from this trial because of the early favorable result and allowance of crossover.
Another misconception about vemurafenib therapy is that most patients will have aggressive disease at relapse, and there will not be an opportunity for a second therapy. In fact, many patients have a localized relapse, undergo resection, and can continue on the vemurafenib. My experience has been that the patients having an aggressive relapse are those who enrolled in the trial with aggressive disease, and would not have been appropriate for other treatments. They did have a great improvement in their quality of life, albeit not for as long as hoped.
With all the limitations of comparisons between trials, vemurafenib compared with ipilimumab has been associated with better rates of overall survival at 1 year (54%-58% vs. 46%) and 2 years (33% vs. 24%). And there is a huge improvement in the disease control rate (86% vs. 44%).
Unquestionably, some patients have highly durable remissions with immunotherapy, but a major frustration is our inability to pick out this small subset of patients up front – prior to therapy.
Vemurafenib is probably the only effective therapy we have ever had for symptomatic patients with a high LDH level, who need rapid improvement in their performance status. I honestly had never seen a patient with those characteristics respond to anything until vemurafenib.
Finally, there may be some science suggesting that giving vemurafenib after ipilimumab is better, but there is not one bit of data to show that you are not able to achieve a durable remission to ipilimumab after failure of a BRAF inhibitor. More research on the sequencing of therapies is needed. In BRIM-2, only about 5% of patients had previously received ipilimumab. And although an analysis is being done among the 24% who got ipilimumab after stopping vemurafenib, it is a post hoc analysis, and the goal is simply to ascertain any contribution to the overall survival result.
In conclusion, in this era of targeted agents, it is essential that all melanomas be genotyped. It is my view that patients with symptomatic bulky disease or an increased LDH level should get vemurafenib. Patients with indolent disease, asymptomatic M1a or M1b disease, could get either; I can’t make any passionate argument that they all should get vemurafenib. But if you’re trying to put a patient into remission for as long as you can with a good quality of life, then vemurafenib is much more likely to do that. Ultimately, we need trials evaluating the issues of sequencing and combination therapy.
Dr. Sosman is professor of medicine and director of the melanoma program at Vanderbilt-Ingram Cancer Center, Nashville, Tenn. He disclosed that he is a consultant to, and receives research support from, GlaxoSmithKline (manufacturer of investigational targeted therapies for melanoma) and Roche (manufacturer of vemurafenib).
Consider immunotherapy as first-line treatment.
Application of immunotherapy is becoming increasingly more sophisticated as we learn to control the many signals that activate and deactivate T cells. We now have two approved immunotherapies for metastatic melanoma – high-dose interleukin-2 (Proleukin) and ipilimumab (Yervoy) – and at least two investigational ones – adoptive T-cell therapy and a novel antibody to PD-1 (MDX-1106; ASCO 2010 annual meeting. Abstract 2506) – that are especially promising.
There is no question that if you give only a single agent and compare agents head to head, ipilimumab will lose to vemurafenib (Zelboraf), a targeted BRAF inhibitor, in terms of response rate, progression-free survival, and probably median survival. But our goal is the best overall outcome in this population, and in that regard, the sequencing of agents comes into play, and there may be advantages to giving immunotherapy first.
The first of several factors to consider when selecting initial therapy is that immunotherapy can produce long-term durable remissions; for example, about 5% of patients given high-dose interleukin-2 have a remission lasting more than 2 years, in some cases more than 10 years, often without maintenance therapy (Clin. Cancer Res. 2006;12:2353s-8s). In contrast, virtually all patients given targeted agents will require some sort of second therapy when they have progression.
Second, the actual treatment duration of immunotherapy is quite limited, and patients having a response may never need a second therapy. But for targeted therapy, as far as we can tell, patients will have to remain on that therapy forever.
A third factor is the speed of response. Reponses to immunotherapy are slow, in some cases taking at least 12 weeks, whereas responses to targeted agents often occur within days. So it may be difficult to give immunotherapy if you wait until a patient has progression on the targeted agent, whereas the targeted agent works so quickly that it may still be an option after failure of immunotherapy.
It is also noteworthy that targeted therapy can be associated with fulminant relapse. Our limited experience has been that a subset of patients has incredibly rapid progression when this therapy begins to fail and the targeted agent is discontinued, so there’s no opportunity to give immunotherapy at that point.
To be sure, patients with high tumor burden and very rapid tumor progression at initial metastatic presentation are generally not good candidates for receiving immunotherapy first. So you really have to look at the overall patient and consider potential predictive markers like low baseline lymphocyte count (which would disfavor immunotherapy) and preexisting autoimmune disease (which would exclude patients from some immunotherapies and favor targeted therapy).
Fourth, it has been proposed that giving immunotherapy first and targeted therapy second may be synergistic. Admittedly, this is purely theoretical. But the idea is that ipilimumab, for instance, remains in the body for up to 3 months, so if a patient starts to develop progression on this agent and a targeted agent is given at that time, you are essentially giving combination therapy.
Also, antitumor immunity may play an important role in the efficacy of targeted therapy. Very interesting preclinical data suggest that some of the activity of targeted agents requires existing immune responses (Cancer Cell. 2010;18:485-98), so inducing or expanding an immune response first may produce a better overall effect from the targeted agent. Clinically, evidence was presented that vemurafenib induces T-cell infiltrates in the tumor.
In conclusion, there are compelling reasons to consider immunotherapy as first-line therapy for melanoma. At present, we sometimes have to make difficult choices between initial therapies based on the individual patient, existing data, and our clinical judgment. But future treatment will likely entail use of these therapies in combination to optimize outcomes. Those trials are being planned. There are all sorts of possible combinations coming down the road that will likely improve outcomes.
Dr. Sznol is professor of medical oncology and codirector of the melanoma program at Yale Cancer Center, New Haven, Conn. He disclosed that he is a consultant to Bristol-Myers Squibb (manufacturer of ipilimumab) and Genesis Biopharma (a developer of adoptive cell therapies).
BRAF-targeted therapy has better overall survival.
About half of melanomas have a mutation of BRAF, providing a target for therapy that at the present time has demonstrated great promise. Vemurafenib achieves rapid, often dramatic responses in most patients. In BRIM-2, a phase II trial among patients who had had a failure of a prior therapy, nearly all had regression of disease on vemurafenib and more than 50% achieved a confirmed RECIST (Response Evaluation Criteria in Solid Tumors) clinical response (N. Engl. J. Med., in press). In BRIM-3, a phase III trial among patients with untreated disease, vemurafenib provided a dramatic improvement in both progression-free survival and overall survival over dacarbazine with hazard ratios of 0.26 and 0.37, respectively (N. Engl. J. Med. 2011;364:2507-16).
One misconception about vemurafenib therapy is that everybody is going to have a relapse in 6-7 months and die. The progression-free survival has a median of 6.8 months, but we are seeing impressive median overall survival, with a 15.9-month median in BRIM-2, probably the longest in any similar trial. The 63% relative reduction in the risk of death in BRIM-3 with vemurafenib benefited nearly all subgroups but was most impressive in patients having high lactate dehydrogenase (LDH) levels or M1c disease. We are unlikely to get good long-term survival data from this trial because of the early favorable result and allowance of crossover.
Another misconception about vemurafenib therapy is that most patients will have aggressive disease at relapse, and there will not be an opportunity for a second therapy. In fact, many patients have a localized relapse, undergo resection, and can continue on the vemurafenib. My experience has been that the patients having an aggressive relapse are those who enrolled in the trial with aggressive disease, and would not have been appropriate for other treatments. They did have a great improvement in their quality of life, albeit not for as long as hoped.
With all the limitations of comparisons between trials, vemurafenib compared with ipilimumab has been associated with better rates of overall survival at 1 year (54%-58% vs. 46%) and 2 years (33% vs. 24%). And there is a huge improvement in the disease control rate (86% vs. 44%).
Unquestionably, some patients have highly durable remissions with immunotherapy, but a major frustration is our inability to pick out this small subset of patients up front – prior to therapy.
Vemurafenib is probably the only effective therapy we have ever had for symptomatic patients with a high LDH level, who need rapid improvement in their performance status. I honestly had never seen a patient with those characteristics respond to anything until vemurafenib.
Finally, there may be some science suggesting that giving vemurafenib after ipilimumab is better, but there is not one bit of data to show that you are not able to achieve a durable remission to ipilimumab after failure of a BRAF inhibitor. More research on the sequencing of therapies is needed. In BRIM-2, only about 5% of patients had previously received ipilimumab. And although an analysis is being done among the 24% who got ipilimumab after stopping vemurafenib, it is a post hoc analysis, and the goal is simply to ascertain any contribution to the overall survival result.
In conclusion, in this era of targeted agents, it is essential that all melanomas be genotyped. It is my view that patients with symptomatic bulky disease or an increased LDH level should get vemurafenib. Patients with indolent disease, asymptomatic M1a or M1b disease, could get either; I can’t make any passionate argument that they all should get vemurafenib. But if you’re trying to put a patient into remission for as long as you can with a good quality of life, then vemurafenib is much more likely to do that. Ultimately, we need trials evaluating the issues of sequencing and combination therapy.
Dr. Sosman is professor of medicine and director of the melanoma program at Vanderbilt-Ingram Cancer Center, Nashville, Tenn. He disclosed that he is a consultant to, and receives research support from, GlaxoSmithKline (manufacturer of investigational targeted therapies for melanoma) and Roche (manufacturer of vemurafenib).
