User login
HIV chemoprophylaxis in U.S. up 738% in recent 3-year period
DURBAN, SOUTH AFRICA – The number of Americans using oral emtricitabine/tenofovir (Truvada) to prevent HIV infection jumped by 738% during a recent 3-year period since the drug’s 2012 marketing approval. However, uptake in certain at-risk populations leaves much room for improvement, Scott McCallister, MD, said at the 21st International AIDS Conference.
“While there are encouraging signs in using Truvada for pre-exposure prophylaxis across the country as a whole, there are certainly barriers that must be addressed in women, those under age 25, and in certain regions of the U.S. where lifetime risk of HIV acquisition is high,” said Dr. McCallister, senior director for clinical research at Gilead Sciences in Foster City, Calif.
He presented key findings from a national survey of 80% of retail pharmacies that showed that more than three-quarters of the 79,684 individuals who started on emtricitabine/tenofovir for HIV chemoprophylaxis during the fourth quarter of 2012 through the end of 2015 were men. Just 11% of the men and 28% of women taking the drug for pre-exposure prophylaxis (PrEP) were under age 25, a particularly high-risk group.
Fifty-one percent of all patients on emtricitabine/tenofovir for PrEP resided in five states: Texas, New York, Florida, Illinois, and California. Yet that doesn’t reflect the distribution of the HIV epidemic. A 2016 Centers for Disease and Prevention report concluded that the highest lifetime risk of HIV infection occurs in eight southeastern states and the District of Columbia, as well as Texas and New York.
“Some of the states with the highest lifetime risks of HIV diagnosis are lagging behind and have low numbers for Truvada for PrEP,” Dr. McCallister observed.
The Centers for Disease Control and Prevention estimates that while the lifetime risk of HIV is 1 in 99 in the United States overall, it’s 1 in 48 among black or Hispanic men, 1 in 2 for black men who have sex with men, and 1 in 4 for Hispanic MSM. Moreover, the CDC has also reported that 44% of all new HIV diagnoses occur in African Americans. And while Dr. McCallister said it’s difficult to analyze race/ethnicity in the Gilead survey because the data were de-identified, it’s his impression from other studies and talking with clinicians that PrEP users are disproportionately white.
Asked why prescribing of emtricitabine/tenofovir to date for PrEP in men far outpaces that for women, Dr. McCallister offered a theory: “Based on conversations I’ve had with community-based physicians who are avid prescribers, I think it’s a matter of men having a greater comfort level in terms of using PrEP because their clinicians and the MSM communities are more knowledgeable about PrEP. Perhaps there are fewer touch points for women to see knowledgeable clinicians. They are probably most often going to an ob.gyn. or a primary care physician who may have slightly less knowledge of PrEP than the HIV specialists or sexual health clinics where many MSMs are going.”
He noted that in addition to the randomized trial evidence of safety and efficacy that won Food and Drug Administration approval of emtricitabine/tenofovir for HIV PrEP, the real-world experience accrued in 32 demonstration projects with a total of more than 7,000 person-years of follow-up showed an HIV seroconversion rate of 0.95% per year in patients on the daily oral medication.
Dr. McCallister is an employee of Gilead Sciences, which sponsored the national PrEP survey and markets emtricitabine/tenofovir.
DURBAN, SOUTH AFRICA – The number of Americans using oral emtricitabine/tenofovir (Truvada) to prevent HIV infection jumped by 738% during a recent 3-year period since the drug’s 2012 marketing approval. However, uptake in certain at-risk populations leaves much room for improvement, Scott McCallister, MD, said at the 21st International AIDS Conference.
“While there are encouraging signs in using Truvada for pre-exposure prophylaxis across the country as a whole, there are certainly barriers that must be addressed in women, those under age 25, and in certain regions of the U.S. where lifetime risk of HIV acquisition is high,” said Dr. McCallister, senior director for clinical research at Gilead Sciences in Foster City, Calif.
He presented key findings from a national survey of 80% of retail pharmacies that showed that more than three-quarters of the 79,684 individuals who started on emtricitabine/tenofovir for HIV chemoprophylaxis during the fourth quarter of 2012 through the end of 2015 were men. Just 11% of the men and 28% of women taking the drug for pre-exposure prophylaxis (PrEP) were under age 25, a particularly high-risk group.
Fifty-one percent of all patients on emtricitabine/tenofovir for PrEP resided in five states: Texas, New York, Florida, Illinois, and California. Yet that doesn’t reflect the distribution of the HIV epidemic. A 2016 Centers for Disease and Prevention report concluded that the highest lifetime risk of HIV infection occurs in eight southeastern states and the District of Columbia, as well as Texas and New York.
“Some of the states with the highest lifetime risks of HIV diagnosis are lagging behind and have low numbers for Truvada for PrEP,” Dr. McCallister observed.
The Centers for Disease Control and Prevention estimates that while the lifetime risk of HIV is 1 in 99 in the United States overall, it’s 1 in 48 among black or Hispanic men, 1 in 2 for black men who have sex with men, and 1 in 4 for Hispanic MSM. Moreover, the CDC has also reported that 44% of all new HIV diagnoses occur in African Americans. And while Dr. McCallister said it’s difficult to analyze race/ethnicity in the Gilead survey because the data were de-identified, it’s his impression from other studies and talking with clinicians that PrEP users are disproportionately white.
Asked why prescribing of emtricitabine/tenofovir to date for PrEP in men far outpaces that for women, Dr. McCallister offered a theory: “Based on conversations I’ve had with community-based physicians who are avid prescribers, I think it’s a matter of men having a greater comfort level in terms of using PrEP because their clinicians and the MSM communities are more knowledgeable about PrEP. Perhaps there are fewer touch points for women to see knowledgeable clinicians. They are probably most often going to an ob.gyn. or a primary care physician who may have slightly less knowledge of PrEP than the HIV specialists or sexual health clinics where many MSMs are going.”
He noted that in addition to the randomized trial evidence of safety and efficacy that won Food and Drug Administration approval of emtricitabine/tenofovir for HIV PrEP, the real-world experience accrued in 32 demonstration projects with a total of more than 7,000 person-years of follow-up showed an HIV seroconversion rate of 0.95% per year in patients on the daily oral medication.
Dr. McCallister is an employee of Gilead Sciences, which sponsored the national PrEP survey and markets emtricitabine/tenofovir.
DURBAN, SOUTH AFRICA – The number of Americans using oral emtricitabine/tenofovir (Truvada) to prevent HIV infection jumped by 738% during a recent 3-year period since the drug’s 2012 marketing approval. However, uptake in certain at-risk populations leaves much room for improvement, Scott McCallister, MD, said at the 21st International AIDS Conference.
“While there are encouraging signs in using Truvada for pre-exposure prophylaxis across the country as a whole, there are certainly barriers that must be addressed in women, those under age 25, and in certain regions of the U.S. where lifetime risk of HIV acquisition is high,” said Dr. McCallister, senior director for clinical research at Gilead Sciences in Foster City, Calif.
He presented key findings from a national survey of 80% of retail pharmacies that showed that more than three-quarters of the 79,684 individuals who started on emtricitabine/tenofovir for HIV chemoprophylaxis during the fourth quarter of 2012 through the end of 2015 were men. Just 11% of the men and 28% of women taking the drug for pre-exposure prophylaxis (PrEP) were under age 25, a particularly high-risk group.
Fifty-one percent of all patients on emtricitabine/tenofovir for PrEP resided in five states: Texas, New York, Florida, Illinois, and California. Yet that doesn’t reflect the distribution of the HIV epidemic. A 2016 Centers for Disease and Prevention report concluded that the highest lifetime risk of HIV infection occurs in eight southeastern states and the District of Columbia, as well as Texas and New York.
“Some of the states with the highest lifetime risks of HIV diagnosis are lagging behind and have low numbers for Truvada for PrEP,” Dr. McCallister observed.
The Centers for Disease Control and Prevention estimates that while the lifetime risk of HIV is 1 in 99 in the United States overall, it’s 1 in 48 among black or Hispanic men, 1 in 2 for black men who have sex with men, and 1 in 4 for Hispanic MSM. Moreover, the CDC has also reported that 44% of all new HIV diagnoses occur in African Americans. And while Dr. McCallister said it’s difficult to analyze race/ethnicity in the Gilead survey because the data were de-identified, it’s his impression from other studies and talking with clinicians that PrEP users are disproportionately white.
Asked why prescribing of emtricitabine/tenofovir to date for PrEP in men far outpaces that for women, Dr. McCallister offered a theory: “Based on conversations I’ve had with community-based physicians who are avid prescribers, I think it’s a matter of men having a greater comfort level in terms of using PrEP because their clinicians and the MSM communities are more knowledgeable about PrEP. Perhaps there are fewer touch points for women to see knowledgeable clinicians. They are probably most often going to an ob.gyn. or a primary care physician who may have slightly less knowledge of PrEP than the HIV specialists or sexual health clinics where many MSMs are going.”
He noted that in addition to the randomized trial evidence of safety and efficacy that won Food and Drug Administration approval of emtricitabine/tenofovir for HIV PrEP, the real-world experience accrued in 32 demonstration projects with a total of more than 7,000 person-years of follow-up showed an HIV seroconversion rate of 0.95% per year in patients on the daily oral medication.
Dr. McCallister is an employee of Gilead Sciences, which sponsored the national PrEP survey and markets emtricitabine/tenofovir.
AT AIDS 2016
Key clinical point: More than 79,000 Americans started taking emtricitabine/tenofovir for prevention of HIV infection in a recent 3-year period.
Major finding: Adoption of HIV chemoprophylaxis via daily oral emtricitabine/tenofovir has risen steeply of late in the United States, although not in close accord with the epidemiology of the HIV epidemic.
Data source: This national survey of patients starting on emtricitabine/tenofovir to prevent HIV infection was based upon de-identified data obtained from 80% of U.S. retail pharmacies for fourth quarter 2012 through 2015.
Disclosures: The national survey of HIV pre-exposure prophylaxis was sponsored by Gilead Sciences, which markets emtricitabine/tenofovir (Truvada). The presenter is a company employee.
Starting antiretroviral therapy on same day as HIV testing improves outcomes
DURBAN, SOUTH AFRICA – Same-day HIV testing and initiation of antiretroviral therapy results in significantly better outcomes at 12 months than does the common practice of delaying ART for days to weeks in order to provide counseling and conduct further tests, Serena Koenig, MD, reported at the 21st International AIDS Conference.
“With standard testing protocols, patients are given the terrible news that they are HIV-positive and then discharged home without treatment. We believe that if we roll out the red carpet on that very challenging first day and provide extra attention and same-day ART, we increase a sense of hope, optimism, and connectedness to health care providers,” explained Dr. Koenig of Brigham and Women’s Hospital, Boston.
She presented a randomized, prospective clinical trial involving 577 patients at the GHESKIO Clinic in Port-au-Prince, Haiti, the world’s oldest HIV/AIDS treatment clinic and the largest provider of HIV/AIDS care in the Caribbean. Participants, all of whom had a positive HIV test, were randomized to same-day initiation of ART or to standard care, which was discharge home with instructions to return once per week for three counseling and clinical evaluation visits, with ART to begin on day 21.
Both groups received identical services and the same number of contacts with physicians and social workers in the first month following their HIV diagnosis. The only difference was the timing of ART initiation.
Study eligibility was restricted to patients with World Health Organization stage 1 or 2 HIV disease and a same-day chest x-ray showing no indication of tuberculosis or pneumonia.
The primary study endpoint was the rate of retention in care with a plasma HIV RNA viral load of less than 50 copies/mL at 12 months follow-up. This target was achieved in 54% of the same-day ART group, compared with 42% of controls.
Same-day ART also proved significantly better than the standard protocol in terms of the secondary study endpoints. It was associated with 3% mortality at 12 months, compared with 7% in the control arm. Also, 61% of patients in the same-day ART group were retained in care with a viral load of less than 1,000 copies/mL at 12 months, compared with 50% of controls.
In a multivariate regression analysis adjusted for patient demographics and clinical factors, same-day ART was independently associated with a 76% greater likelihood of retention in care with a viral load below 50 copies/mL at 12 months, a 65% reduction in the risk of mortality, and a 67% greater likelihood of retention in care with a viral load of less than 1,000 copies/mL.
The usual rationale for scheduling multiple pre-ART clinic visits is that patients may need time to come to grips with the reality that they require lifelong treatment, coupled with a feeling among many clinicians that there is no real urgency regarding ART initiation. But Dr. Koenig characterized the standard practice of requiring several clinic visits before starting ART as “a missed opportunity.”
“Delays in treatment are associated with increased mortality, diminished recovery of CD4+ cells, higher cost of treatment for opportunistic infections, and ongoing HIV transmission,” she said. “In addition to making things logistically easy, a big part of our study of same-day ART was the foundation of hope.”
Dr. Koenig reported having no financial conflicts with regard to this study, which was funded by the National Institute of Allergy and Infectious Diseases.
DURBAN, SOUTH AFRICA – Same-day HIV testing and initiation of antiretroviral therapy results in significantly better outcomes at 12 months than does the common practice of delaying ART for days to weeks in order to provide counseling and conduct further tests, Serena Koenig, MD, reported at the 21st International AIDS Conference.