Consider immunotherapy as first-line treatment.
Application of immunotherapy is becoming increasingly more sophisticated as we learn to control the many signals that activate and deactivate T cells. We now have two approved immunotherapies for metastatic melanoma – high-dose interleukin-2 (Proleukin) and ipilimumab (Yervoy) – and at least two investigational ones – adoptive T-cell therapy and a novel antibody to PD-1 (MDX-1106; ASCO 2010 annual meeting. Abstract 2506) – that are especially promising.
There is no question that if you give only a single agent and compare agents head to head, ipilimumab will lose to vemurafenib (Zelboraf), a targeted BRAF inhibitor, in terms of response rate, progression-free survival, and probably median survival. But our goal is the best overall outcome in this population, and in that regard, the sequencing of agents comes into play, and there may be advantages to giving immunotherapy first.
The first of several factors to consider when selecting initial therapy is that immunotherapy can produce long-term durable remissions; for example, about 5% of patients given high-dose interleukin-2 have a remission lasting more than 2 years, in some cases more than 10 years, often without maintenance therapy (Clin. Cancer Res. 2006;12:2353s-8s). In contrast, virtually all patients given targeted agents will require some sort of second therapy when they have progression.
Second, the actual treatment duration of immunotherapy is quite limited, and patients having a response may never need a second therapy. But for targeted therapy, as far as we can tell, patients will have to remain on that therapy forever.
A third factor is the speed of response. Reponses to immunotherapy are slow, in some cases taking at least 12 weeks, whereas responses to targeted agents often occur within days. So it may be difficult to give immunotherapy if you wait until a patient has progression on the targeted agent, whereas the targeted agent works so quickly that it may still be an option after failure of immunotherapy.
It is also noteworthy that targeted therapy can be associated with fulminant relapse. Our limited experience has been that a subset of patients has incredibly rapid progression when this therapy begins to fail and the targeted agent is discontinued, so there’s no opportunity to give immunotherapy at that point.
To be sure, patients with high tumor burden and very rapid tumor progression at initial metastatic presentation are generally not good candidates for receiving immunotherapy first. So you really have to look at the overall patient and consider potential predictive markers like low baseline lymphocyte count (which would disfavor immunotherapy) and preexisting autoimmune disease (which would exclude patients from some immunotherapies and favor targeted therapy).
Fourth, it has been proposed that giving immunotherapy first and targeted therapy second may be synergistic. Admittedly, this is purely theoretical. But the idea is that ipilimumab, for instance, remains in the body for up to 3 months, so if a patient starts to develop progression on this agent and a targeted agent is given at that time, you are essentially giving combination therapy.
Also, antitumor immunity may play an important role in the efficacy of targeted therapy. Very interesting preclinical data suggest that some of the activity of targeted agents requires existing immune responses (Cancer Cell. 2010;18:485-98), so inducing or expanding an immune response first may produce a better overall effect from the targeted agent. Clinically, evidence was presented that vemurafenib induces T-cell infiltrates in the tumor.
In conclusion, there are compelling reasons to consider immunotherapy as first-line therapy for melanoma. At present, we sometimes have to make difficult choices between initial therapies based on the individual patient, existing data, and our clinical judgment. But future treatment will likely entail use of these therapies in combination to optimize outcomes. Those trials are being planned. There are all sorts of possible combinations coming down the road that will likely improve outcomes.
Dr. Sznol is professor of medical oncology and codirector of the melanoma program at Yale Cancer Center, New Haven, Conn. He disclosed that he is a consultant to Bristol-Myers Squibb (manufacturer of ipilimumab) and Genesis Biopharma (a developer of adoptive cell therapies).
BRAF-targeted therapy has better overall survival.
About half of melanomas have a mutation of BRAF, providing a target for therapy that at the present time has demonstrated great promise. Vemurafenib achieves rapid, often dramatic responses in most patients. In BRIM-2, a phase II trial among patients who had had a failure of a prior therapy, nearly all had regression of disease on vemurafenib and more than 50% achieved a confirmed RECIST (Response Evaluation Criteria in Solid Tumors) clinical response (N. Engl. J. Med., in press). In BRIM-3, a phase III trial among patients with untreated disease, vemurafenib provided a dramatic improvement in both progression-free survival and overall survival over dacarbazine with hazard ratios of 0.26 and 0.37, respectively (N. Engl. J. Med. 2011;364:2507-16).
One misconception about vemurafenib therapy is that everybody is going to have a relapse in 6-7 months and die. The progression-free survival has a median of 6.8 months, but we are seeing impressive median overall survival, with a 15.9-month median in BRIM-2, probably the longest in any similar trial. The 63% relative reduction in the risk of death in BRIM-3 with vemurafenib benefited nearly all subgroups but was most impressive in patients having high lactate dehydrogenase (LDH) levels or M1c disease. We are unlikely to get good long-term survival data from this trial because of the early favorable result and allowance of crossover.
Another misconception about vemurafenib therapy is that most patients will have aggressive disease at relapse, and there will not be an opportunity for a second therapy. In fact, many patients have a localized relapse, undergo resection, and can continue on the vemurafenib. My experience has been that the patients having an aggressive relapse are those who enrolled in the trial with aggressive disease, and would not have been appropriate for other treatments. They did have a great improvement in their quality of life, albeit not for as long as hoped.
With all the limitations of comparisons between trials, vemurafenib compared with ipilimumab has been associated with better rates of overall survival at 1 year (54%-58% vs. 46%) and 2 years (33% vs. 24%). And there is a huge improvement in the disease control rate (86% vs. 44%).
Unquestionably, some patients have highly durable remissions with immunotherapy, but a major frustration is our inability to pick out this small subset of patients up front – prior to therapy.
Vemurafenib is probably the only effective therapy we have ever had for symptomatic patients with a high LDH level, who need rapid improvement in their performance status. I honestly had never seen a patient with those characteristics respond to anything until vemurafenib.
Finally, there may be some science suggesting that giving vemurafenib after ipilimumab is better, but there is not one bit of data to show that you are not able to achieve a durable remission to ipilimumab after failure of a BRAF inhibitor. More research on the sequencing of therapies is needed. In BRIM-2, only about 5% of patients had previously received ipilimumab. And although an analysis is being done among the 24% who got ipilimumab after stopping vemurafenib, it is a post hoc analysis, and the goal is simply to ascertain any contribution to the overall survival result.
In conclusion, in this era of targeted agents, it is essential that all melanomas be genotyped. It is my view that patients with symptomatic bulky disease or an increased LDH level should get vemurafenib. Patients with indolent disease, asymptomatic M1a or M1b disease, could get either; I can’t make any passionate argument that they all should get vemurafenib. But if you’re trying to put a patient into remission for as long as you can with a good quality of life, then vemurafenib is much more likely to do that. Ultimately, we need trials evaluating the issues of sequencing and combination therapy.
Dr. Sosman is professor of medicine and director of the melanoma program at Vanderbilt-Ingram Cancer Center, Nashville, Tenn. He disclosed that he is a consultant to, and receives research support from, GlaxoSmithKline (manufacturer of investigational targeted therapies for melanoma) and Roche (manufacturer of vemurafenib).
Novel Therapies Put Multiple Myeloma 'On the Ropes'
SAN FRANCISCO – A sweep of new agents are poised to deliver what could be a knock-out blow to multiple myeloma, according to the director of the myeloma program at the University of California, San Francisco.
Some are second- or third-generation agents in a mainstay class that appear to have less toxicity than and/or overcome resistance to their predecessors, Dr. Jeffrey L. Wolf said at the annual Oncology Congress here. Others come from classes not previously used in this disease.
"There is a rush to develop new drugs in myeloma," Dr. Wolf told attendees. "We [understand] some mechanisms that the disease seems to favor, so we can interfere with those."
The prospects, in turn, are excellent: "We have made such tremendous headway in myeloma, except for those exceptional cases with 17p deletions and some other adverse prognostic features," he said. "As a disease, it seems to be on the ropes."
A Less-Toxic Proteasome Inhibitor
The first-generation proteasome inhibitor bortezomib (Velcade) improves myeloma outcomes, but maximizing its benefit will require addressing the peripheral neuropathy that limits its use. Three strategies may lessen this toxicity without compromising efficacy, Dr. Wolf suggested: modestly reducing the standard dose, adjusting the schedule from twice to once weekly, and altering the route of administration from intravenous to subcutaneous.
For example, in patients with pretreated myeloma, giving bortezomib subcutaneously instead of intravenously results in an identical overall response rate of 52% (Lancet Oncol. 2011;12:431-40). But there are significant reductions in rates of peripheral neuropathy of any grade (38% vs. 53%) and grade 3 or higher (6% vs. 16%).
"Practically everybody that we see now at UCSF gets subcutaneous [bortezomib]," Dr. Wolf said. It’s a great way to go back and treat patients who maybe otherwise have stopped their therapy because of their neuropathy."
Carfilzomib, an investigational next-generation proteasome inhibitor in phase III trials, is showing good antimyeloma activity and a low rate of peripheral neuropathy. Among patients with pretreated, relapsed, or refractory disease, carfilzomib monotherapy achieved overall response rates of 42% to 53% in a bortezomib-naive group (ASCO 2011 annual meeting. Abstract 8026) and 21% in a bortezomib-exposed group (Haematologica 2010;95:452 Abstract 1099). Median time to progression was at least 8 months for both.
Dr. Wolf said that unpublished data suggest that the response rate was still 17% specifically among patients who had had progression on bortezomib, "so there appears to be some activity in patients who are already refractory to a prior proteasome inhibitor."
Meanwhile, the rates of grade 1/2 and grade 3 peripheral neuropathy were 6% and 1%, respectively. And only a single patient of the 155 had to stop treatment because of this adverse effect.
When carfilzomib is combined with lenalidomide-dexamethasone (the so-called CRd regimen), the overall response rate in patients with pretreated, relapsed, and refractory disease is 78%, and the rate of complete or near complete response is 24% (ASCO 2011 meeting. Abstract 8025).
And, in newly diagnosed myeloma, among 18 patients receiving eight cycles of CRd, the overall response rate was 100%, and the rate of stringent-complete, complete, or near-complete response was 61% (2011 International Myeloma Workshop. Poster P-253). "This is very, very exciting—I don’t think we’ve seen this in any other combination," Dr. Wolf commented. "But these are small numbers of patients; we still need to increase the numbers of patients studied with this combination."
Bortezomib and carfilzomib may soon have company from several investigational proteasome inhibitors available in oral formulations that have shown promise in preclinical testing or have advanced to clinical trials: CEP-18770 (Cephalon), ONX-0912 (Onyx), and MLN-9708 (Millenium).
A Third-Generation IMiD in Trials
Pomalidomide, a third-generation immunomodulatory drug (IMiD), coming after thalidomide (Thalomid), and lenalidomide (Revlimid), is also showing good antimyeloma activity in clinical trials, according to Dr. Wolf.
Among patients with pretreated myeloma, the rate of partial or better response when pomalidomide is combined with dexamethasone has ranged from 25% to 42%, depending on the trial and patient population. Adverse events are primarily hematologic.
And in patients who have previously received lenalidomide, the response rate is similar, at 35% (ASCO 2011 annual meeting. Abstract 8067). "So, as with carfilzomib, where there appear to be responses in patients who have prior resistance to bortezomib, it appears that pomalidomide can give us responses in patients who have already had resistance to lenalidomide," he said.
HDAC Inhibitors Show Activity
The histone deacetylase (HDAC) inhibitor vorinostat (Zolinza) is approved for treatment of lymphoma. But it is being tested in clinical trials for myeloma, in combination therapy, with promising results, according to Dr. Wolf. Overall response rates have ranged from 40% to 94%, depending on the patient population and combination.
Similarly, the HDAC inhibitor romidepsin (Istodax) is approved for lymphoma treatment but is also being studied for antimyeloma activity. And panobinostat, an investigational member of this drug class, is being evaluated as a component of combination therapy in phase II and III myeloma trials.
Monoclonal Antibodies Tested
Elotuzumab is an investigational monoclonal antibody directed against CS1, a glycoprotein that is highly expressed on the surface of plasma cells and implicated in myeloma pathogenesis.
In a phase I trial among patients with relapsed or refractory myeloma, the combination of elotuzumab with lenalidomide and low-dose dexamethasone yielded an overall response rate of 82% (ASCO 2011 meeting. Abstract 8076). The rate was 83% among the subset of patients whose disease was refractory to the most recent therapy and 95% among the subset of lenalidomide-naive patients.
The combination of elotuzumab with bortezomib has also been tested in patients with relapsed or refractory myeloma. But the overall response rate with this combination was less impressive, at 48% (ASH 2010 meeting. Abstract 3023).
Other Agents and Pathways
Several other agents are being eyed for roles in myeloma therapy as well. They include bendamustine (Treanda), an old drug initially revived for lymphoma treatment; aurora kinase inhibitors (for example, MLN-8237); and inhibitors of the mammalian target of rapamycin, or mTOR (for example, INK-128).
Additionally, there is considerable interest in agents that target fibroblast growth factor receptor 3 (FGFR3) for one subgroup. "In patients with the 4;14 translocation, FGFR3 is overexpressed," Dr. Wolf explained. "Finding an inhibitor for that or a direct antibody ... may be quite effective in those patients."
Investigators are also assessing the impact of targeting certain signaling pathways, such as the Jak/Stat pathway and the AKT pathway. For instance, a phase III trial is testing perifosine, an investigational AKT inhibitor, in combination therapy among patients with relapsed or refractory myeloma (NCT01002248).
The Oncology Congress is presented by Reed Medical Education. Reed Medical Education and this news organization are owned by Reed Elsevier Inc.
Dr. Wolf disclosed that he is on the speakers bureaus of Millenium, Celgene, and Ortho-Biotech, and is a consultant to and speaker for Onyx.
SAN FRANCISCO – A sweep of new agents are poised to deliver what could be a knock-out blow to multiple myeloma, according to the director of the myeloma program at the University of California, San Francisco.
Some are second- or third-generation agents in a mainstay class that appear to have less toxicity than and/or overcome resistance to their predecessors, Dr. Jeffrey L. Wolf said at the annual Oncology Congress here. Others come from classes not previously used in this disease.
"There is a rush to develop new drugs in myeloma," Dr. Wolf told attendees. "We [understand] some mechanisms that the disease seems to favor, so we can interfere with those."
The prospects, in turn, are excellent: "We have made such tremendous headway in myeloma, except for those exceptional cases with 17p deletions and some other adverse prognostic features," he said. "As a disease, it seems to be on the ropes."
A Less-Toxic Proteasome Inhibitor
The first-generation proteasome inhibitor bortezomib (Velcade) improves myeloma outcomes, but maximizing its benefit will require addressing the peripheral neuropathy that limits its use. Three strategies may lessen this toxicity without compromising efficacy, Dr. Wolf suggested: modestly reducing the standard dose, adjusting the schedule from twice to once weekly, and altering the route of administration from intravenous to subcutaneous.
For example, in patients with pretreated myeloma, giving bortezomib subcutaneously instead of intravenously results in an identical overall response rate of 52% (Lancet Oncol. 2011;12:431-40). But there are significant reductions in rates of peripheral neuropathy of any grade (38% vs. 53%) and grade 3 or higher (6% vs. 16%).
"Practically everybody that we see now at UCSF gets subcutaneous [bortezomib]," Dr. Wolf said. It’s a great way to go back and treat patients who maybe otherwise have stopped their therapy because of their neuropathy."
Carfilzomib, an investigational next-generation proteasome inhibitor in phase III trials, is showing good antimyeloma activity and a low rate of peripheral neuropathy. Among patients with pretreated, relapsed, or refractory disease, carfilzomib monotherapy achieved overall response rates of 42% to 53% in a bortezomib-naive group (ASCO 2011 annual meeting. Abstract 8026) and 21% in a bortezomib-exposed group (Haematologica 2010;95:452 Abstract 1099). Median time to progression was at least 8 months for both.
Dr. Wolf said that unpublished data suggest that the response rate was still 17% specifically among patients who had had progression on bortezomib, "so there appears to be some activity in patients who are already refractory to a prior proteasome inhibitor."
Meanwhile, the rates of grade 1/2 and grade 3 peripheral neuropathy were 6% and 1%, respectively. And only a single patient of the 155 had to stop treatment because of this adverse effect.
When carfilzomib is combined with lenalidomide-dexamethasone (the so-called CRd regimen), the overall response rate in patients with pretreated, relapsed, and refractory disease is 78%, and the rate of complete or near complete response is 24% (ASCO 2011 meeting. Abstract 8025).
And, in newly diagnosed myeloma, among 18 patients receiving eight cycles of CRd, the overall response rate was 100%, and the rate of stringent-complete, complete, or near-complete response was 61% (2011 International Myeloma Workshop. Poster P-253). "This is very, very exciting—I don’t think we’ve seen this in any other combination," Dr. Wolf commented. "But these are small numbers of patients; we still need to increase the numbers of patients studied with this combination."
Bortezomib and carfilzomib may soon have company from several investigational proteasome inhibitors available in oral formulations that have shown promise in preclinical testing or have advanced to clinical trials: CEP-18770 (Cephalon), ONX-0912 (Onyx), and MLN-9708 (Millenium).
A Third-Generation IMiD in Trials
Pomalidomide, a third-generation immunomodulatory drug (IMiD), coming after thalidomide (Thalomid), and lenalidomide (Revlimid), is also showing good antimyeloma activity in clinical trials, according to Dr. Wolf.
Among patients with pretreated myeloma, the rate of partial or better response when pomalidomide is combined with dexamethasone has ranged from 25% to 42%, depending on the trial and patient population. Adverse events are primarily hematologic.
And in patients who have previously received lenalidomide, the response rate is similar, at 35% (ASCO 2011 annual meeting. Abstract 8067). "So, as with carfilzomib, where there appear to be responses in patients who have prior resistance to bortezomib, it appears that pomalidomide can give us responses in patients who have already had resistance to lenalidomide," he said.
HDAC Inhibitors Show Activity
The histone deacetylase (HDAC) inhibitor vorinostat (Zolinza) is approved for treatment of lymphoma. But it is being tested in clinical trials for myeloma, in combination therapy, with promising results, according to Dr. Wolf. Overall response rates have ranged from 40% to 94%, depending on the patient population and combination.
Similarly, the HDAC inhibitor romidepsin (Istodax) is approved for lymphoma treatment but is also being studied for antimyeloma activity. And panobinostat, an investigational member of this drug class, is being evaluated as a component of combination therapy in phase II and III myeloma trials.