“With standard testing protocols, patients are given the terrible news that they are HIV-positive and then discharged home without treatment. We believe that if we roll out the red carpet on that very challenging first day and provide extra attention and same-day ART, we increase a sense of hope, optimism, and connectedness to health care providers,” explained Dr. Koenig of Brigham and Women’s Hospital, Boston.
She presented a randomized, prospective clinical trial involving 577 patients at the GHESKIO Clinic in Port-au-Prince, Haiti, the world’s oldest HIV/AIDS treatment clinic and the largest provider of HIV/AIDS care in the Caribbean. Participants, all of whom had a positive HIV test, were randomized to same-day initiation of ART or to standard care, which was discharge home with instructions to return once per week for three counseling and clinical evaluation visits, with ART to begin on day 21.
Both groups received identical services and the same number of contacts with physicians and social workers in the first month following their HIV diagnosis. The only difference was the timing of ART initiation.
Study eligibility was restricted to patients with World Health Organization stage 1 or 2 HIV disease and a same-day chest x-ray showing no indication of tuberculosis or pneumonia.
The primary study endpoint was the rate of retention in care with a plasma HIV RNA viral load of less than 50 copies/mL at 12 months follow-up. This target was achieved in 54% of the same-day ART group, compared with 42% of controls.
Same-day ART also proved significantly better than the standard protocol in terms of the secondary study endpoints. It was associated with 3% mortality at 12 months, compared with 7% in the control arm. Also, 61% of patients in the same-day ART group were retained in care with a viral load of less than 1,000 copies/mL at 12 months, compared with 50% of controls.
In a multivariate regression analysis adjusted for patient demographics and clinical factors, same-day ART was independently associated with a 76% greater likelihood of retention in care with a viral load below 50 copies/mL at 12 months, a 65% reduction in the risk of mortality, and a 67% greater likelihood of retention in care with a viral load of less than 1,000 copies/mL.
The usual rationale for scheduling multiple pre-ART clinic visits is that patients may need time to come to grips with the reality that they require lifelong treatment, coupled with a feeling among many clinicians that there is no real urgency regarding ART initiation. But Dr. Koenig characterized the standard practice of requiring several clinic visits before starting ART as “a missed opportunity.”
“Delays in treatment are associated with increased mortality, diminished recovery of CD4+ cells, higher cost of treatment for opportunistic infections, and ongoing HIV transmission,” she said. “In addition to making things logistically easy, a big part of our study of same-day ART was the foundation of hope.”
Dr. Koenig reported having no financial conflicts with regard to this study, which was funded by the National Institute of Allergy and Infectious Diseases.
DURBAN, SOUTH AFRICA – Same-day HIV testing and initiation of antiretroviral therapy results in significantly better outcomes at 12 months than does the common practice of delaying ART for days to weeks in order to provide counseling and conduct further tests, Serena Koenig, MD, reported at the 21st International AIDS Conference.
“With standard testing protocols, patients are given the terrible news that they are HIV-positive and then discharged home without treatment. We believe that if we roll out the red carpet on that very challenging first day and provide extra attention and same-day ART, we increase a sense of hope, optimism, and connectedness to health care providers,” explained Dr. Koenig of Brigham and Women’s Hospital, Boston.
She presented a randomized, prospective clinical trial involving 577 patients at the GHESKIO Clinic in Port-au-Prince, Haiti, the world’s oldest HIV/AIDS treatment clinic and the largest provider of HIV/AIDS care in the Caribbean. Participants, all of whom had a positive HIV test, were randomized to same-day initiation of ART or to standard care, which was discharge home with instructions to return once per week for three counseling and clinical evaluation visits, with ART to begin on day 21.
Both groups received identical services and the same number of contacts with physicians and social workers in the first month following their HIV diagnosis. The only difference was the timing of ART initiation.
Study eligibility was restricted to patients with World Health Organization stage 1 or 2 HIV disease and a same-day chest x-ray showing no indication of tuberculosis or pneumonia.
The primary study endpoint was the rate of retention in care with a plasma HIV RNA viral load of less than 50 copies/mL at 12 months follow-up. This target was achieved in 54% of the same-day ART group, compared with 42% of controls.
Same-day ART also proved significantly better than the standard protocol in terms of the secondary study endpoints. It was associated with 3% mortality at 12 months, compared with 7% in the control arm. Also, 61% of patients in the same-day ART group were retained in care with a viral load of less than 1,000 copies/mL at 12 months, compared with 50% of controls.
In a multivariate regression analysis adjusted for patient demographics and clinical factors, same-day ART was independently associated with a 76% greater likelihood of retention in care with a viral load below 50 copies/mL at 12 months, a 65% reduction in the risk of mortality, and a 67% greater likelihood of retention in care with a viral load of less than 1,000 copies/mL.
The usual rationale for scheduling multiple pre-ART clinic visits is that patients may need time to come to grips with the reality that they require lifelong treatment, coupled with a feeling among many clinicians that there is no real urgency regarding ART initiation. But Dr. Koenig characterized the standard practice of requiring several clinic visits before starting ART as “a missed opportunity.”
“Delays in treatment are associated with increased mortality, diminished recovery of CD4+ cells, higher cost of treatment for opportunistic infections, and ongoing HIV transmission,” she said. “In addition to making things logistically easy, a big part of our study of same-day ART was the foundation of hope.”
Dr. Koenig reported having no financial conflicts with regard to this study, which was funded by the National Institute of Allergy and Infectious Diseases.
AT AIDS 2016
Key clinical point: Starting antiretroviral therapy on the same day a patient undergoes HIV testing is clinically advantageous.
Major finding: Patients who started antiretroviral therapy the same day they tested positive for HIV were 76% more likely to remain in care with a plasma HIV RNA viral load of less than 50 copies/mL at 12 months follow-up than were those whose treatment start was delayed 3 weeks for pre-treatment counseling sessions and further testing.
Data source: This prospective, randomized trial included 577 HIV-positive patients who were randomized to initiation of antiretroviral therapy on the same day they underwent HIV testing or to initiation of therapy 21 days later, after three additional clinic visits.
Disclosures: The presenter reported having no financial conflicts with regard to this study, which was funded by the National Institute of Allergy and Infectious Diseases.
Extended-release naltrexone helps alcohol-dependent HIV-positive prisoners transition to community
DURBAN, SOUTH AFRICA – Extended-release naltrexone provides clinically meaningful benefits in HIV-infected prisoners with alcohol use disorder and multiple comorbid conditions as they transition back into the community, according to the findings of a double-blind randomized clinical trial.
“I think it’s important to know that a very effective medication, which has not previously been given to this population, was accepted by this group. It may be a feasible conduit to care as they transition to the community, even among those with severe psychosocial disparities like homelessness and mental illness,” Sandra A. Springer, MD, said in presenting the study findings at the 21st International AIDS Conference.
Extended-release naltrexone (Vivitrol) is a mu-opioid receptor antagonist approved for the treatment of alcohol use disorder, where it has been shown to decrease consumption. But prior to her study, the once-monthly injectable drug hadn’t been studied in alcohol-dependent prisoners living with HIV who are transitioning from jail or prison into the community, noted Dr. Springer, an infectious disease specialist at Yale University in New Haven, Conn.
This is a large, important, and seriously neglected patient population, she observed. The United States has the highest incarceration rate in the world. The prevalence of HIV infection is at least three times greater in U.S. criminal justice settings than in the general population. Alcohol use disorders are eightfold more common. Release from prison or jail in affected individuals often is complicated by relapse to alcohol use, which in turn is associated with poor HIV treatment outcomes.
Dr. Springer reported on 100 HIV-positive adult prisoners with alcohol use disorder diagnosed by DSM-IV criteria who were randomized double-blind two-to-one to 6 monthly 380-mg intramuscular injections of extended-release naltrexone or placebo, with the first dose given 3-7 days prior to release. Participants were required to have no baseline clinical evidence of cirrhosis or very high liver enzyme levels.
Half of participants had chronic hepatitis C. Eighty-seven percent of subjects scored 20 or higher on the Alcohol Use Disorders Identification test, indicating alcohol dependence. On the Mini International Neuropsychiatric Interview, 15% of participants met criteria for major depressive disorder, 16% for bipolar disorder, 59% for cocaine use disorder, 16% for narcotic use disorder, and 16% for cannabis use disorder. Most of the subjects were homeless or had an unstable housing situation.
Alcohol outcomes were assessed monthly during the 6-month trial. Not surprisingly, the better the treatment adherence, the better the outcomes. During the 90 days before incarceration, patients self-reported that 70% of those days were heavy drinking days, defined in men as having five or more drinks per day and in women as four or more. Their average consumption on those heavy drinking days was 28 drinks per day. In contrast, patients who accepted four or more extended-release naltrexone injections during 180 days of follow-up after release from custody drank heavily on just 7.6% of days, with an average of 8.6 drinks per day on those heavy drinking days. Subjects who received four or more placebo injections drank heavily on 11.6% of days, consuming an average of 12 drinks per heavy drinking day.
The time to first heavy drinking day was longer in patients who accepted 4-6 monthly injections of extended-release naltrexone than in those with 4-6 placebo injections. However, the difference achieved statistical significance only in the younger subgroup of participants aged 21-29 years. In that subgroup, the average time to the first heavy drinking day was 24.1 days, compared with 9.5 days with placebo.
On a composite alcohol consumption index comprised of time to first heavy drinking day after release, mean number of drinks per drinking day, change from before to after incarceration in average number of drinks per drinking day, alcohol craving score, and total number of drinking days, subjects who received four or more extended-release naltrexone injections had a significantly more favorable result, with a mean score of 3.15, compared with 2.93 in patients who took four or more placebo injections.
Moreover, consistent use of extended-release naltrexone was associated with significantly lower HIV viral load counts, compared with placebo-treated controls.
Treatment with extended-release naltrexone was safe. No serious side effects occurred, even in patients with comorbid hepatitis C who were on antiretroviral therapy. The most common side effects were the same as in seen in studies of the drug in other populations: mild to moderate nausea, headache, decreased appetite, fatigue, and dizziness.
Elsewhere at AIDS 2016, Chris Beyrer, MD, president of the International AIDS Society, included prisoners on his list of the populations most vulnerable to HIV because of discriminatory laws and policies in many parts of the world. Others on the list were transgender people, sex workers, men who have sex with men, and injection drug users.
“We’ll never be able to end AIDS without addressing the needs of these most vulnerable individuals and communities, and yet we know in 2016 far too many are being left behind,” said Dr. Beyrer, professor of epidemiology at Johns Hopkins University, Baltimore.
Transgender individuals, for example, are 49 times more likely to have HIV infection than other adults. Injection drug users and men who have sex with men are each 24-fold more likely to become HIV infected than the general population. Sex workers are 10 times more likely to acquire HIV infection than others in their reproductive years. And prisoners have a fivefold greater prevalence of HIV.
“In 2014 these vulnerable groups accounted for more than one-third of all new HIV infections. That’s an extraordinary proportion of HIV,” he observed. “This truly is the undone work of the HIV response. If there’s any silver lining in this cloud, it’s this: We’re talking about a relatively small number of people who are at high risk of infection relative to the world’s population. And that means that turning this around doesn’t require massive new commitments to very large populations. What it does require is an honest acknowledgment of where the epidemic is hitting hardest and directing resources to that need.”
Unfortunately, screening and treatment programs are rarely tailored to reach these highly vulnerable groups effectively, he added.
Dr. Beyrer was a contributor to a special issue of the Lancet devoted to HIV infection among prisoners published with the AIDS 2016 conference.
Dr. Springer’s study was funded by the National Institute on Alcohol Abuse and Alcoholism, and the National Institute on Drug Abuse. She reported having no financial conflicts of interest.
DURBAN, SOUTH AFRICA – Extended-release naltrexone provides clinically meaningful benefits in HIV-infected prisoners with alcohol use disorder and multiple comorbid conditions as they transition back into the community, according to the findings of a double-blind randomized clinical trial.
“I think it’s important to know that a very effective medication, which has not previously been given to this population, was accepted by this group. It may be a feasible conduit to care as they transition to the community, even among those with severe psychosocial disparities like homelessness and mental illness,” Sandra A. Springer, MD, said in presenting the study findings at the 21st International AIDS Conference.
Extended-release naltrexone (Vivitrol) is a mu-opioid receptor antagonist approved for the treatment of alcohol use disorder, where it has been shown to decrease consumption. But prior to her study, the once-monthly injectable drug hadn’t been studied in alcohol-dependent prisoners living with HIV who are transitioning from jail or prison into the community, noted Dr. Springer, an infectious disease specialist at Yale University in New Haven, Conn.
This is a large, important, and seriously neglected patient population, she observed. The United States has the highest incarceration rate in the world. The prevalence of HIV infection is at least three times greater in U.S. criminal justice settings than in the general population. Alcohol use disorders are eightfold more common. Release from prison or jail in affected individuals often is complicated by relapse to alcohol use, which in turn is associated with poor HIV treatment outcomes.