Monoclonal Antibodies Tested
Elotuzumab is an investigational monoclonal antibody directed against CS1, a glycoprotein that is highly expressed on the surface of plasma cells and implicated in myeloma pathogenesis.
In a phase I trial among patients with relapsed or refractory myeloma, the combination of elotuzumab with lenalidomide and low-dose dexamethasone yielded an overall response rate of 82% (ASCO 2011 meeting. Abstract 8076). The rate was 83% among the subset of patients whose disease was refractory to the most recent therapy and 95% among the subset of lenalidomide-naive patients.
The combination of elotuzumab with bortezomib has also been tested in patients with relapsed or refractory myeloma. But the overall response rate with this combination was less impressive, at 48% (ASH 2010 meeting. Abstract 3023).
Other Agents and Pathways
Several other agents are being eyed for roles in myeloma therapy as well. They include bendamustine (Treanda), an old drug initially revived for lymphoma treatment; aurora kinase inhibitors (for example, MLN-8237); and inhibitors of the mammalian target of rapamycin, or mTOR (for example, INK-128).
Additionally, there is considerable interest in agents that target fibroblast growth factor receptor 3 (FGFR3) for one subgroup. "In patients with the 4;14 translocation, FGFR3 is overexpressed," Dr. Wolf explained. "Finding an inhibitor for that or a direct antibody ... may be quite effective in those patients."
Investigators are also assessing the impact of targeting certain signaling pathways, such as the Jak/Stat pathway and the AKT pathway. For instance, a phase III trial is testing perifosine, an investigational AKT inhibitor, in combination therapy among patients with relapsed or refractory myeloma (NCT01002248).
The Oncology Congress is presented by Reed Medical Education. Reed Medical Education and this news organization are owned by Reed Elsevier Inc.
Dr. Wolf disclosed that he is on the speakers bureaus of Millenium, Celgene, and Ortho-Biotech, and is a consultant to and speaker for Onyx.
SAN FRANCISCO – A sweep of new agents are poised to deliver what could be a knock-out blow to multiple myeloma, according to the director of the myeloma program at the University of California, San Francisco.
Some are second- or third-generation agents in a mainstay class that appear to have less toxicity than and/or overcome resistance to their predecessors, Dr. Jeffrey L. Wolf said at the annual Oncology Congress here. Others come from classes not previously used in this disease.
"There is a rush to develop new drugs in myeloma," Dr. Wolf told attendees. "We [understand] some mechanisms that the disease seems to favor, so we can interfere with those."
The prospects, in turn, are excellent: "We have made such tremendous headway in myeloma, except for those exceptional cases with 17p deletions and some other adverse prognostic features," he said. "As a disease, it seems to be on the ropes."
A Less-Toxic Proteasome Inhibitor
The first-generation proteasome inhibitor bortezomib (Velcade) improves myeloma outcomes, but maximizing its benefit will require addressing the peripheral neuropathy that limits its use. Three strategies may lessen this toxicity without compromising efficacy, Dr. Wolf suggested: modestly reducing the standard dose, adjusting the schedule from twice to once weekly, and altering the route of administration from intravenous to subcutaneous.
For example, in patients with pretreated myeloma, giving bortezomib subcutaneously instead of intravenously results in an identical overall response rate of 52% (Lancet Oncol. 2011;12:431-40). But there are significant reductions in rates of peripheral neuropathy of any grade (38% vs. 53%) and grade 3 or higher (6% vs. 16%).
"Practically everybody that we see now at UCSF gets subcutaneous [bortezomib]," Dr. Wolf said. It’s a great way to go back and treat patients who maybe otherwise have stopped their therapy because of their neuropathy."
Carfilzomib, an investigational next-generation proteasome inhibitor in phase III trials, is showing good antimyeloma activity and a low rate of peripheral neuropathy. Among patients with pretreated, relapsed, or refractory disease, carfilzomib monotherapy achieved overall response rates of 42% to 53% in a bortezomib-naive group (ASCO 2011 annual meeting. Abstract 8026) and 21% in a bortezomib-exposed group (Haematologica 2010;95:452 Abstract 1099). Median time to progression was at least 8 months for both.
Dr. Wolf said that unpublished data suggest that the response rate was still 17% specifically among patients who had had progression on bortezomib, "so there appears to be some activity in patients who are already refractory to a prior proteasome inhibitor."
Meanwhile, the rates of grade 1/2 and grade 3 peripheral neuropathy were 6% and 1%, respectively. And only a single patient of the 155 had to stop treatment because of this adverse effect.
When carfilzomib is combined with lenalidomide-dexamethasone (the so-called CRd regimen), the overall response rate in patients with pretreated, relapsed, and refractory disease is 78%, and the rate of complete or near complete response is 24% (ASCO 2011 meeting. Abstract 8025).
And, in newly diagnosed myeloma, among 18 patients receiving eight cycles of CRd, the overall response rate was 100%, and the rate of stringent-complete, complete, or near-complete response was 61% (2011 International Myeloma Workshop. Poster P-253). "This is very, very exciting—I don’t think we’ve seen this in any other combination," Dr. Wolf commented. "But these are small numbers of patients; we still need to increase the numbers of patients studied with this combination."
Bortezomib and carfilzomib may soon have company from several investigational proteasome inhibitors available in oral formulations that have shown promise in preclinical testing or have advanced to clinical trials: CEP-18770 (Cephalon), ONX-0912 (Onyx), and MLN-9708 (Millenium).
A Third-Generation IMiD in Trials
Pomalidomide, a third-generation immunomodulatory drug (IMiD), coming after thalidomide (Thalomid), and lenalidomide (Revlimid), is also showing good antimyeloma activity in clinical trials, according to Dr. Wolf.
Among patients with pretreated myeloma, the rate of partial or better response when pomalidomide is combined with dexamethasone has ranged from 25% to 42%, depending on the trial and patient population. Adverse events are primarily hematologic.
And in patients who have previously received lenalidomide, the response rate is similar, at 35% (ASCO 2011 annual meeting. Abstract 8067). "So, as with carfilzomib, where there appear to be responses in patients who have prior resistance to bortezomib, it appears that pomalidomide can give us responses in patients who have already had resistance to lenalidomide," he said.
HDAC Inhibitors Show Activity
The histone deacetylase (HDAC) inhibitor vorinostat (Zolinza) is approved for treatment of lymphoma. But it is being tested in clinical trials for myeloma, in combination therapy, with promising results, according to Dr. Wolf. Overall response rates have ranged from 40% to 94%, depending on the patient population and combination.
Similarly, the HDAC inhibitor romidepsin (Istodax) is approved for lymphoma treatment but is also being studied for antimyeloma activity. And panobinostat, an investigational member of this drug class, is being evaluated as a component of combination therapy in phase II and III myeloma trials.
Monoclonal Antibodies Tested
Elotuzumab is an investigational monoclonal antibody directed against CS1, a glycoprotein that is highly expressed on the surface of plasma cells and implicated in myeloma pathogenesis.
In a phase I trial among patients with relapsed or refractory myeloma, the combination of elotuzumab with lenalidomide and low-dose dexamethasone yielded an overall response rate of 82% (ASCO 2011 meeting. Abstract 8076). The rate was 83% among the subset of patients whose disease was refractory to the most recent therapy and 95% among the subset of lenalidomide-naive patients.
The combination of elotuzumab with bortezomib has also been tested in patients with relapsed or refractory myeloma. But the overall response rate with this combination was less impressive, at 48% (ASH 2010 meeting. Abstract 3023).
Other Agents and Pathways
Several other agents are being eyed for roles in myeloma therapy as well. They include bendamustine (Treanda), an old drug initially revived for lymphoma treatment; aurora kinase inhibitors (for example, MLN-8237); and inhibitors of the mammalian target of rapamycin, or mTOR (for example, INK-128).
Additionally, there is considerable interest in agents that target fibroblast growth factor receptor 3 (FGFR3) for one subgroup. "In patients with the 4;14 translocation, FGFR3 is overexpressed," Dr. Wolf explained. "Finding an inhibitor for that or a direct antibody ... may be quite effective in those patients."
Investigators are also assessing the impact of targeting certain signaling pathways, such as the Jak/Stat pathway and the AKT pathway. For instance, a phase III trial is testing perifosine, an investigational AKT inhibitor, in combination therapy among patients with relapsed or refractory myeloma (NCT01002248).
The Oncology Congress is presented by Reed Medical Education. Reed Medical Education and this news organization are owned by Reed Elsevier Inc.
Dr. Wolf disclosed that he is on the speakers bureaus of Millenium, Celgene, and Ortho-Biotech, and is a consultant to and speaker for Onyx.
EXPERT ANALYSIS FROM THE ANNUAL ONCOLOGY CONGRESS
Making Inroads in Treatment of Adult ALL
SAN FRANCISCO – Several areas of active research are improving the outlook for adults with acute lymphoblastic leukemia, Dr. Partow Kebriaei told attendees of the annual Oncology Congress here.
Investigators are exploring the impact of treatment intensification, stem cell transplantation, use of minimal residual disease to guide therapy, and a host of new agents, according to Dr. Kebriaei of the department of stem cell transplantation and cellular therapy at the University of Texas M.D. Anderson Cancer Center, Houston.
More effective and less toxic treatments are especially needed in this age group, she explained at the congress, which is presented by Reed Medical Education. "We have had great success in eradicating acute lymphoblastic leukemia (ALL) in children but still have a ways to go in adults," Dr. Kebraiei said.
In particular, although both incidence and mortality increase sharply over the age of 50 years, the median age of enrollees in major ALL adult trials has been 30-40 years. "So one thing that we need to think about as we devise more new therapies is how to make them more tolerable or relevant to the older patient," she commented.
Intensified Treatment for Young Adults
The observation that younger adults with ALL, up to the age of 40, have better long-term remission when treated with the more intensive pediatric regimens vs. adult regimens has raised interest in this approach for adults generally, Dr. Kebriaei noted.
A trial of intensified chemotherapy in adults found a significantly better response rate, event-free survival, and overall survival compared with outcomes seen historically with standard-intensity chemotherapy (J. Clin. Oncol. 2009;27:911-8).
But in age-stratified analyses, any survival benefit among patients older than 45 was offset by higher treatment-related mortality. "So, for that older subgroup, treatment intensification still does not really impact outcome favorably," she said.
Additionally, a caveat for the younger adults was that the trial allowed crossover to transplantation for patients having a donor. "So it’s a little bit difficult to ascertain whether these improved results are from the availability of transplant or the intensification of treatment," Dr. Kebriaei commented. "But, I think in general, one can conclude that treatment intensification is effective up to a certain age."
Stem Cell Transplantation
The International ALL Trial assessed the role of stem cell transplantation in adults with ALL in first complete remission. Patients younger than 55 years of age having a sibling donor were allocated to allogeneic transplantation, whereas older patients or those without a donor were randomized to autologous transplantation or chemotherapy.
Among patients not having the Philadelphia chromosome, which confers poorer prognosis, those with a donor were less likely to have a relapse than were those without a donor, whether they had standard- or high-risk disease (Blood 2008;111:1827-33). But treatment-related mortality was increased; it canceled out the relapse benefit in the high-risk group, so that in contrast to the standard-risk group, there was no net improvement in survival.
The higher treatment-related mortality was mainly due to higher rates of infection and graft-vs.-host disease. "So if we want transplant to afford a better outcome to all patients, we are going to have to impact these complications," Dr. Kebriaei maintained.
Of note, in additional trial analyses, chemotherapy was associated with significantly better event-free and overall survival than autologous transplantation.
Efforts to reduce the treatment-related mortality of transplantation include reduced-intensity conditioning regimens that, for example, omit total-body irradiation. Studies of this approach have indeed found that compared with the standard one including total-body irradiation, it achieves a lower rate of deaths due to treatment, and among patients transplanted in first remission, similar overall survival.
"But the most important thing is that if you look at the median age of patients [in these studies], we are now approaching 55, which is more reflective of what we see in the clinic," she noted.
Use of MRD in Risk-Oriented Therapy
"Minimal residual disease [MRD] really defines a submicroscopic ALL and in patients that we would normally term as remission patients," Dr. Kebriaei explained. "It is evaluated by multichannel flow cytometry or PCR [polymerase chain reaction], and it’s really becoming one of the most significant prognostic markers that we have."
For example, in the International ALL Trial, persistent MRD after induction or early consolidation was significantly associated with an increased risk of relapse in patients receiving chemotherapy alone (Br. J. Haematol. 2010;148:80-9). Yet, that was not the whole story.
"Interestingly, here, when they looked at MRD in patients prior to going forward on allogeneic stem cell transplant, they did not see any impact of MRD," she noted. "They also didn’t see an impact of MRD in helping to predict which patients would develop CNS relapse."
Targeted Therapies
Several therapies that target tumors’ molecular vulnerabilities are being incorporated into the treatment of adult ALL to clinical benefit. "I think the most dramatic improvement has been seen with the use of tyrosine kinase inhibitors (TKIs) for the Philadelphia chromosome–positive subset," Dr. Kebriaei commented.
In trials, use of TKIs in these patients has led to higher rates of complete remission, transplantation eligibility, and survival without transplantation. "Now all of these trials allowed transplant as well, so it’s hard to ascertain how much of this impact is coming from more transplant eligibility and how much of it is coming from the impact of the TKIs," she cautioned.
The SWOG S0805 Intergroup trial, open to patients up 50 years old with newly diagnosed Philadelphia chromosome–positive ALL, is looking more closely at the issue, as well as the issue of using TKIs as maintenance therapy. Patients receive dasatinib (Sprycel)-containing induction therapy and, if they undergo transplantation, dasatinib thereafter as well.
In clinical practice, "there is great variation in practice patterns, ranging from providing no TKI in maintenance all the way up to providing 2 years of TKI," Dr. Kebriaei observed. "And unfortunately, there isn’t good data yet published to determine what the best approach will be."
A second targeted therapy being used is rituximab (Rituxan), for ALL expressing CD20, another poor prognostic marker. Adding rituximab to the hyper-CVAD regimen significantly improves survival for patients younger than 60 years (J. Clin. Oncol. 2010;28:3880-9). But in older patients, there was no survival benefit, in part because of increased induction-related mortality.
Novel Agents and Cellular Therapies
The Food and Drug Administration has approved three novel agents for ALL treatment: pegylated asparaginase (Oncaspar), clofarabine (Clolar), and nelarabine (Arranon), and has received a new drug application for liposomal vincristine (Marqibo).
Nelarabine, for example, is a prodrug of ara-G that achieves good response and survival rates in patients with relapsed T-cell ALL, with a dose-limiting toxicity of neurotoxicity. It is now being evaluated when added to hyper-CVAD up front for patients with T-cell ALL, according to Dr. Kebriaei.
The investigational agent inotuzumab ozogamicin, an anti-CD22 antibody conjugated to calicheamicin, achieves an overall response rate of 61% when used as monotherapy in a trial among patients with refractory ALL (Jabbour et al. ASCO 2011 meeting. Abstract 6507).
"Interestingly, almost half of these patients were then able to go on to transplant in complete remission," she noted. Adverse effects included hepatotoxicity and veno-occlusive disease.
Another investigational antibody, blinatumomab, redirects T cells toward lysis of CD19-expressing cells and has been found to achieve complete remission in 13 of 16 patients with precursor B-ALL having persistent MRD (Topp et al. ASH 2009 meeting. Abstract 840).
A final novel strategy being tested is cellular therapy using chimeric antigen receptors (CARs), which are produced by fusing an extracellular single-chain antibody to an intracellular signaling domain. When expressed in T cells, these receptors redirect the cells’ antigen recognition toward a desired target, such as CD19.
"What’s very effective and interesting about this CAR strategy is that it doesn’t rely on preexisting antitumor immunity to generate an antitumor effector response," Dr. Kebriaei noted. "This is particularly important in the setting of ALL, where you have immune dysfunction."
Trials are assessing the use of CARs in the setting of stem cell transplantation, whereby donor T cells are transduced with anti-CD19 CARs and given as a targeted donor lymphocyte infusion.
"Perhaps by incorporating these targeted therapies and cellular therapies with the traditional cytotoxic therapies, we may be able to improve treatment outcomes without adding toxicity," concluded Dr. Kebriaei, who reported having no conflicts of interest related to her presentation.
The Oncology Congress is presented by Reed Medical Education. Reed Medical Education and this news organization are owned by Reed Elsevier Inc.
SAN FRANCISCO – Several areas of active research are improving the outlook for adults with acute lymphoblastic leukemia, Dr. Partow Kebriaei told attendees of the annual Oncology Congress here.
Investigators are exploring the impact of treatment intensification, stem cell transplantation, use of minimal residual disease to guide therapy, and a host of new agents, according to Dr. Kebriaei of the department of stem cell transplantation and cellular therapy at the University of Texas M.D. Anderson Cancer Center, Houston.
More effective and less toxic treatments are especially needed in this age group, she explained at the congress, which is presented by Reed Medical Education. "We have had great success in eradicating acute lymphoblastic leukemia (ALL) in children but still have a ways to go in adults," Dr. Kebraiei said.
In particular, although both incidence and mortality increase sharply over the age of 50 years, the median age of enrollees in major ALL adult trials has been 30-40 years. "So one thing that we need to think about as we devise more new therapies is how to make them more tolerable or relevant to the older patient," she commented.
Intensified Treatment for Young Adults
The observation that younger adults with ALL, up to the age of 40, have better long-term remission when treated with the more intensive pediatric regimens vs. adult regimens has raised interest in this approach for adults generally, Dr. Kebriaei noted.
A trial of intensified chemotherapy in adults found a significantly better response rate, event-free survival, and overall survival compared with outcomes seen historically with standard-intensity chemotherapy (J. Clin. Oncol. 2009;27:911-8).
But in age-stratified analyses, any survival benefit among patients older than 45 was offset by higher treatment-related mortality. "So, for that older subgroup, treatment intensification still does not really impact outcome favorably," she said.
Additionally, a caveat for the younger adults was that the trial allowed crossover to transplantation for patients having a donor. "So it’s a little bit difficult to ascertain whether these improved results are from the availability of transplant or the intensification of treatment," Dr. Kebriaei commented. "But, I think in general, one can conclude that treatment intensification is effective up to a certain age."
Stem Cell Transplantation
The International ALL Trial assessed the role of stem cell transplantation in adults with ALL in first complete remission. Patients younger than 55 years of age having a sibling donor were allocated to allogeneic transplantation, whereas older patients or those without a donor were randomized to autologous transplantation or chemotherapy.