Dr. Springer reported on 100 HIV-positive adult prisoners with alcohol use disorder diagnosed by DSM-IV criteria who were randomized double-blind two-to-one to 6 monthly 380-mg intramuscular injections of extended-release naltrexone or placebo, with the first dose given 3-7 days prior to release. Participants were required to have no baseline clinical evidence of cirrhosis or very high liver enzyme levels.
Half of participants had chronic hepatitis C. Eighty-seven percent of subjects scored 20 or higher on the Alcohol Use Disorders Identification test, indicating alcohol dependence. On the Mini International Neuropsychiatric Interview, 15% of participants met criteria for major depressive disorder, 16% for bipolar disorder, 59% for cocaine use disorder, 16% for narcotic use disorder, and 16% for cannabis use disorder. Most of the subjects were homeless or had an unstable housing situation.
Alcohol outcomes were assessed monthly during the 6-month trial. Not surprisingly, the better the treatment adherence, the better the outcomes. During the 90 days before incarceration, patients self-reported that 70% of those days were heavy drinking days, defined in men as having five or more drinks per day and in women as four or more. Their average consumption on those heavy drinking days was 28 drinks per day. In contrast, patients who accepted four or more extended-release naltrexone injections during 180 days of follow-up after release from custody drank heavily on just 7.6% of days, with an average of 8.6 drinks per day on those heavy drinking days. Subjects who received four or more placebo injections drank heavily on 11.6% of days, consuming an average of 12 drinks per heavy drinking day.
The time to first heavy drinking day was longer in patients who accepted 4-6 monthly injections of extended-release naltrexone than in those with 4-6 placebo injections. However, the difference achieved statistical significance only in the younger subgroup of participants aged 21-29 years. In that subgroup, the average time to the first heavy drinking day was 24.1 days, compared with 9.5 days with placebo.
On a composite alcohol consumption index comprised of time to first heavy drinking day after release, mean number of drinks per drinking day, change from before to after incarceration in average number of drinks per drinking day, alcohol craving score, and total number of drinking days, subjects who received four or more extended-release naltrexone injections had a significantly more favorable result, with a mean score of 3.15, compared with 2.93 in patients who took four or more placebo injections.
Moreover, consistent use of extended-release naltrexone was associated with significantly lower HIV viral load counts, compared with placebo-treated controls.
Treatment with extended-release naltrexone was safe. No serious side effects occurred, even in patients with comorbid hepatitis C who were on antiretroviral therapy. The most common side effects were the same as in seen in studies of the drug in other populations: mild to moderate nausea, headache, decreased appetite, fatigue, and dizziness.
Elsewhere at AIDS 2016, Chris Beyrer, MD, president of the International AIDS Society, included prisoners on his list of the populations most vulnerable to HIV because of discriminatory laws and policies in many parts of the world. Others on the list were transgender people, sex workers, men who have sex with men, and injection drug users.
“We’ll never be able to end AIDS without addressing the needs of these most vulnerable individuals and communities, and yet we know in 2016 far too many are being left behind,” said Dr. Beyrer, professor of epidemiology at Johns Hopkins University, Baltimore.
Transgender individuals, for example, are 49 times more likely to have HIV infection than other adults. Injection drug users and men who have sex with men are each 24-fold more likely to become HIV infected than the general population. Sex workers are 10 times more likely to acquire HIV infection than others in their reproductive years. And prisoners have a fivefold greater prevalence of HIV.
“In 2014 these vulnerable groups accounted for more than one-third of all new HIV infections. That’s an extraordinary proportion of HIV,” he observed. “This truly is the undone work of the HIV response. If there’s any silver lining in this cloud, it’s this: We’re talking about a relatively small number of people who are at high risk of infection relative to the world’s population. And that means that turning this around doesn’t require massive new commitments to very large populations. What it does require is an honest acknowledgment of where the epidemic is hitting hardest and directing resources to that need.”
Unfortunately, screening and treatment programs are rarely tailored to reach these highly vulnerable groups effectively, he added.
Dr. Beyrer was a contributor to a special issue of the Lancet devoted to HIV infection among prisoners published with the AIDS 2016 conference.
Dr. Springer’s study was funded by the National Institute on Alcohol Abuse and Alcoholism, and the National Institute on Drug Abuse. She reported having no financial conflicts of interest.
DURBAN, SOUTH AFRICA – Extended-release naltrexone provides clinically meaningful benefits in HIV-infected prisoners with alcohol use disorder and multiple comorbid conditions as they transition back into the community, according to the findings of a double-blind randomized clinical trial.
“I think it’s important to know that a very effective medication, which has not previously been given to this population, was accepted by this group. It may be a feasible conduit to care as they transition to the community, even among those with severe psychosocial disparities like homelessness and mental illness,” Sandra A. Springer, MD, said in presenting the study findings at the 21st International AIDS Conference.
Extended-release naltrexone (Vivitrol) is a mu-opioid receptor antagonist approved for the treatment of alcohol use disorder, where it has been shown to decrease consumption. But prior to her study, the once-monthly injectable drug hadn’t been studied in alcohol-dependent prisoners living with HIV who are transitioning from jail or prison into the community, noted Dr. Springer, an infectious disease specialist at Yale University in New Haven, Conn.
This is a large, important, and seriously neglected patient population, she observed. The United States has the highest incarceration rate in the world. The prevalence of HIV infection is at least three times greater in U.S. criminal justice settings than in the general population. Alcohol use disorders are eightfold more common. Release from prison or jail in affected individuals often is complicated by relapse to alcohol use, which in turn is associated with poor HIV treatment outcomes.
Dr. Springer reported on 100 HIV-positive adult prisoners with alcohol use disorder diagnosed by DSM-IV criteria who were randomized double-blind two-to-one to 6 monthly 380-mg intramuscular injections of extended-release naltrexone or placebo, with the first dose given 3-7 days prior to release. Participants were required to have no baseline clinical evidence of cirrhosis or very high liver enzyme levels.
Half of participants had chronic hepatitis C. Eighty-seven percent of subjects scored 20 or higher on the Alcohol Use Disorders Identification test, indicating alcohol dependence. On the Mini International Neuropsychiatric Interview, 15% of participants met criteria for major depressive disorder, 16% for bipolar disorder, 59% for cocaine use disorder, 16% for narcotic use disorder, and 16% for cannabis use disorder. Most of the subjects were homeless or had an unstable housing situation.
Alcohol outcomes were assessed monthly during the 6-month trial. Not surprisingly, the better the treatment adherence, the better the outcomes. During the 90 days before incarceration, patients self-reported that 70% of those days were heavy drinking days, defined in men as having five or more drinks per day and in women as four or more. Their average consumption on those heavy drinking days was 28 drinks per day. In contrast, patients who accepted four or more extended-release naltrexone injections during 180 days of follow-up after release from custody drank heavily on just 7.6% of days, with an average of 8.6 drinks per day on those heavy drinking days. Subjects who received four or more placebo injections drank heavily on 11.6% of days, consuming an average of 12 drinks per heavy drinking day.
The time to first heavy drinking day was longer in patients who accepted 4-6 monthly injections of extended-release naltrexone than in those with 4-6 placebo injections. However, the difference achieved statistical significance only in the younger subgroup of participants aged 21-29 years. In that subgroup, the average time to the first heavy drinking day was 24.1 days, compared with 9.5 days with placebo.
On a composite alcohol consumption index comprised of time to first heavy drinking day after release, mean number of drinks per drinking day, change from before to after incarceration in average number of drinks per drinking day, alcohol craving score, and total number of drinking days, subjects who received four or more extended-release naltrexone injections had a significantly more favorable result, with a mean score of 3.15, compared with 2.93 in patients who took four or more placebo injections.
Moreover, consistent use of extended-release naltrexone was associated with significantly lower HIV viral load counts, compared with placebo-treated controls.
Treatment with extended-release naltrexone was safe. No serious side effects occurred, even in patients with comorbid hepatitis C who were on antiretroviral therapy. The most common side effects were the same as in seen in studies of the drug in other populations: mild to moderate nausea, headache, decreased appetite, fatigue, and dizziness.
Elsewhere at AIDS 2016, Chris Beyrer, MD, president of the International AIDS Society, included prisoners on his list of the populations most vulnerable to HIV because of discriminatory laws and policies in many parts of the world. Others on the list were transgender people, sex workers, men who have sex with men, and injection drug users.
“We’ll never be able to end AIDS without addressing the needs of these most vulnerable individuals and communities, and yet we know in 2016 far too many are being left behind,” said Dr. Beyrer, professor of epidemiology at Johns Hopkins University, Baltimore.
Transgender individuals, for example, are 49 times more likely to have HIV infection than other adults. Injection drug users and men who have sex with men are each 24-fold more likely to become HIV infected than the general population. Sex workers are 10 times more likely to acquire HIV infection than others in their reproductive years. And prisoners have a fivefold greater prevalence of HIV.
“In 2014 these vulnerable groups accounted for more than one-third of all new HIV infections. That’s an extraordinary proportion of HIV,” he observed. “This truly is the undone work of the HIV response. If there’s any silver lining in this cloud, it’s this: We’re talking about a relatively small number of people who are at high risk of infection relative to the world’s population. And that means that turning this around doesn’t require massive new commitments to very large populations. What it does require is an honest acknowledgment of where the epidemic is hitting hardest and directing resources to that need.”
Unfortunately, screening and treatment programs are rarely tailored to reach these highly vulnerable groups effectively, he added.
Dr. Beyrer was a contributor to a special issue of the Lancet devoted to HIV infection among prisoners published with the AIDS 2016 conference.
Dr. Springer’s study was funded by the National Institute on Alcohol Abuse and Alcoholism, and the National Institute on Drug Abuse. She reported having no financial conflicts of interest.
AT AIDS 2016
Key clinical point: Extended-release naltrexone helps HIV-infected prisoners with alcohol use disorder in transitioning to the community.
Major finding: The mean time to the first heavy drinking day among 21- to 29-year-old HIV-infected prisoners with an alcohol use disorder was 24.1 days following release from prison or jail in those on extended-release naltrexone versus 9.5 days with placebo.
Data source: This randomized, double-blind clinical trial included 100 HIV-positive prisoners with alcohol use disorder who were released into the community. Two-thirds received six monthly injections of extended-release naltrexone, the rest placebo.
Disclosures: The study was funded by the National Institute on Alcohol Abuse and Alcoholism and the National Institute on Drug Abuse. The presenter reported having no financial conflicts of interest.
Psychiatric disorders often impair antiretroviral adherence in perinatally HIV-infected teens
DURBAN, SOUTH AFRICA – Adolescents who were perinatally infected with HIV have a high prevalence of selected psychiatric disorders that impede their adherence to antiretroviral therapy, Claude Ann Mellins, PhD, reported at the 21st International AIDS Conference.
Those psychiatric diagnoses were predictive of viremia over the ensuing 2-3 years in a new analysis from the ongoing Child and Adolescent Self-Awareness and Health (CASAH) study, according to Dr. Mellins, professor of medical psychology at Columbia University, New York, and codirector of CASAH.
The clinical implications of the CASAH findings are clear, she added. “Assessing and treating specific categories of psychiatric and substance abuse problems may enhance efforts to improve adherence and prevent poor health outcomes in these adolescents and young adults, who are especially vulnerable due to their very challenging circumstances,” Dr. Mellins said.
CASAH is a longitudinal study of perinatally HIV-infected and perinatally HIV-exposed but uninfected New York City youth. They were enrolled during 2003-2008, when they were 9-16 years old. They and their caregivers undergo detailed psychosocial interviews every 12-18 months. The goal is to identify risk factors as well as protective factors influencing their behavioral health outcomes, the clinical psychologist explained.
She reported on 179 perinatally infected adolescents who were at least 13 years old at the first of their three interviews conducted over a 2.7-year period. Of note, 53% of them met Diagnostic Interview Schedule for Children (DISC-IV) criteria for one or more psychiatric diagnoses at all three time points. The pattern of psychopathology was somewhat different from that previously described in adults with HIV, who have been studied much more extensively than perinatally infected teens.
“Much of the literature on adults has focused on depression and mood disorders as predictors of poor health outcomes. Our data suggest that among youth, disruptive behavioral disorders – things like [attention-deficit/hyperactivity disorder], conduct disorder, or oppositional defiant disorder – may be just as important, if not more so. Substance abuse was also a critical factor,” Dr. Mellins said.
In a cross-sectional multivariate logistic regression analysis, a behavior disorder diagnosed at the first interview was associated with a 2.57-fold increased likelihood of contemporaneous viremia as evidenced by a plasma HIV RNA viral load greater than 1,000 copies/mL, and with a threefold increased likelihood of self-reported missed doses of antiretroviral medications during the previous week.
Anxiety disorder was the most common psychiatric diagnosis at the initial interview, followed by disruptive behavior disorder and substance use disorder. A diagnosis of any psychiatric disorder at the time of the first interview was associated with a significantly increased risk of viremia across the next 2.7 years. Forty-seven percent of subjects had viremia at 2.7 years of follow-up, reflective of chronic suboptimal medication adherence.
She noted that the pattern of psychiatric disorders in perinatally infected patients shifts between adolescence and young adulthood.
“By the time perinatally infected adolescents become young adults, I will say that anxiety and mood disorders become much more prevalent. But the number of psychiatric problems actually goes down by young adulthood,” according to Dr. Mellins.