Among patients not having the Philadelphia chromosome, which confers poorer prognosis, those with a donor were less likely to have a relapse than were those without a donor, whether they had standard- or high-risk disease (Blood 2008;111:1827-33). But treatment-related mortality was increased; it canceled out the relapse benefit in the high-risk group, so that in contrast to the standard-risk group, there was no net improvement in survival.
The higher treatment-related mortality was mainly due to higher rates of infection and graft-vs.-host disease. "So if we want transplant to afford a better outcome to all patients, we are going to have to impact these complications," Dr. Kebriaei maintained.
Of note, in additional trial analyses, chemotherapy was associated with significantly better event-free and overall survival than autologous transplantation.
Efforts to reduce the treatment-related mortality of transplantation include reduced-intensity conditioning regimens that, for example, omit total-body irradiation. Studies of this approach have indeed found that compared with the standard one including total-body irradiation, it achieves a lower rate of deaths due to treatment, and among patients transplanted in first remission, similar overall survival.
"But the most important thing is that if you look at the median age of patients [in these studies], we are now approaching 55, which is more reflective of what we see in the clinic," she noted.
Use of MRD in Risk-Oriented Therapy
"Minimal residual disease [MRD] really defines a submicroscopic ALL and in patients that we would normally term as remission patients," Dr. Kebriaei explained. "It is evaluated by multichannel flow cytometry or PCR [polymerase chain reaction], and it’s really becoming one of the most significant prognostic markers that we have."
For example, in the International ALL Trial, persistent MRD after induction or early consolidation was significantly associated with an increased risk of relapse in patients receiving chemotherapy alone (Br. J. Haematol. 2010;148:80-9). Yet, that was not the whole story.
"Interestingly, here, when they looked at MRD in patients prior to going forward on allogeneic stem cell transplant, they did not see any impact of MRD," she noted. "They also didn’t see an impact of MRD in helping to predict which patients would develop CNS relapse."
Targeted Therapies
Several therapies that target tumors’ molecular vulnerabilities are being incorporated into the treatment of adult ALL to clinical benefit. "I think the most dramatic improvement has been seen with the use of tyrosine kinase inhibitors (TKIs) for the Philadelphia chromosome–positive subset," Dr. Kebriaei commented.
In trials, use of TKIs in these patients has led to higher rates of complete remission, transplantation eligibility, and survival without transplantation. "Now all of these trials allowed transplant as well, so it’s hard to ascertain how much of this impact is coming from more transplant eligibility and how much of it is coming from the impact of the TKIs," she cautioned.
The SWOG S0805 Intergroup trial, open to patients up 50 years old with newly diagnosed Philadelphia chromosome–positive ALL, is looking more closely at the issue, as well as the issue of using TKIs as maintenance therapy. Patients receive dasatinib (Sprycel)-containing induction therapy and, if they undergo transplantation, dasatinib thereafter as well.
In clinical practice, "there is great variation in practice patterns, ranging from providing no TKI in maintenance all the way up to providing 2 years of TKI," Dr. Kebriaei observed. "And unfortunately, there isn’t good data yet published to determine what the best approach will be."
A second targeted therapy being used is rituximab (Rituxan), for ALL expressing CD20, another poor prognostic marker. Adding rituximab to the hyper-CVAD regimen significantly improves survival for patients younger than 60 years (J. Clin. Oncol. 2010;28:3880-9). But in older patients, there was no survival benefit, in part because of increased induction-related mortality.
Novel Agents and Cellular Therapies
The Food and Drug Administration has approved three novel agents for ALL treatment: pegylated asparaginase (Oncaspar), clofarabine (Clolar), and nelarabine (Arranon), and has received a new drug application for liposomal vincristine (Marqibo).
Nelarabine, for example, is a prodrug of ara-G that achieves good response and survival rates in patients with relapsed T-cell ALL, with a dose-limiting toxicity of neurotoxicity. It is now being evaluated when added to hyper-CVAD up front for patients with T-cell ALL, according to Dr. Kebriaei.
The investigational agent inotuzumab ozogamicin, an anti-CD22 antibody conjugated to calicheamicin, achieves an overall response rate of 61% when used as monotherapy in a trial among patients with refractory ALL (Jabbour et al. ASCO 2011 meeting. Abstract 6507).
"Interestingly, almost half of these patients were then able to go on to transplant in complete remission," she noted. Adverse effects included hepatotoxicity and veno-occlusive disease.
Another investigational antibody, blinatumomab, redirects T cells toward lysis of CD19-expressing cells and has been found to achieve complete remission in 13 of 16 patients with precursor B-ALL having persistent MRD (Topp et al. ASH 2009 meeting. Abstract 840).
A final novel strategy being tested is cellular therapy using chimeric antigen receptors (CARs), which are produced by fusing an extracellular single-chain antibody to an intracellular signaling domain. When expressed in T cells, these receptors redirect the cells’ antigen recognition toward a desired target, such as CD19.
"What’s very effective and interesting about this CAR strategy is that it doesn’t rely on preexisting antitumor immunity to generate an antitumor effector response," Dr. Kebriaei noted. "This is particularly important in the setting of ALL, where you have immune dysfunction."
Trials are assessing the use of CARs in the setting of stem cell transplantation, whereby donor T cells are transduced with anti-CD19 CARs and given as a targeted donor lymphocyte infusion.
"Perhaps by incorporating these targeted therapies and cellular therapies with the traditional cytotoxic therapies, we may be able to improve treatment outcomes without adding toxicity," concluded Dr. Kebriaei, who reported having no conflicts of interest related to her presentation.
The Oncology Congress is presented by Reed Medical Education. Reed Medical Education and this news organization are owned by Reed Elsevier Inc.
SAN FRANCISCO – Several areas of active research are improving the outlook for adults with acute lymphoblastic leukemia, Dr. Partow Kebriaei told attendees of the annual Oncology Congress here.
Investigators are exploring the impact of treatment intensification, stem cell transplantation, use of minimal residual disease to guide therapy, and a host of new agents, according to Dr. Kebriaei of the department of stem cell transplantation and cellular therapy at the University of Texas M.D. Anderson Cancer Center, Houston.
More effective and less toxic treatments are especially needed in this age group, she explained at the congress, which is presented by Reed Medical Education. "We have had great success in eradicating acute lymphoblastic leukemia (ALL) in children but still have a ways to go in adults," Dr. Kebraiei said.
In particular, although both incidence and mortality increase sharply over the age of 50 years, the median age of enrollees in major ALL adult trials has been 30-40 years. "So one thing that we need to think about as we devise more new therapies is how to make them more tolerable or relevant to the older patient," she commented.
Intensified Treatment for Young Adults
The observation that younger adults with ALL, up to the age of 40, have better long-term remission when treated with the more intensive pediatric regimens vs. adult regimens has raised interest in this approach for adults generally, Dr. Kebriaei noted.
A trial of intensified chemotherapy in adults found a significantly better response rate, event-free survival, and overall survival compared with outcomes seen historically with standard-intensity chemotherapy (J. Clin. Oncol. 2009;27:911-8).
But in age-stratified analyses, any survival benefit among patients older than 45 was offset by higher treatment-related mortality. "So, for that older subgroup, treatment intensification still does not really impact outcome favorably," she said.
Additionally, a caveat for the younger adults was that the trial allowed crossover to transplantation for patients having a donor. "So it’s a little bit difficult to ascertain whether these improved results are from the availability of transplant or the intensification of treatment," Dr. Kebriaei commented. "But, I think in general, one can conclude that treatment intensification is effective up to a certain age."
Stem Cell Transplantation
The International ALL Trial assessed the role of stem cell transplantation in adults with ALL in first complete remission. Patients younger than 55 years of age having a sibling donor were allocated to allogeneic transplantation, whereas older patients or those without a donor were randomized to autologous transplantation or chemotherapy.
Among patients not having the Philadelphia chromosome, which confers poorer prognosis, those with a donor were less likely to have a relapse than were those without a donor, whether they had standard- or high-risk disease (Blood 2008;111:1827-33). But treatment-related mortality was increased; it canceled out the relapse benefit in the high-risk group, so that in contrast to the standard-risk group, there was no net improvement in survival.
The higher treatment-related mortality was mainly due to higher rates of infection and graft-vs.-host disease. "So if we want transplant to afford a better outcome to all patients, we are going to have to impact these complications," Dr. Kebriaei maintained.
Of note, in additional trial analyses, chemotherapy was associated with significantly better event-free and overall survival than autologous transplantation.
Efforts to reduce the treatment-related mortality of transplantation include reduced-intensity conditioning regimens that, for example, omit total-body irradiation. Studies of this approach have indeed found that compared with the standard one including total-body irradiation, it achieves a lower rate of deaths due to treatment, and among patients transplanted in first remission, similar overall survival.
"But the most important thing is that if you look at the median age of patients [in these studies], we are now approaching 55, which is more reflective of what we see in the clinic," she noted.
Use of MRD in Risk-Oriented Therapy
"Minimal residual disease [MRD] really defines a submicroscopic ALL and in patients that we would normally term as remission patients," Dr. Kebriaei explained. "It is evaluated by multichannel flow cytometry or PCR [polymerase chain reaction], and it’s really becoming one of the most significant prognostic markers that we have."
For example, in the International ALL Trial, persistent MRD after induction or early consolidation was significantly associated with an increased risk of relapse in patients receiving chemotherapy alone (Br. J. Haematol. 2010;148:80-9). Yet, that was not the whole story.