Indeed, in another CASAH analysis she presented at AIDS 2016, this one involving 136 perinatally infected young adults and 86 perinatally exposed but uninfected controls, the vast majority living in impoverished communities, there was no difference between the two groups in rates of psychiatric or substance use disorders, although the 27% prevalence of substance use disorders is higher than that found in the age-matched general population.
Eighty-four percent of the perinatally infected 18- to 28-year-olds had graduated from high school, 94% were in a stable housing situation, 59% were currently working or in school, 54% were paying rent, and 95% reported ever being in a romantic relationship. Rates were similar in the perinatally exposed but uninfected group with the exception that these individuals were less likely to be paying rent.
“In spite of substantive risks, there is a relatively large portion of both groups with positive behavioral health outcomes, achieving normative young adult transition milestones. We need to understand why. Identification of protective factors conferring resilience can inform evidence-based prevention efforts, which are critical given the staggering numbers of children and young adolescents worldwide affected by HIV who will be transitioning to adulthood,” she said.
Dr. Mellins said the CASAH findings constitute a persuasive argument in favor of integrating mental health as a component of HIV care.
“Young people don’t always go to mental health appointments that are separate from medical care, so integrating mental health as a component of HIV care might be one of the most effective ways to identify and treat mental health problems in infected youth while simultaneously improving medication adherence and health outcomes,” Dr. Mellins said.
The ongoing CASAH study is funded by the National Institute of Mental Health. Dr. Mellins reported having no relevant financial conflicts.
DURBAN, SOUTH AFRICA – Adolescents who were perinatally infected with HIV have a high prevalence of selected psychiatric disorders that impede their adherence to antiretroviral therapy, Claude Ann Mellins, PhD, reported at the 21st International AIDS Conference.
Those psychiatric diagnoses were predictive of viremia over the ensuing 2-3 years in a new analysis from the ongoing Child and Adolescent Self-Awareness and Health (CASAH) study, according to Dr. Mellins, professor of medical psychology at Columbia University, New York, and codirector of CASAH.
The clinical implications of the CASAH findings are clear, she added. “Assessing and treating specific categories of psychiatric and substance abuse problems may enhance efforts to improve adherence and prevent poor health outcomes in these adolescents and young adults, who are especially vulnerable due to their very challenging circumstances,” Dr. Mellins said.
CASAH is a longitudinal study of perinatally HIV-infected and perinatally HIV-exposed but uninfected New York City youth. They were enrolled during 2003-2008, when they were 9-16 years old. They and their caregivers undergo detailed psychosocial interviews every 12-18 months. The goal is to identify risk factors as well as protective factors influencing their behavioral health outcomes, the clinical psychologist explained.
She reported on 179 perinatally infected adolescents who were at least 13 years old at the first of their three interviews conducted over a 2.7-year period. Of note, 53% of them met Diagnostic Interview Schedule for Children (DISC-IV) criteria for one or more psychiatric diagnoses at all three time points. The pattern of psychopathology was somewhat different from that previously described in adults with HIV, who have been studied much more extensively than perinatally infected teens.
“Much of the literature on adults has focused on depression and mood disorders as predictors of poor health outcomes. Our data suggest that among youth, disruptive behavioral disorders – things like [attention-deficit/hyperactivity disorder], conduct disorder, or oppositional defiant disorder – may be just as important, if not more so. Substance abuse was also a critical factor,” Dr. Mellins said.
In a cross-sectional multivariate logistic regression analysis, a behavior disorder diagnosed at the first interview was associated with a 2.57-fold increased likelihood of contemporaneous viremia as evidenced by a plasma HIV RNA viral load greater than 1,000 copies/mL, and with a threefold increased likelihood of self-reported missed doses of antiretroviral medications during the previous week.
Anxiety disorder was the most common psychiatric diagnosis at the initial interview, followed by disruptive behavior disorder and substance use disorder. A diagnosis of any psychiatric disorder at the time of the first interview was associated with a significantly increased risk of viremia across the next 2.7 years. Forty-seven percent of subjects had viremia at 2.7 years of follow-up, reflective of chronic suboptimal medication adherence.
She noted that the pattern of psychiatric disorders in perinatally infected patients shifts between adolescence and young adulthood.
“By the time perinatally infected adolescents become young adults, I will say that anxiety and mood disorders become much more prevalent. But the number of psychiatric problems actually goes down by young adulthood,” according to Dr. Mellins.
Indeed, in another CASAH analysis she presented at AIDS 2016, this one involving 136 perinatally infected young adults and 86 perinatally exposed but uninfected controls, the vast majority living in impoverished communities, there was no difference between the two groups in rates of psychiatric or substance use disorders, although the 27% prevalence of substance use disorders is higher than that found in the age-matched general population.
Eighty-four percent of the perinatally infected 18- to 28-year-olds had graduated from high school, 94% were in a stable housing situation, 59% were currently working or in school, 54% were paying rent, and 95% reported ever being in a romantic relationship. Rates were similar in the perinatally exposed but uninfected group with the exception that these individuals were less likely to be paying rent.
“In spite of substantive risks, there is a relatively large portion of both groups with positive behavioral health outcomes, achieving normative young adult transition milestones. We need to understand why. Identification of protective factors conferring resilience can inform evidence-based prevention efforts, which are critical given the staggering numbers of children and young adolescents worldwide affected by HIV who will be transitioning to adulthood,” she said.
Dr. Mellins said the CASAH findings constitute a persuasive argument in favor of integrating mental health as a component of HIV care.
“Young people don’t always go to mental health appointments that are separate from medical care, so integrating mental health as a component of HIV care might be one of the most effective ways to identify and treat mental health problems in infected youth while simultaneously improving medication adherence and health outcomes,” Dr. Mellins said.
The ongoing CASAH study is funded by the National Institute of Mental Health. Dr. Mellins reported having no relevant financial conflicts.
DURBAN, SOUTH AFRICA – Adolescents who were perinatally infected with HIV have a high prevalence of selected psychiatric disorders that impede their adherence to antiretroviral therapy, Claude Ann Mellins, PhD, reported at the 21st International AIDS Conference.
Those psychiatric diagnoses were predictive of viremia over the ensuing 2-3 years in a new analysis from the ongoing Child and Adolescent Self-Awareness and Health (CASAH) study, according to Dr. Mellins, professor of medical psychology at Columbia University, New York, and codirector of CASAH.
The clinical implications of the CASAH findings are clear, she added. “Assessing and treating specific categories of psychiatric and substance abuse problems may enhance efforts to improve adherence and prevent poor health outcomes in these adolescents and young adults, who are especially vulnerable due to their very challenging circumstances,” Dr. Mellins said.
CASAH is a longitudinal study of perinatally HIV-infected and perinatally HIV-exposed but uninfected New York City youth. They were enrolled during 2003-2008, when they were 9-16 years old. They and their caregivers undergo detailed psychosocial interviews every 12-18 months. The goal is to identify risk factors as well as protective factors influencing their behavioral health outcomes, the clinical psychologist explained.
She reported on 179 perinatally infected adolescents who were at least 13 years old at the first of their three interviews conducted over a 2.7-year period. Of note, 53% of them met Diagnostic Interview Schedule for Children (DISC-IV) criteria for one or more psychiatric diagnoses at all three time points. The pattern of psychopathology was somewhat different from that previously described in adults with HIV, who have been studied much more extensively than perinatally infected teens.
“Much of the literature on adults has focused on depression and mood disorders as predictors of poor health outcomes. Our data suggest that among youth, disruptive behavioral disorders – things like [attention-deficit/hyperactivity disorder], conduct disorder, or oppositional defiant disorder – may be just as important, if not more so. Substance abuse was also a critical factor,” Dr. Mellins said.
In a cross-sectional multivariate logistic regression analysis, a behavior disorder diagnosed at the first interview was associated with a 2.57-fold increased likelihood of contemporaneous viremia as evidenced by a plasma HIV RNA viral load greater than 1,000 copies/mL, and with a threefold increased likelihood of self-reported missed doses of antiretroviral medications during the previous week.
Anxiety disorder was the most common psychiatric diagnosis at the initial interview, followed by disruptive behavior disorder and substance use disorder. A diagnosis of any psychiatric disorder at the time of the first interview was associated with a significantly increased risk of viremia across the next 2.7 years. Forty-seven percent of subjects had viremia at 2.7 years of follow-up, reflective of chronic suboptimal medication adherence.
She noted that the pattern of psychiatric disorders in perinatally infected patients shifts between adolescence and young adulthood.
“By the time perinatally infected adolescents become young adults, I will say that anxiety and mood disorders become much more prevalent. But the number of psychiatric problems actually goes down by young adulthood,” according to Dr. Mellins.
Indeed, in another CASAH analysis she presented at AIDS 2016, this one involving 136 perinatally infected young adults and 86 perinatally exposed but uninfected controls, the vast majority living in impoverished communities, there was no difference between the two groups in rates of psychiatric or substance use disorders, although the 27% prevalence of substance use disorders is higher than that found in the age-matched general population.
Eighty-four percent of the perinatally infected 18- to 28-year-olds had graduated from high school, 94% were in a stable housing situation, 59% were currently working or in school, 54% were paying rent, and 95% reported ever being in a romantic relationship. Rates were similar in the perinatally exposed but uninfected group with the exception that these individuals were less likely to be paying rent.
“In spite of substantive risks, there is a relatively large portion of both groups with positive behavioral health outcomes, achieving normative young adult transition milestones. We need to understand why. Identification of protective factors conferring resilience can inform evidence-based prevention efforts, which are critical given the staggering numbers of children and young adolescents worldwide affected by HIV who will be transitioning to adulthood,” she said.
Dr. Mellins said the CASAH findings constitute a persuasive argument in favor of integrating mental health as a component of HIV care.
“Young people don’t always go to mental health appointments that are separate from medical care, so integrating mental health as a component of HIV care might be one of the most effective ways to identify and treat mental health problems in infected youth while simultaneously improving medication adherence and health outcomes,” Dr. Mellins said.
The ongoing CASAH study is funded by the National Institute of Mental Health. Dr. Mellins reported having no relevant financial conflicts.
AT AIDS 2016
Key clinical point: Look for and treat psychiatric disorders in perinatally HIV-infected adolescents as a means of optimizing their antiretroviral medication adherence.
Major finding: A majority of perinatally HIV-infected adolescents meet the criteria for at least one psychiatric diagnosis, and they are at significantly increased risk for poor medication adherence and viremia during the next 2-3 years.
Data source: The longitudinal CASAH study involving prospective follow-up of several hundred perinatally HIV-infected and perinatally exposed but uninfected subjects through adolescence and young adulthood.
Disclosures: The ongoing CASAH study is funded by the National Institute of Mental Health. Dr. Mellins reported having no relevant financial conflicts.
Who benefits most from immediate HIV therapy?
DURBAN, SOUTH AFRICA – Immediate initiation of antiretroviral therapy in asymptomatic treatment-naive HIV-infected adults with a CD4+ cell count greater than 500/mL brings considerably more bang for the buck in selected patient subgroups, according to a secondary analysis from the landmark START trial.
Four subgroups in START stood out as having larger absolute risk reductions and lower numbers-needed-to-treat with a strategy of immediate treatment: patients above age 50, those with a baseline Framingham Risk Score in excess of 10%, individuals whose plasma HIV RNA level exceeds 50,000 copies/mL, and patients with a CD4:CD8 ratio below 0.5, Dr. Jean-Michel Molina reported at the 21st International AIDS Conference.
“These patients might be prioritized for immediate access to ART,” observed Dr. Molina, professor of infectious diseases at the University of Paris-Diderot and head of the infectious diseases department at Saint-Louis Hospital, also in Paris.
The START (Strategic Timing of AntiRetroviral Treatment) study was a major clinical trial conducted in 35 countries. Investigators randomized 4,685 treatment-naive HIV-infected men and women with CD4+ cell counts in the normal range to immediate antiretroviral therapy or to deferral of treatment until their CD4+ cell count dropped to 350 cells/mL. After 3 years of prospective follow-up, the immediate-treatment strategy was associated with a 47% reduction in risk for the primary endpoint, a composite of AIDS, major cardiovascular or other non-AIDS events, and death. The number needed to treat immediately for 1 year in order to prevent one major event was 128 (N Engl J Med. 2015;373:795-807).
The START findings prompted a revision in World Health Organization guidelines, which now recommend universal antiretroviral treatment (ART) in patients with HIV infection regardless of their CD4+ cell count.
But some patients are reluctant to go on lifetime ART, particularly since they still feel normal while in the initial phases of HIV infection. In such cases, these new subgroup data may tip the balance in decision-making. Moreover, the new START findings should help physicians and policy makers in prioritizing access to immediate ART in settings where it isn’t universally available, according to Dr. Molina.
In the prespecified subgroup analysis, patients aged 50 and up at enrollment had a 2.9% incidence of the primary composite endpoint at 3 years if randomized to immediate ART and an 11.7% rate if they were assigned to deferred ART. The number of 50-plus-year-olds needed to treat (NNT) immediately for 1 year in order to prevent one additional case of AIDS, a major non-AIDS event, or death was just 45, compared to NNTs of 151 and 206 in patients aged 30-49 and younger than 30, respectively. Patients aged 50 and older accounted for nearly 12% of the overall study population.
For the roughly 28% of START participants whose baseline CD4:CD8 ratio was less than 0.5, the NNT for immediate rather than deferred therapy was 60, substantially more favorable than the NNTs of 214 in patients with a baseline ratio of 0.5-0.8 and 248 in patients with a CD4:CD8 ratio greater than 0.8. The incidence of the primary endpoint at 3 years of follow-up in patients with a CD4:CD8 ratio of less than 0.5 was 0.5% in the immediate ART group and 6.3% with deferred therapy.
Similarly, patients with a baseline 10-year Framingham Risk Score (FRS) of 10% or higher had an NNT of 69, compared with NNTs of 111 in subjects with an FRS of 1%-9.9% and 276 in those with an FRS of less than 1%. Patients with an FRS of 10% or more had a 2.4% incidence of the primary endpoint at 3 years if assigned to immediate ART and a 10.1% rate with deferred therapy. Patients with an FRS of 10% or more comprised only 9.6% of the study population, Dr. Molina continued.
Patients with a heavy baseline viral load as evidenced by a plasma HIV RNA level of at least 50,000 copies/mL accounted for roughly 22% of the total study sample. Their 3-year rate of the primary outcome was 2.1% with immediate ART and 6.9% with deferred treatment. The NNT was 67, compared to an NNT of 122 in patients with 3,000-49,999 copies/mL and 992 in the one-quarter of START participants with a baseline plasma HIV RNA level of less than 3,000 copies/mL.
Variables that weren’t related to the magnitude of absolute risk reduction and NNT in the START subgroup analysis were race, gender, geographic region, baseline CD4 cell count, and whether an individual resided in a high- or lower-income country.
Several audience members rose to assert that the CD4:CD8 ratio and viral load might very well be redundant predictors measuring the same thing, since they are typically tightly correlated. Dr. Molina replied that the START investigators are planning to conduct a multivariate analysis of the data in the near future, which should provide a definitive answer.
Another audience member expressed surprise at what struck him as a low cardiovascular event rate in the START study, given that HIV infection is known to be associated with accelerated atherosclerosis. Dr. Molina said the explanation for the low number of cardiovascular events lies in the fact that cardiovascular risk is so heavily age-dependent, and START participants were relatively young, with a median age of 36 years.
The START trial was carried out by the International Network for Strategic Initiatives in Global HIV Trials (INSIGHT) with funding provided mainly by the National Institutes of Health. Dr. Molina reported having no financial conflicts of interest.
DURBAN, SOUTH AFRICA – Immediate initiation of antiretroviral therapy in asymptomatic treatment-naive HIV-infected adults with a CD4+ cell count greater than 500/mL brings considerably more bang for the buck in selected patient subgroups, according to a secondary analysis from the landmark START trial.
Four subgroups in START stood out as having larger absolute risk reductions and lower numbers-needed-to-treat with a strategy of immediate treatment: patients above age 50, those with a baseline Framingham Risk Score in excess of 10%, individuals whose plasma HIV RNA level exceeds 50,000 copies/mL, and patients with a CD4:CD8 ratio below 0.5, Dr. Jean-Michel Molina reported at the 21st International AIDS Conference.
“These patients might be prioritized for immediate access to ART,” observed Dr. Molina, professor of infectious diseases at the University of Paris-Diderot and head of the infectious diseases department at Saint-Louis Hospital, also in Paris.
The START (Strategic Timing of AntiRetroviral Treatment) study was a major clinical trial conducted in 35 countries. Investigators randomized 4,685 treatment-naive HIV-infected men and women with CD4+ cell counts in the normal range to immediate antiretroviral therapy or to deferral of treatment until their CD4+ cell count dropped to 350 cells/mL. After 3 years of prospective follow-up, the immediate-treatment strategy was associated with a 47% reduction in risk for the primary endpoint, a composite of AIDS, major cardiovascular or other non-AIDS events, and death. The number needed to treat immediately for 1 year in order to prevent one major event was 128 (N Engl J Med. 2015;373:795-807).
The START findings prompted a revision in World Health Organization guidelines, which now recommend universal antiretroviral treatment (ART) in patients with HIV infection regardless of their CD4+ cell count.
But some patients are reluctant to go on lifetime ART, particularly since they still feel normal while in the initial phases of HIV infection. In such cases, these new subgroup data may tip the balance in decision-making. Moreover, the new START findings should help physicians and policy makers in prioritizing access to immediate ART in settings where it isn’t universally available, according to Dr. Molina.
In the prespecified subgroup analysis, patients aged 50 and up at enrollment had a 2.9% incidence of the primary composite endpoint at 3 years if randomized to immediate ART and an 11.7% rate if they were assigned to deferred ART. The number of 50-plus-year-olds needed to treat (NNT) immediately for 1 year in order to prevent one additional case of AIDS, a major non-AIDS event, or death was just 45, compared to NNTs of 151 and 206 in patients aged 30-49 and younger than 30, respectively. Patients aged 50 and older accounted for nearly 12% of the overall study population.
For the roughly 28% of START participants whose baseline CD4:CD8 ratio was less than 0.5, the NNT for immediate rather than deferred therapy was 60, substantially more favorable than the NNTs of 214 in patients with a baseline ratio of 0.5-0.8 and 248 in patients with a CD4:CD8 ratio greater than 0.8. The incidence of the primary endpoint at 3 years of follow-up in patients with a CD4:CD8 ratio of less than 0.5 was 0.5% in the immediate ART group and 6.3% with deferred therapy.
Similarly, patients with a baseline 10-year Framingham Risk Score (FRS) of 10% or higher had an NNT of 69, compared with NNTs of 111 in subjects with an FRS of 1%-9.9% and 276 in those with an FRS of less than 1%. Patients with an FRS of 10% or more had a 2.4% incidence of the primary endpoint at 3 years if assigned to immediate ART and a 10.1% rate with deferred therapy. Patients with an FRS of 10% or more comprised only 9.6% of the study population, Dr. Molina continued.
Patients with a heavy baseline viral load as evidenced by a plasma HIV RNA level of at least 50,000 copies/mL accounted for roughly 22% of the total study sample. Their 3-year rate of the primary outcome was 2.1% with immediate ART and 6.9% with deferred treatment. The NNT was 67, compared to an NNT of 122 in patients with 3,000-49,999 copies/mL and 992 in the one-quarter of START participants with a baseline plasma HIV RNA level of less than 3,000 copies/mL.
Variables that weren’t related to the magnitude of absolute risk reduction and NNT in the START subgroup analysis were race, gender, geographic region, baseline CD4 cell count, and whether an individual resided in a high- or lower-income country.
Several audience members rose to assert that the CD4:CD8 ratio and viral load might very well be redundant predictors measuring the same thing, since they are typically tightly correlated. Dr. Molina replied that the START investigators are planning to conduct a multivariate analysis of the data in the near future, which should provide a definitive answer.
Another audience member expressed surprise at what struck him as a low cardiovascular event rate in the START study, given that HIV infection is known to be associated with accelerated atherosclerosis. Dr. Molina said the explanation for the low number of cardiovascular events lies in the fact that cardiovascular risk is so heavily age-dependent, and START participants were relatively young, with a median age of 36 years.
The START trial was carried out by the International Network for Strategic Initiatives in Global HIV Trials (INSIGHT) with funding provided mainly by the National Institutes of Health. Dr. Molina reported having no financial conflicts of interest.
DURBAN, SOUTH AFRICA – Immediate initiation of antiretroviral therapy in asymptomatic treatment-naive HIV-infected adults with a CD4+ cell count greater than 500/mL brings considerably more bang for the buck in selected patient subgroups, according to a secondary analysis from the landmark START trial.
Four subgroups in START stood out as having larger absolute risk reductions and lower numbers-needed-to-treat with a strategy of immediate treatment: patients above age 50, those with a baseline Framingham Risk Score in excess of 10%, individuals whose plasma HIV RNA level exceeds 50,000 copies/mL, and patients with a CD4:CD8 ratio below 0.5, Dr. Jean-Michel Molina reported at the 21st International AIDS Conference.
“These patients might be prioritized for immediate access to ART,” observed Dr. Molina, professor of infectious diseases at the University of Paris-Diderot and head of the infectious diseases department at Saint-Louis Hospital, also in Paris.
The START (Strategic Timing of AntiRetroviral Treatment) study was a major clinical trial conducted in 35 countries. Investigators randomized 4,685 treatment-naive HIV-infected men and women with CD4+ cell counts in the normal range to immediate antiretroviral therapy or to deferral of treatment until their CD4+ cell count dropped to 350 cells/mL. After 3 years of prospective follow-up, the immediate-treatment strategy was associated with a 47% reduction in risk for the primary endpoint, a composite of AIDS, major cardiovascular or other non-AIDS events, and death. The number needed to treat immediately for 1 year in order to prevent one major event was 128 (N Engl J Med. 2015;373:795-807).
The START findings prompted a revision in World Health Organization guidelines, which now recommend universal antiretroviral treatment (ART) in patients with HIV infection regardless of their CD4+ cell count.
But some patients are reluctant to go on lifetime ART, particularly since they still feel normal while in the initial phases of HIV infection. In such cases, these new subgroup data may tip the balance in decision-making. Moreover, the new START findings should help physicians and policy makers in prioritizing access to immediate ART in settings where it isn’t universally available, according to Dr. Molina.
In the prespecified subgroup analysis, patients aged 50 and up at enrollment had a 2.9% incidence of the primary composite endpoint at 3 years if randomized to immediate ART and an 11.7% rate if they were assigned to deferred ART. The number of 50-plus-year-olds needed to treat (NNT) immediately for 1 year in order to prevent one additional case of AIDS, a major non-AIDS event, or death was just 45, compared to NNTs of 151 and 206 in patients aged 30-49 and younger than 30, respectively. Patients aged 50 and older accounted for nearly 12% of the overall study population.
For the roughly 28% of START participants whose baseline CD4:CD8 ratio was less than 0.5, the NNT for immediate rather than deferred therapy was 60, substantially more favorable than the NNTs of 214 in patients with a baseline ratio of 0.5-0.8 and 248 in patients with a CD4:CD8 ratio greater than 0.8. The incidence of the primary endpoint at 3 years of follow-up in patients with a CD4:CD8 ratio of less than 0.5 was 0.5% in the immediate ART group and 6.3% with deferred therapy.
Similarly, patients with a baseline 10-year Framingham Risk Score (FRS) of 10% or higher had an NNT of 69, compared with NNTs of 111 in subjects with an FRS of 1%-9.9% and 276 in those with an FRS of less than 1%. Patients with an FRS of 10% or more had a 2.4% incidence of the primary endpoint at 3 years if assigned to immediate ART and a 10.1% rate with deferred therapy. Patients with an FRS of 10% or more comprised only 9.6% of the study population, Dr. Molina continued.
Patients with a heavy baseline viral load as evidenced by a plasma HIV RNA level of at least 50,000 copies/mL accounted for roughly 22% of the total study sample. Their 3-year rate of the primary outcome was 2.1% with immediate ART and 6.9% with deferred treatment. The NNT was 67, compared to an NNT of 122 in patients with 3,000-49,999 copies/mL and 992 in the one-quarter of START participants with a baseline plasma HIV RNA level of less than 3,000 copies/mL.
Variables that weren’t related to the magnitude of absolute risk reduction and NNT in the START subgroup analysis were race, gender, geographic region, baseline CD4 cell count, and whether an individual resided in a high- or lower-income country.
Several audience members rose to assert that the CD4:CD8 ratio and viral load might very well be redundant predictors measuring the same thing, since they are typically tightly correlated. Dr. Molina replied that the START investigators are planning to conduct a multivariate analysis of the data in the near future, which should provide a definitive answer.
Another audience member expressed surprise at what struck him as a low cardiovascular event rate in the START study, given that HIV infection is known to be associated with accelerated atherosclerosis. Dr. Molina said the explanation for the low number of cardiovascular events lies in the fact that cardiovascular risk is so heavily age-dependent, and START participants were relatively young, with a median age of 36 years.
The START trial was carried out by the International Network for Strategic Initiatives in Global HIV Trials (INSIGHT) with funding provided mainly by the National Institutes of Health. Dr. Molina reported having no financial conflicts of interest.
AT AIDS 2016
Key clinical point: Four specific subgroups of asymptomatic HIV-infected adults who obtain the most clinical benefit from immediate rather than deferred antiretroviral therapy have been identified.
Major finding: The number of asymptomatic HIV-infected adults needed to treat immediately with antiretroviral therapy for 1 year instead of deferring treatment in order to avoid one case of AIDS or serious non-AIDS illness is 45 in patients aged 50 and older, compared with NNTs of 151 in 30- to 49-year-olds and 206 in patients younger than age 30.
Data source: This was a prespecified subgroup analysis of the landmark START trial, in which 4,685 treatment-naive asymptomatic HIV-infected adults with more than 500 CD4+ cells/mL were randomized to immediate or deferred antiretroviral therapy.
Disclosures: The START trial was funded chiefly by the National Institutes of Health. The presenter reported having no financial conflicts of interest.