"Interestingly, here, when they looked at MRD in patients prior to going forward on allogeneic stem cell transplant, they did not see any impact of MRD," she noted. "They also didn’t see an impact of MRD in helping to predict which patients would develop CNS relapse."
Targeted Therapies
Several therapies that target tumors’ molecular vulnerabilities are being incorporated into the treatment of adult ALL to clinical benefit. "I think the most dramatic improvement has been seen with the use of tyrosine kinase inhibitors (TKIs) for the Philadelphia chromosome–positive subset," Dr. Kebriaei commented.
In trials, use of TKIs in these patients has led to higher rates of complete remission, transplantation eligibility, and survival without transplantation. "Now all of these trials allowed transplant as well, so it’s hard to ascertain how much of this impact is coming from more transplant eligibility and how much of it is coming from the impact of the TKIs," she cautioned.
The SWOG S0805 Intergroup trial, open to patients up 50 years old with newly diagnosed Philadelphia chromosome–positive ALL, is looking more closely at the issue, as well as the issue of using TKIs as maintenance therapy. Patients receive dasatinib (Sprycel)-containing induction therapy and, if they undergo transplantation, dasatinib thereafter as well.
In clinical practice, "there is great variation in practice patterns, ranging from providing no TKI in maintenance all the way up to providing 2 years of TKI," Dr. Kebriaei observed. "And unfortunately, there isn’t good data yet published to determine what the best approach will be."
A second targeted therapy being used is rituximab (Rituxan), for ALL expressing CD20, another poor prognostic marker. Adding rituximab to the hyper-CVAD regimen significantly improves survival for patients younger than 60 years (J. Clin. Oncol. 2010;28:3880-9). But in older patients, there was no survival benefit, in part because of increased induction-related mortality.
Novel Agents and Cellular Therapies
The Food and Drug Administration has approved three novel agents for ALL treatment: pegylated asparaginase (Oncaspar), clofarabine (Clolar), and nelarabine (Arranon), and has received a new drug application for liposomal vincristine (Marqibo).
Nelarabine, for example, is a prodrug of ara-G that achieves good response and survival rates in patients with relapsed T-cell ALL, with a dose-limiting toxicity of neurotoxicity. It is now being evaluated when added to hyper-CVAD up front for patients with T-cell ALL, according to Dr. Kebriaei.
The investigational agent inotuzumab ozogamicin, an anti-CD22 antibody conjugated to calicheamicin, achieves an overall response rate of 61% when used as monotherapy in a trial among patients with refractory ALL (Jabbour et al. ASCO 2011 meeting. Abstract 6507).
"Interestingly, almost half of these patients were then able to go on to transplant in complete remission," she noted. Adverse effects included hepatotoxicity and veno-occlusive disease.
Another investigational antibody, blinatumomab, redirects T cells toward lysis of CD19-expressing cells and has been found to achieve complete remission in 13 of 16 patients with precursor B-ALL having persistent MRD (Topp et al. ASH 2009 meeting. Abstract 840).
A final novel strategy being tested is cellular therapy using chimeric antigen receptors (CARs), which are produced by fusing an extracellular single-chain antibody to an intracellular signaling domain. When expressed in T cells, these receptors redirect the cells’ antigen recognition toward a desired target, such as CD19.
"What’s very effective and interesting about this CAR strategy is that it doesn’t rely on preexisting antitumor immunity to generate an antitumor effector response," Dr. Kebriaei noted. "This is particularly important in the setting of ALL, where you have immune dysfunction."
Trials are assessing the use of CARs in the setting of stem cell transplantation, whereby donor T cells are transduced with anti-CD19 CARs and given as a targeted donor lymphocyte infusion.
"Perhaps by incorporating these targeted therapies and cellular therapies with the traditional cytotoxic therapies, we may be able to improve treatment outcomes without adding toxicity," concluded Dr. Kebriaei, who reported having no conflicts of interest related to her presentation.
The Oncology Congress is presented by Reed Medical Education. Reed Medical Education and this news organization are owned by Reed Elsevier Inc.
EXPERT ANALYSIS FROM THE ANNUAL ONCOLOGY CONGRESS
Refined Risk Stratification Guides Leukemia Transplant Decisions
SAN FRANCISCO – Risk stratification is becoming progressively more refined in adults with acute leukemia, helping to identify patients most likely to benefit from transplantation, according to Dr. Robert S. Negrin.
"What has become clear is that there is important prognostic information that one can gain from the patient at the time of diagnosis that can really help guide therapy," Dr. Negrin, a professor of medicine and chief of the division of blood and marrow transplantation at Stanford (Calif.) University, said at the annual Oncology Congress.
"Clearly, one can identify patients who are at higher risk for [poor outcome]. They can be split, cytogenetics being the first pass and then molecular markers being the second pass," he told attendees.
AML Status Predicts Outcome
Typically, three groups of adults with acute myeloid leukemia (AML) are offered transplantation, he said: those having a failure of induction chemotherapy, those in a first complete remission but having an intermediate or high risk for relapse, and those beyond first complete remission.
"The No. 1 predictor of outcome is the status of the disease at the time of transplant consideration, by far and away," noted Dr. Negrin. With transplantation, the 10-year overall survival rate is only 17% for the patients with induction failure or relapsed disease, in the Stanford experience. But patients in first complete remission fare better, at 57%.
Outcomes among patients in first complete remission are varied, however, with cytogenetics identifying distinct subgroups: better risk (10%-15% of these patients), poor risk (20%-30%), and intermediate risk (all the rest).
The better-risk subgroup does fairly well with chemotherapy alone, according to Dr. Negrin. "Those are patients that we generally would recommend not to consider transplant in first complete remission. One would only consider transplant at time of relapse or second remission." At the other extreme, the poor-risk subgroup "should clearly be considered for transplant up front."
Then there is the large subgroup having intermediate risk, many of whom have normal cytogenetics. Molecular markers have shown these cytogenetically normal AMLs to be highly heterogeneous (Blood 2010;115:453-74) – information that is now being used to guide transplant decisions.
For example, patients with mutation of the nucleophosmin (NPM1) gene have a favorable prognosis and are generally managed with chemotherapy alone. In contrast, their counterparts with a mutation of the FMS-like tyrosine kinase 3 (FLT3) gene have an unfavorable prognosis with chemotherapy and may fare better with transplantation.
"So this [molecular analysis] is very helpful because it helps split those patients with cytogenetically normal AML into favorable and unfavorable groups of patients," he commented. And he predicted that such molecular risk stratification will likely be even further refined in the future.
Research is also showing that molecular prognostic information may modify cytogenetic prognostic information. For instance, in the better-risk subgroup in first remission, among patients having the favorable inversion 16 cytogenetic profile, those with a KIT mutation have poorer survival with chemotherapy than do their counterparts with wild-type KIT (J. Clin. Oncol. 2006;24:3904-11).
"By and large, unfortunately, negative markers overcome the positive ones. That’s obviously a gross generalization, but unfortunately, it is reasonably accurate," Dr. Negrin commented. "So just finding a favorable cytogenetic abnormality does not tell the whole story. One needs to do the molecular studies as well."
And doing them early is key.
"Cytogenetic and molecular studies should be done on all leukemic patients," he stressed. "When we see patients in referral, a lot of patients still are not having these molecular studies done on a routine basis, and that’s unfortunate because it’s very important that we do the best we can to try to [evaluate] patients with the most advanced technologies we have. ... It’s very important that we identify these patients up front to treat them as appropriately as we can."
Know bcr-abl Status in ALL
Risk stratification is also improving among adults with acute lymphoblastic leukemia (ALL). In these cases as well, three groups are typically offered transplantation: those having a failure of induction chemotherapy, those in first complete remission having high-risk disease, and those in either a second complete remission or first relapse.
"Clearly, one can identify patients who are at higher risk for [poor outcome]. They can be split."
Disease status at the time of transplantation is also the best predictor of outcome in ALL. In the Stanford experience, the 10-year rate of overall survival is 62% for patients who undergo transplantation in first complete remission, compared with 43% for patients having relapsed or refractory disease at transplantation.
In terms of cytogenetics, the bcr-abl translocation (Philadelphia chromosome) is "a very ominous" finding among patients with B cell–lineage ALL, according to Dr. Negrin. These patients are not cured by intensive chemotherapy and derive only short-term benefit from tyrosine kinase inhibitors. Transplantation can achieve cure, however, although less often than in other ALL subtypes.
At Stanford, the 10-year rate of overall survival for patients having this cytogenetic abnormality is about 55% among those in first complete remission at transplantation, and 20% among those beyond first complete remission.
"Clearly, patients with Philadelphia chromosome–positive ALL are at extraordinary risk and are those who do benefit from transplant," he said.
Dr. Negrin reported that he sits on the data safety monitoring boards for Abbott Pharmaceuticals and Ziopharm, and is a consultant to Genzyme and Baxter. The Oncology Congress is presented by Reed Medical Education. Reed Medical Education and this news organization are owned by Reed Elsevier Inc.
SAN FRANCISCO – Risk stratification is becoming progressively more refined in adults with acute leukemia, helping to identify patients most likely to benefit from transplantation, according to Dr. Robert S. Negrin.
"What has become clear is that there is important prognostic information that one can gain from the patient at the time of diagnosis that can really help guide therapy," Dr. Negrin, a professor of medicine and chief of the division of blood and marrow transplantation at Stanford (Calif.) University, said at the annual Oncology Congress.