Gay and Bisexual Male High Schoolers Have High Injected Drug Use
DURBAN, SOUTH AFRICA – The first-ever nationally representative survey on sexual identity and behaviors in U.S. male high school students has exposed a high prevalence of illicit injected drug use among those who have sex with males or with both males and females.
About 10% of high school boys who identify as gay or bisexual reported having ever used a needle to inject any illegal drug into their bodies, compared with 1.5% of high school boys who identify as heterosexual.
“This is a cause for great concern because of the efficiency with which injected drug use transmits not only HIV but hepatitis and other diseases,” Laura Kann, PhD, said in reporting the results of the 2015 National Youth Risk Behavior Survey (YRBS) at the 21st International AIDS Conference.
Dr. Kann of the Centers for Disease Control and Prevention’s division of adolescent and school health directs the YRBS, an annual, multiple-choice, anonymous voluntary survey that addresses six areas of risk: behaviors that contribute to unintentional injuries and violence, tobacco use, alcohol and other drug use, sexual behaviors, inadequate physical activity, and unhealthy diet behaviors.
The 2015 survey was the first ever to include questions regarding sexual identity and behaviors.
“Twenty-two percent of all new HIV diagnoses in the U.S. occur in 13- to 24-year-olds. Most occur in males who have sex with males. This makes young males an important focus for HIV prevention efforts,” Dr. Kann said.
Yet until the 2015 YRBS, no reliable estimates existed regarding the number of gay or bisexual male high school students. “Reducing HIV infection among young sexual minority males is key to reducing HIV infection in the U.S.,” she said. “It’s hard to respond appropriately to a population that has not been counted.”
The 15,624 male students in public and private school grades 9-12 who participated in the YRBS were demographically representative of the nation’s roughly 8 million male high schoolers.
About 2% of male students identified themselves as gay, another 2.4% declared themselves bisexual, 93.1% said they were heterosexual, and 2.6% were unsure. With regard to sexual behaviors, 53.3% of respondents indicated they had sexual contact with females only, 1.3% with males only, and 1.9% with both males and females; 43.6% reported they hadn’t ever had sexual contact. Respondents could interpret “sexual contact” as they wished, ranging from kissing and hugging to intercourse.
Large differences in illicit drug use behaviors were found between the sexual minorities – that is, males who had sexual contact with males or with females as well as males – and those with only heterosexual contacts.
“We need to conduct new research on injected drug use among young males who have sex with males and determine what can be done to minimize, if not eliminate, this very high-risk behavior that is occurring at alarming rates,” Dr. Kann said.
The sexual minority students were also more likely to have engaged in various forms of noninjected illicit drug use known to boost the risk of HIV infection indirectly by increasing the likelihood of having unsafe sex. The sexual minority males had significantly higher rates of having ever used cocaine in any form, of having ever used amphetamines, and of having taken prescription drugs without a physician’s prescription. For instance, 14.8% of sexual minority males reported ever using amphetamines, compared with 2.5% of heterosexual males surveyed.
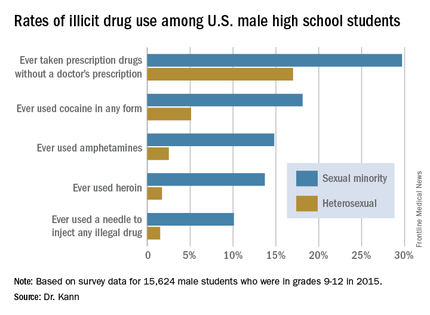
She noted that the YRBS results provided no evidence that the disparity in HIV diagnoses between young males who have sex with males and young males who have sex with females are caused by differences in the prevalence of HIV-related sexual behaviors. There were no statistically significant differences between heterosexual and sexual minority students in rates of ever having had sexual intercourse, having had intercourse with four or more persons, having sexual activity currently, or having used a condom at last intercourse.
Dr. Kann called for more research in the neglected area of social issues affecting young sexual minority males that likely increase their sense of marginalization and may promote behaviors placing them at increased risk for HIV acquisition.
“Many young males who have sex with males suffer from social isolation, stress from self-concealment and from coming out, discrimination, and even hatred, which may occur at home, at school, and in communities among both their peers and the adults responsible for their care and protection,” she said. “These factors may lead to harmful coping behaviors, such as drug use, that can further increase risk for HIV infection. It’s hard to imagine how even the best intervention technology will be able to eliminate the disparities in HIV diagnosis unless these social issues are addressed first and foremost.”
One clinician from New York rose from the audience to chastise the CDC for taking decades to incorporate questions about sexual minority youth into the YRBS.
“It’s important for the rest of the world to know how long it took the U.S. to collect this data. It’s not right. For those of you in the rest of the world who struggle to get information about your youth, don’t think that America is way ahead of you because it took us a long time,” she said.
Dr. Kann noted that such data have been collected for some time by some state and city public health agencies. As local officials began clamoring for a more comprehensive national picture, “it reached a tipping point” at the CDC.
Dr. Kann reported having no relevant financial conflicts.
DURBAN, SOUTH AFRICA – The first-ever nationally representative survey on sexual identity and behaviors in U.S. male high school students has exposed a high prevalence of illicit injected drug use among those who have sex with males or with both males and females.
About 10% of high school boys who identify as gay or bisexual reported having ever used a needle to inject any illegal drug into their bodies, compared with 1.5% of high school boys who identify as heterosexual.
“This is a cause for great concern because of the efficiency with which injected drug use transmits not only HIV but hepatitis and other diseases,” Laura Kann, PhD, said in reporting the results of the 2015 National Youth Risk Behavior Survey (YRBS) at the 21st International AIDS Conference.
Dr. Kann of the Centers for Disease Control and Prevention’s division of adolescent and school health directs the YRBS, an annual, multiple-choice, anonymous voluntary survey that addresses six areas of risk: behaviors that contribute to unintentional injuries and violence, tobacco use, alcohol and other drug use, sexual behaviors, inadequate physical activity, and unhealthy diet behaviors.
The 2015 survey was the first ever to include questions regarding sexual identity and behaviors.
“Twenty-two percent of all new HIV diagnoses in the U.S. occur in 13- to 24-year-olds. Most occur in males who have sex with males. This makes young males an important focus for HIV prevention efforts,” Dr. Kann said.
Yet until the 2015 YRBS, no reliable estimates existed regarding the number of gay or bisexual male high school students. “Reducing HIV infection among young sexual minority males is key to reducing HIV infection in the U.S.,” she said. “It’s hard to respond appropriately to a population that has not been counted.”
The 15,624 male students in public and private school grades 9-12 who participated in the YRBS were demographically representative of the nation’s roughly 8 million male high schoolers.
About 2% of male students identified themselves as gay, another 2.4% declared themselves bisexual, 93.1% said they were heterosexual, and 2.6% were unsure. With regard to sexual behaviors, 53.3% of respondents indicated they had sexual contact with females only, 1.3% with males only, and 1.9% with both males and females; 43.6% reported they hadn’t ever had sexual contact. Respondents could interpret “sexual contact” as they wished, ranging from kissing and hugging to intercourse.
Large differences in illicit drug use behaviors were found between the sexual minorities – that is, males who had sexual contact with males or with females as well as males – and those with only heterosexual contacts.
“We need to conduct new research on injected drug use among young males who have sex with males and determine what can be done to minimize, if not eliminate, this very high-risk behavior that is occurring at alarming rates,” Dr. Kann said.
The sexual minority students were also more likely to have engaged in various forms of noninjected illicit drug use known to boost the risk of HIV infection indirectly by increasing the likelihood of having unsafe sex. The sexual minority males had significantly higher rates of having ever used cocaine in any form, of having ever used amphetamines, and of having taken prescription drugs without a physician’s prescription. For instance, 14.8% of sexual minority males reported ever using amphetamines, compared with 2.5% of heterosexual males surveyed.

She noted that the YRBS results provided no evidence that the disparity in HIV diagnoses between young males who have sex with males and young males who have sex with females are caused by differences in the prevalence of HIV-related sexual behaviors. There were no statistically significant differences between heterosexual and sexual minority students in rates of ever having had sexual intercourse, having had intercourse with four or more persons, having sexual activity currently, or having used a condom at last intercourse.
Dr. Kann called for more research in the neglected area of social issues affecting young sexual minority males that likely increase their sense of marginalization and may promote behaviors placing them at increased risk for HIV acquisition.
“Many young males who have sex with males suffer from social isolation, stress from self-concealment and from coming out, discrimination, and even hatred, which may occur at home, at school, and in communities among both their peers and the adults responsible for their care and protection,” she said. “These factors may lead to harmful coping behaviors, such as drug use, that can further increase risk for HIV infection. It’s hard to imagine how even the best intervention technology will be able to eliminate the disparities in HIV diagnosis unless these social issues are addressed first and foremost.”
One clinician from New York rose from the audience to chastise the CDC for taking decades to incorporate questions about sexual minority youth into the YRBS.
“It’s important for the rest of the world to know how long it took the U.S. to collect this data. It’s not right. For those of you in the rest of the world who struggle to get information about your youth, don’t think that America is way ahead of you because it took us a long time,” she said.
Dr. Kann noted that such data have been collected for some time by some state and city public health agencies. As local officials began clamoring for a more comprehensive national picture, “it reached a tipping point” at the CDC.
Dr. Kann reported having no relevant financial conflicts.
DURBAN, SOUTH AFRICA – The first-ever nationally representative survey on sexual identity and behaviors in U.S. male high school students has exposed a high prevalence of illicit injected drug use among those who have sex with males or with both males and females.
About 10% of high school boys who identify as gay or bisexual reported having ever used a needle to inject any illegal drug into their bodies, compared with 1.5% of high school boys who identify as heterosexual.
“This is a cause for great concern because of the efficiency with which injected drug use transmits not only HIV but hepatitis and other diseases,” Laura Kann, PhD, said in reporting the results of the 2015 National Youth Risk Behavior Survey (YRBS) at the 21st International AIDS Conference.
Dr. Kann of the Centers for Disease Control and Prevention’s division of adolescent and school health directs the YRBS, an annual, multiple-choice, anonymous voluntary survey that addresses six areas of risk: behaviors that contribute to unintentional injuries and violence, tobacco use, alcohol and other drug use, sexual behaviors, inadequate physical activity, and unhealthy diet behaviors.
The 2015 survey was the first ever to include questions regarding sexual identity and behaviors.
“Twenty-two percent of all new HIV diagnoses in the U.S. occur in 13- to 24-year-olds. Most occur in males who have sex with males. This makes young males an important focus for HIV prevention efforts,” Dr. Kann said.
Yet until the 2015 YRBS, no reliable estimates existed regarding the number of gay or bisexual male high school students. “Reducing HIV infection among young sexual minority males is key to reducing HIV infection in the U.S.,” she said. “It’s hard to respond appropriately to a population that has not been counted.”
The 15,624 male students in public and private school grades 9-12 who participated in the YRBS were demographically representative of the nation’s roughly 8 million male high schoolers.
About 2% of male students identified themselves as gay, another 2.4% declared themselves bisexual, 93.1% said they were heterosexual, and 2.6% were unsure. With regard to sexual behaviors, 53.3% of respondents indicated they had sexual contact with females only, 1.3% with males only, and 1.9% with both males and females; 43.6% reported they hadn’t ever had sexual contact. Respondents could interpret “sexual contact” as they wished, ranging from kissing and hugging to intercourse.
Large differences in illicit drug use behaviors were found between the sexual minorities – that is, males who had sexual contact with males or with females as well as males – and those with only heterosexual contacts.
“We need to conduct new research on injected drug use among young males who have sex with males and determine what can be done to minimize, if not eliminate, this very high-risk behavior that is occurring at alarming rates,” Dr. Kann said.
The sexual minority students were also more likely to have engaged in various forms of noninjected illicit drug use known to boost the risk of HIV infection indirectly by increasing the likelihood of having unsafe sex. The sexual minority males had significantly higher rates of having ever used cocaine in any form, of having ever used amphetamines, and of having taken prescription drugs without a physician’s prescription. For instance, 14.8% of sexual minority males reported ever using amphetamines, compared with 2.5% of heterosexual males surveyed.

She noted that the YRBS results provided no evidence that the disparity in HIV diagnoses between young males who have sex with males and young males who have sex with females are caused by differences in the prevalence of HIV-related sexual behaviors. There were no statistically significant differences between heterosexual and sexual minority students in rates of ever having had sexual intercourse, having had intercourse with four or more persons, having sexual activity currently, or having used a condom at last intercourse.
Dr. Kann called for more research in the neglected area of social issues affecting young sexual minority males that likely increase their sense of marginalization and may promote behaviors placing them at increased risk for HIV acquisition.
“Many young males who have sex with males suffer from social isolation, stress from self-concealment and from coming out, discrimination, and even hatred, which may occur at home, at school, and in communities among both their peers and the adults responsible for their care and protection,” she said. “These factors may lead to harmful coping behaviors, such as drug use, that can further increase risk for HIV infection. It’s hard to imagine how even the best intervention technology will be able to eliminate the disparities in HIV diagnosis unless these social issues are addressed first and foremost.”
One clinician from New York rose from the audience to chastise the CDC for taking decades to incorporate questions about sexual minority youth into the YRBS.
“It’s important for the rest of the world to know how long it took the U.S. to collect this data. It’s not right. For those of you in the rest of the world who struggle to get information about your youth, don’t think that America is way ahead of you because it took us a long time,” she said.
Dr. Kann noted that such data have been collected for some time by some state and city public health agencies. As local officials began clamoring for a more comprehensive national picture, “it reached a tipping point” at the CDC.
Dr. Kann reported having no relevant financial conflicts.
AT AIDS 2016
Gay and bisexual male high schoolers have high injected drug use
DURBAN, SOUTH AFRICA – The first-ever nationally representative survey on sexual identity and behaviors in U.S. male high school students has exposed a high prevalence of illicit injected drug use among those who have sex with males or with both males and females.
About 10% of high school boys who identify as gay or bisexual reported having ever used a needle to inject any illegal drug into their bodies, compared with 1.5% of high school boys who identify as heterosexual.
“This is a cause for great concern because of the efficiency with which injected drug use transmits not only HIV but hepatitis and other diseases,” Laura Kann, PhD, said in reporting the results of the 2015 National Youth Risk Behavior Survey (YRBS) at the 21st International AIDS Conference.
Dr. Kann of the Centers for Disease Control and Prevention’s division of adolescent and school health directs the YRBS, an annual, multiple-choice, anonymous voluntary survey that addresses six areas of risk: behaviors that contribute to unintentional injuries and violence, tobacco use, alcohol and other drug use, sexual behaviors, inadequate physical activity, and unhealthy diet behaviors.
The 2015 survey was the first ever to include questions regarding sexual identity and behaviors.
“Twenty-two percent of all new HIV diagnoses in the U.S. occur in 13- to 24-year-olds. Most occur in males who have sex with males. This makes young males an important focus for HIV prevention efforts,” Dr. Kann said.
Yet until the 2015 YRBS, no reliable estimates existed regarding the number of gay or bisexual male high school students. “Reducing HIV infection among young sexual minority males is key to reducing HIV infection in the U.S.,” she said. “It’s hard to respond appropriately to a population that has not been counted.”
The 15,624 male students in public and private school grades 9-12 who participated in the YRBS were demographically representative of the nation’s roughly 8 million male high schoolers.
About 2% of male students identified themselves as gay, another 2.4% declared themselves bisexual, 93.1% said they were heterosexual, and 2.6% were unsure. With regard to sexual behaviors, 53.3% of respondents indicated they had sexual contact with females only, 1.3% with males only, and 1.9% with both males and females; 43.6% reported they hadn’t ever had sexual contact. Respondents could interpret “sexual contact” as they wished, ranging from kissing and hugging to intercourse.
Large differences in illicit drug use behaviors were found between the sexual minorities – that is, males who had sexual contact with males or with females as well as males – and those with only heterosexual contacts.
“We need to conduct new research on injected drug use among young males who have sex with males and determine what can be done to minimize, if not eliminate, this very high-risk behavior that is occurring at alarming rates,” Dr. Kann said.
The sexual minority students were also more likely to have engaged in various forms of noninjected illicit drug use known to boost the risk of HIV infection indirectly by increasing the likelihood of having unsafe sex. The sexual minority males had significantly higher rates of having ever used cocaine in any form, of having ever used amphetamines, and of having taken prescription drugs without a physician’s prescription. For instance, 14.8% of sexual minority males reported ever using amphetamines, compared with 2.5% of heterosexual males surveyed.
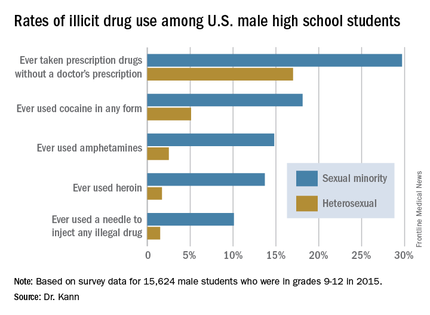
She noted that the YRBS results provided no evidence that the disparity in HIV diagnoses between young males who have sex with males and young males who have sex with females are caused by differences in the prevalence of HIV-related sexual behaviors. There were no statistically significant differences between heterosexual and sexual minority students in rates of ever having had sexual intercourse, having had intercourse with four or more persons, having sexual activity currently, or having used a condom at last intercourse.
Dr. Kann called for more research in the neglected area of social issues affecting young sexual minority males that likely increase their sense of marginalization and may promote behaviors placing them at increased risk for HIV acquisition.
“Many young males who have sex with males suffer from social isolation, stress from self-concealment and from coming out, discrimination, and even hatred, which may occur at home, at school, and in communities among both their peers and the adults responsible for their care and protection,” she said. “These factors may lead to harmful coping behaviors, such as drug use, that can further increase risk for HIV infection. It’s hard to imagine how even the best intervention technology will be able to eliminate the disparities in HIV diagnosis unless these social issues are addressed first and foremost.”
One clinician from New York rose from the audience to chastise the CDC for taking decades to incorporate questions about sexual minority youth into the YRBS.
“It’s important for the rest of the world to know how long it took the U.S. to collect this data. It’s not right. For those of you in the rest of the world who struggle to get information about your youth, don’t think that America is way ahead of you because it took us a long time,” she said.
Dr. Kann noted that such data have been collected for some time by some state and city public health agencies. As local officials began clamoring for a more comprehensive national picture, “it reached a tipping point” at the CDC.
Dr. Kann reported having no relevant financial conflicts.
DURBAN, SOUTH AFRICA – The first-ever nationally representative survey on sexual identity and behaviors in U.S. male high school students has exposed a high prevalence of illicit injected drug use among those who have sex with males or with both males and females.
About 10% of high school boys who identify as gay or bisexual reported having ever used a needle to inject any illegal drug into their bodies, compared with 1.5% of high school boys who identify as heterosexual.
“This is a cause for great concern because of the efficiency with which injected drug use transmits not only HIV but hepatitis and other diseases,” Laura Kann, PhD, said in reporting the results of the 2015 National Youth Risk Behavior Survey (YRBS) at the 21st International AIDS Conference.
Dr. Kann of the Centers for Disease Control and Prevention’s division of adolescent and school health directs the YRBS, an annual, multiple-choice, anonymous voluntary survey that addresses six areas of risk: behaviors that contribute to unintentional injuries and violence, tobacco use, alcohol and other drug use, sexual behaviors, inadequate physical activity, and unhealthy diet behaviors.
The 2015 survey was the first ever to include questions regarding sexual identity and behaviors.
“Twenty-two percent of all new HIV diagnoses in the U.S. occur in 13- to 24-year-olds. Most occur in males who have sex with males. This makes young males an important focus for HIV prevention efforts,” Dr. Kann said.
Yet until the 2015 YRBS, no reliable estimates existed regarding the number of gay or bisexual male high school students. “Reducing HIV infection among young sexual minority males is key to reducing HIV infection in the U.S.,” she said. “It’s hard to respond appropriately to a population that has not been counted.”
The 15,624 male students in public and private school grades 9-12 who participated in the YRBS were demographically representative of the nation’s roughly 8 million male high schoolers.
About 2% of male students identified themselves as gay, another 2.4% declared themselves bisexual, 93.1% said they were heterosexual, and 2.6% were unsure. With regard to sexual behaviors, 53.3% of respondents indicated they had sexual contact with females only, 1.3% with males only, and 1.9% with both males and females; 43.6% reported they hadn’t ever had sexual contact. Respondents could interpret “sexual contact” as they wished, ranging from kissing and hugging to intercourse.
Large differences in illicit drug use behaviors were found between the sexual minorities – that is, males who had sexual contact with males or with females as well as males – and those with only heterosexual contacts.
“We need to conduct new research on injected drug use among young males who have sex with males and determine what can be done to minimize, if not eliminate, this very high-risk behavior that is occurring at alarming rates,” Dr. Kann said.
The sexual minority students were also more likely to have engaged in various forms of noninjected illicit drug use known to boost the risk of HIV infection indirectly by increasing the likelihood of having unsafe sex. The sexual minority males had significantly higher rates of having ever used cocaine in any form, of having ever used amphetamines, and of having taken prescription drugs without a physician’s prescription. For instance, 14.8% of sexual minority males reported ever using amphetamines, compared with 2.5% of heterosexual males surveyed.

She noted that the YRBS results provided no evidence that the disparity in HIV diagnoses between young males who have sex with males and young males who have sex with females are caused by differences in the prevalence of HIV-related sexual behaviors. There were no statistically significant differences between heterosexual and sexual minority students in rates of ever having had sexual intercourse, having had intercourse with four or more persons, having sexual activity currently, or having used a condom at last intercourse.
Dr. Kann called for more research in the neglected area of social issues affecting young sexual minority males that likely increase their sense of marginalization and may promote behaviors placing them at increased risk for HIV acquisition.
“Many young males who have sex with males suffer from social isolation, stress from self-concealment and from coming out, discrimination, and even hatred, which may occur at home, at school, and in communities among both their peers and the adults responsible for their care and protection,” she said. “These factors may lead to harmful coping behaviors, such as drug use, that can further increase risk for HIV infection. It’s hard to imagine how even the best intervention technology will be able to eliminate the disparities in HIV diagnosis unless these social issues are addressed first and foremost.”
One clinician from New York rose from the audience to chastise the CDC for taking decades to incorporate questions about sexual minority youth into the YRBS.
“It’s important for the rest of the world to know how long it took the U.S. to collect this data. It’s not right. For those of you in the rest of the world who struggle to get information about your youth, don’t think that America is way ahead of you because it took us a long time,” she said.
Dr. Kann noted that such data have been collected for some time by some state and city public health agencies. As local officials began clamoring for a more comprehensive national picture, “it reached a tipping point” at the CDC.
Dr. Kann reported having no relevant financial conflicts.
DURBAN, SOUTH AFRICA – The first-ever nationally representative survey on sexual identity and behaviors in U.S. male high school students has exposed a high prevalence of illicit injected drug use among those who have sex with males or with both males and females.
About 10% of high school boys who identify as gay or bisexual reported having ever used a needle to inject any illegal drug into their bodies, compared with 1.5% of high school boys who identify as heterosexual.
“This is a cause for great concern because of the efficiency with which injected drug use transmits not only HIV but hepatitis and other diseases,” Laura Kann, PhD, said in reporting the results of the 2015 National Youth Risk Behavior Survey (YRBS) at the 21st International AIDS Conference.
Dr. Kann of the Centers for Disease Control and Prevention’s division of adolescent and school health directs the YRBS, an annual, multiple-choice, anonymous voluntary survey that addresses six areas of risk: behaviors that contribute to unintentional injuries and violence, tobacco use, alcohol and other drug use, sexual behaviors, inadequate physical activity, and unhealthy diet behaviors.
The 2015 survey was the first ever to include questions regarding sexual identity and behaviors.
“Twenty-two percent of all new HIV diagnoses in the U.S. occur in 13- to 24-year-olds. Most occur in males who have sex with males. This makes young males an important focus for HIV prevention efforts,” Dr. Kann said.
Yet until the 2015 YRBS, no reliable estimates existed regarding the number of gay or bisexual male high school students. “Reducing HIV infection among young sexual minority males is key to reducing HIV infection in the U.S.,” she said. “It’s hard to respond appropriately to a population that has not been counted.”
The 15,624 male students in public and private school grades 9-12 who participated in the YRBS were demographically representative of the nation’s roughly 8 million male high schoolers.
About 2% of male students identified themselves as gay, another 2.4% declared themselves bisexual, 93.1% said they were heterosexual, and 2.6% were unsure. With regard to sexual behaviors, 53.3% of respondents indicated they had sexual contact with females only, 1.3% with males only, and 1.9% with both males and females; 43.6% reported they hadn’t ever had sexual contact. Respondents could interpret “sexual contact” as they wished, ranging from kissing and hugging to intercourse.
Large differences in illicit drug use behaviors were found between the sexual minorities – that is, males who had sexual contact with males or with females as well as males – and those with only heterosexual contacts.
“We need to conduct new research on injected drug use among young males who have sex with males and determine what can be done to minimize, if not eliminate, this very high-risk behavior that is occurring at alarming rates,” Dr. Kann said.
The sexual minority students were also more likely to have engaged in various forms of noninjected illicit drug use known to boost the risk of HIV infection indirectly by increasing the likelihood of having unsafe sex. The sexual minority males had significantly higher rates of having ever used cocaine in any form, of having ever used amphetamines, and of having taken prescription drugs without a physician’s prescription. For instance, 14.8% of sexual minority males reported ever using amphetamines, compared with 2.5% of heterosexual males surveyed.

She noted that the YRBS results provided no evidence that the disparity in HIV diagnoses between young males who have sex with males and young males who have sex with females are caused by differences in the prevalence of HIV-related sexual behaviors. There were no statistically significant differences between heterosexual and sexual minority students in rates of ever having had sexual intercourse, having had intercourse with four or more persons, having sexual activity currently, or having used a condom at last intercourse.
Dr. Kann called for more research in the neglected area of social issues affecting young sexual minority males that likely increase their sense of marginalization and may promote behaviors placing them at increased risk for HIV acquisition.