"Clearly, one can identify patients who are at higher risk for [poor outcome]. They can be split, cytogenetics being the first pass and then molecular markers being the second pass," he told attendees.
AML Status Predicts Outcome
Typically, three groups of adults with acute myeloid leukemia (AML) are offered transplantation, he said: those having a failure of induction chemotherapy, those in a first complete remission but having an intermediate or high risk for relapse, and those beyond first complete remission.
"The No. 1 predictor of outcome is the status of the disease at the time of transplant consideration, by far and away," noted Dr. Negrin. With transplantation, the 10-year overall survival rate is only 17% for the patients with induction failure or relapsed disease, in the Stanford experience. But patients in first complete remission fare better, at 57%.
Outcomes among patients in first complete remission are varied, however, with cytogenetics identifying distinct subgroups: better risk (10%-15% of these patients), poor risk (20%-30%), and intermediate risk (all the rest).
The better-risk subgroup does fairly well with chemotherapy alone, according to Dr. Negrin. "Those are patients that we generally would recommend not to consider transplant in first complete remission. One would only consider transplant at time of relapse or second remission." At the other extreme, the poor-risk subgroup "should clearly be considered for transplant up front."
Then there is the large subgroup having intermediate risk, many of whom have normal cytogenetics. Molecular markers have shown these cytogenetically normal AMLs to be highly heterogeneous (Blood 2010;115:453-74) – information that is now being used to guide transplant decisions.
For example, patients with mutation of the nucleophosmin (NPM1) gene have a favorable prognosis and are generally managed with chemotherapy alone. In contrast, their counterparts with a mutation of the FMS-like tyrosine kinase 3 (FLT3) gene have an unfavorable prognosis with chemotherapy and may fare better with transplantation.
"So this [molecular analysis] is very helpful because it helps split those patients with cytogenetically normal AML into favorable and unfavorable groups of patients," he commented. And he predicted that such molecular risk stratification will likely be even further refined in the future.
Research is also showing that molecular prognostic information may modify cytogenetic prognostic information. For instance, in the better-risk subgroup in first remission, among patients having the favorable inversion 16 cytogenetic profile, those with a KIT mutation have poorer survival with chemotherapy than do their counterparts with wild-type KIT (J. Clin. Oncol. 2006;24:3904-11).
"By and large, unfortunately, negative markers overcome the positive ones. That’s obviously a gross generalization, but unfortunately, it is reasonably accurate," Dr. Negrin commented. "So just finding a favorable cytogenetic abnormality does not tell the whole story. One needs to do the molecular studies as well."
And doing them early is key.
"Cytogenetic and molecular studies should be done on all leukemic patients," he stressed. "When we see patients in referral, a lot of patients still are not having these molecular studies done on a routine basis, and that’s unfortunate because it’s very important that we do the best we can to try to [evaluate] patients with the most advanced technologies we have. ... It’s very important that we identify these patients up front to treat them as appropriately as we can."
Know bcr-abl Status in ALL
Risk stratification is also improving among adults with acute lymphoblastic leukemia (ALL). In these cases as well, three groups are typically offered transplantation: those having a failure of induction chemotherapy, those in first complete remission having high-risk disease, and those in either a second complete remission or first relapse.
"Clearly, one can identify patients who are at higher risk for [poor outcome]. They can be split."
Disease status at the time of transplantation is also the best predictor of outcome in ALL. In the Stanford experience, the 10-year rate of overall survival is 62% for patients who undergo transplantation in first complete remission, compared with 43% for patients having relapsed or refractory disease at transplantation.
In terms of cytogenetics, the bcr-abl translocation (Philadelphia chromosome) is "a very ominous" finding among patients with B cell–lineage ALL, according to Dr. Negrin. These patients are not cured by intensive chemotherapy and derive only short-term benefit from tyrosine kinase inhibitors. Transplantation can achieve cure, however, although less often than in other ALL subtypes.
At Stanford, the 10-year rate of overall survival for patients having this cytogenetic abnormality is about 55% among those in first complete remission at transplantation, and 20% among those beyond first complete remission.
"Clearly, patients with Philadelphia chromosome–positive ALL are at extraordinary risk and are those who do benefit from transplant," he said.
Dr. Negrin reported that he sits on the data safety monitoring boards for Abbott Pharmaceuticals and Ziopharm, and is a consultant to Genzyme and Baxter. The Oncology Congress is presented by Reed Medical Education. Reed Medical Education and this news organization are owned by Reed Elsevier Inc.
SAN FRANCISCO – Risk stratification is becoming progressively more refined in adults with acute leukemia, helping to identify patients most likely to benefit from transplantation, according to Dr. Robert S. Negrin.
"What has become clear is that there is important prognostic information that one can gain from the patient at the time of diagnosis that can really help guide therapy," Dr. Negrin, a professor of medicine and chief of the division of blood and marrow transplantation at Stanford (Calif.) University, said at the annual Oncology Congress.
"Clearly, one can identify patients who are at higher risk for [poor outcome]. They can be split, cytogenetics being the first pass and then molecular markers being the second pass," he told attendees.
AML Status Predicts Outcome
Typically, three groups of adults with acute myeloid leukemia (AML) are offered transplantation, he said: those having a failure of induction chemotherapy, those in a first complete remission but having an intermediate or high risk for relapse, and those beyond first complete remission.
"The No. 1 predictor of outcome is the status of the disease at the time of transplant consideration, by far and away," noted Dr. Negrin. With transplantation, the 10-year overall survival rate is only 17% for the patients with induction failure or relapsed disease, in the Stanford experience. But patients in first complete remission fare better, at 57%.
Outcomes among patients in first complete remission are varied, however, with cytogenetics identifying distinct subgroups: better risk (10%-15% of these patients), poor risk (20%-30%), and intermediate risk (all the rest).
The better-risk subgroup does fairly well with chemotherapy alone, according to Dr. Negrin. "Those are patients that we generally would recommend not to consider transplant in first complete remission. One would only consider transplant at time of relapse or second remission." At the other extreme, the poor-risk subgroup "should clearly be considered for transplant up front."
Then there is the large subgroup having intermediate risk, many of whom have normal cytogenetics. Molecular markers have shown these cytogenetically normal AMLs to be highly heterogeneous (Blood 2010;115:453-74) – information that is now being used to guide transplant decisions.
For example, patients with mutation of the nucleophosmin (NPM1) gene have a favorable prognosis and are generally managed with chemotherapy alone. In contrast, their counterparts with a mutation of the FMS-like tyrosine kinase 3 (FLT3) gene have an unfavorable prognosis with chemotherapy and may fare better with transplantation.
"So this [molecular analysis] is very helpful because it helps split those patients with cytogenetically normal AML into favorable and unfavorable groups of patients," he commented. And he predicted that such molecular risk stratification will likely be even further refined in the future.
Research is also showing that molecular prognostic information may modify cytogenetic prognostic information. For instance, in the better-risk subgroup in first remission, among patients having the favorable inversion 16 cytogenetic profile, those with a KIT mutation have poorer survival with chemotherapy than do their counterparts with wild-type KIT (J. Clin. Oncol. 2006;24:3904-11).
"By and large, unfortunately, negative markers overcome the positive ones. That’s obviously a gross generalization, but unfortunately, it is reasonably accurate," Dr. Negrin commented. "So just finding a favorable cytogenetic abnormality does not tell the whole story. One needs to do the molecular studies as well."
And doing them early is key.
"Cytogenetic and molecular studies should be done on all leukemic patients," he stressed. "When we see patients in referral, a lot of patients still are not having these molecular studies done on a routine basis, and that’s unfortunate because it’s very important that we do the best we can to try to [evaluate] patients with the most advanced technologies we have. ... It’s very important that we identify these patients up front to treat them as appropriately as we can."
Know bcr-abl Status in ALL
Risk stratification is also improving among adults with acute lymphoblastic leukemia (ALL). In these cases as well, three groups are typically offered transplantation: those having a failure of induction chemotherapy, those in first complete remission having high-risk disease, and those in either a second complete remission or first relapse.
"Clearly, one can identify patients who are at higher risk for [poor outcome]. They can be split."
Disease status at the time of transplantation is also the best predictor of outcome in ALL. In the Stanford experience, the 10-year rate of overall survival is 62% for patients who undergo transplantation in first complete remission, compared with 43% for patients having relapsed or refractory disease at transplantation.
In terms of cytogenetics, the bcr-abl translocation (Philadelphia chromosome) is "a very ominous" finding among patients with B cell–lineage ALL, according to Dr. Negrin. These patients are not cured by intensive chemotherapy and derive only short-term benefit from tyrosine kinase inhibitors. Transplantation can achieve cure, however, although less often than in other ALL subtypes.
At Stanford, the 10-year rate of overall survival for patients having this cytogenetic abnormality is about 55% among those in first complete remission at transplantation, and 20% among those beyond first complete remission.
"Clearly, patients with Philadelphia chromosome–positive ALL are at extraordinary risk and are those who do benefit from transplant," he said.
Dr. Negrin reported that he sits on the data safety monitoring boards for Abbott Pharmaceuticals and Ziopharm, and is a consultant to Genzyme and Baxter. The Oncology Congress is presented by Reed Medical Education. Reed Medical Education and this news organization are owned by Reed Elsevier Inc.
EXPERT ANALYSIS FROM THE ANNUAL ONCOLOGY CONGRESS