“Many young males who have sex with males suffer from social isolation, stress from self-concealment and from coming out, discrimination, and even hatred, which may occur at home, at school, and in communities among both their peers and the adults responsible for their care and protection,” she said. “These factors may lead to harmful coping behaviors, such as drug use, that can further increase risk for HIV infection. It’s hard to imagine how even the best intervention technology will be able to eliminate the disparities in HIV diagnosis unless these social issues are addressed first and foremost.”
One clinician from New York rose from the audience to chastise the CDC for taking decades to incorporate questions about sexual minority youth into the YRBS.
“It’s important for the rest of the world to know how long it took the U.S. to collect this data. It’s not right. For those of you in the rest of the world who struggle to get information about your youth, don’t think that America is way ahead of you because it took us a long time,” she said.
Dr. Kann noted that such data have been collected for some time by some state and city public health agencies. As local officials began clamoring for a more comprehensive national picture, “it reached a tipping point” at the CDC.
Dr. Kann reported having no relevant financial conflicts.
AT AIDS 2016
Key clinical point: Male U.S. students in grades 9-12 who identify themselves as sexual minorities have markedly higher rates of illicit drug use than their heterosexual peers.
Major finding: A total of 4.4% of U.S. male high school students identify themselves as gay or bisexual, and they are about sevenfold more likely than heterosexual students to have ever injected illegal drugs.
Data source: A national population-based survey of 15,624 male students in grades 9-12.
Disclosures: The presenter is an employee of the Centers for Disease Control and Prevention, which sponsored the annual Youth Risk Behavior Survey.
Dapivirine vaginal ring sharply reduces HIV infection risk
DURBAN, SOUTH AFRICA – Women who consistently used a monthly vaginal ring containing the antiretroviral drug dapivirine reduced their risk of acquiring HIV infection by 75%-91% in exploratory analyses of the phase III ASPIRE study, Elizabeth R. Brown, ScD, reported at the 21st International AIDS Conference.
ASPIRE (A Study to Prevent Infection with a Ring for Extended use), also known as the Microbicide Trials Network-020 trial, was a multicenter, randomized, double-blind, placebo-controlled, phase III trial of a silicone vaginal ring containing 25 mg of the non-nucleoside reverse transcriptase inhibitor dapivirine. ASPIRE involved 2,629 HIV-uninfected women aged 18-45 years from four Southern African countries with extremely high HIV infection rates: South Africa, Uganda, Malawi, and Zimbabwe. They were prospectively followed monthly for 12-33 months.
As reported at a conference earlier in 2016, seroconversion occurred in 71 women assigned to the dapivirine ring and 97 on a placebo ring during 4,280 person-years of follow-up. The resultant 27% relative risk reduction in an intent-to-treat analysis, while statistically significant, was less than hoped for by investigators.
However, the device only works if it’s used consistently, so the investigators decided to conduct exploratory analyses using an objective measure of adherence: the amount of dapivirine remaining in a ring after a month’s use. This was possible because the rings had been stored for analysis in a central laboratory.
The vaginal ring is self-inserted, then meant to be kept in place continuously for a month before being self-removed and replaced with a fresh ring. Dapivirine is released continuously while the ring is in place, so the less residual dapivirine contained in a used ring, the greater the adherence during that month, explained Dr. Brown, a biostatistician at the University of Washington, Seattle.
A dose-response effect was observed. In the most encouraging of the exploratory time-dependent use analyses, the rate of HIV acquisition was 4.7 cases per 100 person-years with placebo, 4.9/100 person-years in women defined as nonadherent based upon a residual dapivirine level of 23.5 mg or more, 3.1 with low use, 1.9 with moderate use, and 0.4 cases/100 person-years with consistent use as directed, meaning a patient left the device in place continuously for a month as reflected in a residual dapivirine rate below 22 mg.
This translates into a 91% reduction in risk, compared with placebo, in the most consistent users, a 58% relative risk reduction with moderate use, and a 29% reduction with low use, she continued.
The new results were hailed as a major advance in preventing HIV infection.
“We’re meeting here at AIDS 2016 in Durban, capital of the KwaZulu-Natal province of South Africa, which is one of the hardest-hit areas of the world for HIV. Over the course of their lifetime, women in Southern Africa have more than a 50% chance of HIV infection. So a new prevention tool that women can use on their own discretely to protect themselves against HIV would be a game changer,” Jared Baeten, MD, PhD, said in an interview.
“I see this as having an important potential role in the [United States] as well for women who face an increased risk of HIV. It’s extremely valuable for them to have a method of protection that they can control and feel good about and safely use for an extended period of time,” added Dr. Baeten, professor and vice chair of global health, as well as professor of allergy and infectious diseases at the University of Washington, Seattle.
The challenges involved in consistent use of the dapivirine ring are less formidable than are those posed by consistent use of a daily oral medication for preexposure prevention, he noted.
Dr. Baeten was a leader of ASPIRE and is codirector of the HOPE (HIV Open-label Prevention Extension) study, which is now underway. The expectation is that adherence to the dapivirine vaginal ring will be better in HOPE than in ASPIRE because participants can now be counseled that the ring works, it’s safe, and there is no chance of getting a placebo device, he said.
“There is an urgent unmet need to expand long-acting HIV prevention options for women and girls,” Zeda Rosenberg, ScD, founder and chief executive officer of the nonprofit International Partnership for Microbicides (IPM) in Silver Spring, Md., which is the sponsor and developer of the ring.
Toward that end, in the next several months the ASPIRE data will be combined with those from the IPM-sponsored Ring Study, another completed large phase III trial of the dapivirine ring conducted in Africa. The goals will be to try to identify potentially modifiable sociodemographic and behavioral correlates of adherence and to further strengthen the safety and efficacy findings. The plan is to submit the full data package to the Food and Drug Administration and regulat ory agencies in other countries in the spring of 2017.
“Hopefully, the product will receive marketing approval in 2018,” Dr. Rosenberg said.
In addition to sponsoring HOPE, IPM is also sponsoring the ongoing open-label DREAM (Dapivirine Ring Extended Access and Monitoring) study. IPM is also partnering with the Microbicides Trial Network to conduct a separate study of the vaginal ring in adolescents and young women in Africa.
A dapivirine vaginal ring that’s active for 3 months rather than 1 month will begin safety studies later in 2016. The potential advantages of this product are reduced annual cost and fewer clinic visits. Also, a 3-month version of the ring with an added contraceptive has been developed and will begin safety studies in the United States in fall 2016. The possibility that suboptimal adherence can be overcome by a ring containing a higher dose of dapivirine will also be explored, according to Dr. Rosenberg.
Dr. Brown, Dr. Baeten, and Dr. Rosenberg reported having no financial conflicts of interest.
DURBAN, SOUTH AFRICA – Women who consistently used a monthly vaginal ring containing the antiretroviral drug dapivirine reduced their risk of acquiring HIV infection by 75%-91% in exploratory analyses of the phase III ASPIRE study, Elizabeth R. Brown, ScD, reported at the 21st International AIDS Conference.
ASPIRE (A Study to Prevent Infection with a Ring for Extended use), also known as the Microbicide Trials Network-020 trial, was a multicenter, randomized, double-blind, placebo-controlled, phase III trial of a silicone vaginal ring containing 25 mg of the non-nucleoside reverse transcriptase inhibitor dapivirine. ASPIRE involved 2,629 HIV-uninfected women aged 18-45 years from four Southern African countries with extremely high HIV infection rates: South Africa, Uganda, Malawi, and Zimbabwe. They were prospectively followed monthly for 12-33 months.
As reported at a conference earlier in 2016, seroconversion occurred in 71 women assigned to the dapivirine ring and 97 on a placebo ring during 4,280 person-years of follow-up. The resultant 27% relative risk reduction in an intent-to-treat analysis, while statistically significant, was less than hoped for by investigators.
However, the device only works if it’s used consistently, so the investigators decided to conduct exploratory analyses using an objective measure of adherence: the amount of dapivirine remaining in a ring after a month’s use. This was possible because the rings had been stored for analysis in a central laboratory.
The vaginal ring is self-inserted, then meant to be kept in place continuously for a month before being self-removed and replaced with a fresh ring. Dapivirine is released continuously while the ring is in place, so the less residual dapivirine contained in a used ring, the greater the adherence during that month, explained Dr. Brown, a biostatistician at the University of Washington, Seattle.
A dose-response effect was observed. In the most encouraging of the exploratory time-dependent use analyses, the rate of HIV acquisition was 4.7 cases per 100 person-years with placebo, 4.9/100 person-years in women defined as nonadherent based upon a residual dapivirine level of 23.5 mg or more, 3.1 with low use, 1.9 with moderate use, and 0.4 cases/100 person-years with consistent use as directed, meaning a patient left the device in place continuously for a month as reflected in a residual dapivirine rate below 22 mg.
This translates into a 91% reduction in risk, compared with placebo, in the most consistent users, a 58% relative risk reduction with moderate use, and a 29% reduction with low use, she continued.
The new results were hailed as a major advance in preventing HIV infection.
“We’re meeting here at AIDS 2016 in Durban, capital of the KwaZulu-Natal province of South Africa, which is one of the hardest-hit areas of the world for HIV. Over the course of their lifetime, women in Southern Africa have more than a 50% chance of HIV infection. So a new prevention tool that women can use on their own discretely to protect themselves against HIV would be a game changer,” Jared Baeten, MD, PhD, said in an interview.
“I see this as having an important potential role in the [United States] as well for women who face an increased risk of HIV. It’s extremely valuable for them to have a method of protection that they can control and feel good about and safely use for an extended period of time,” added Dr. Baeten, professor and vice chair of global health, as well as professor of allergy and infectious diseases at the University of Washington, Seattle.
The challenges involved in consistent use of the dapivirine ring are less formidable than are those posed by consistent use of a daily oral medication for preexposure prevention, he noted.
Dr. Baeten was a leader of ASPIRE and is codirector of the HOPE (HIV Open-label Prevention Extension) study, which is now underway. The expectation is that adherence to the dapivirine vaginal ring will be better in HOPE than in ASPIRE because participants can now be counseled that the ring works, it’s safe, and there is no chance of getting a placebo device, he said.
“There is an urgent unmet need to expand long-acting HIV prevention options for women and girls,” Zeda Rosenberg, ScD, founder and chief executive officer of the nonprofit International Partnership for Microbicides (IPM) in Silver Spring, Md., which is the sponsor and developer of the ring.
Toward that end, in the next several months the ASPIRE data will be combined with those from the IPM-sponsored Ring Study, another completed large phase III trial of the dapivirine ring conducted in Africa. The goals will be to try to identify potentially modifiable sociodemographic and behavioral correlates of adherence and to further strengthen the safety and efficacy findings. The plan is to submit the full data package to the Food and Drug Administration and regulat ory agencies in other countries in the spring of 2017.
“Hopefully, the product will receive marketing approval in 2018,” Dr. Rosenberg said.
In addition to sponsoring HOPE, IPM is also sponsoring the ongoing open-label DREAM (Dapivirine Ring Extended Access and Monitoring) study. IPM is also partnering with the Microbicides Trial Network to conduct a separate study of the vaginal ring in adolescents and young women in Africa.
A dapivirine vaginal ring that’s active for 3 months rather than 1 month will begin safety studies later in 2016. The potential advantages of this product are reduced annual cost and fewer clinic visits. Also, a 3-month version of the ring with an added contraceptive has been developed and will begin safety studies in the United States in fall 2016. The possibility that suboptimal adherence can be overcome by a ring containing a higher dose of dapivirine will also be explored, according to Dr. Rosenberg.
Dr. Brown, Dr. Baeten, and Dr. Rosenberg reported having no financial conflicts of interest.
DURBAN, SOUTH AFRICA – Women who consistently used a monthly vaginal ring containing the antiretroviral drug dapivirine reduced their risk of acquiring HIV infection by 75%-91% in exploratory analyses of the phase III ASPIRE study, Elizabeth R. Brown, ScD, reported at the 21st International AIDS Conference.
ASPIRE (A Study to Prevent Infection with a Ring for Extended use), also known as the Microbicide Trials Network-020 trial, was a multicenter, randomized, double-blind, placebo-controlled, phase III trial of a silicone vaginal ring containing 25 mg of the non-nucleoside reverse transcriptase inhibitor dapivirine. ASPIRE involved 2,629 HIV-uninfected women aged 18-45 years from four Southern African countries with extremely high HIV infection rates: South Africa, Uganda, Malawi, and Zimbabwe. They were prospectively followed monthly for 12-33 months.
As reported at a conference earlier in 2016, seroconversion occurred in 71 women assigned to the dapivirine ring and 97 on a placebo ring during 4,280 person-years of follow-up. The resultant 27% relative risk reduction in an intent-to-treat analysis, while statistically significant, was less than hoped for by investigators.
However, the device only works if it’s used consistently, so the investigators decided to conduct exploratory analyses using an objective measure of adherence: the amount of dapivirine remaining in a ring after a month’s use. This was possible because the rings had been stored for analysis in a central laboratory.
The vaginal ring is self-inserted, then meant to be kept in place continuously for a month before being self-removed and replaced with a fresh ring. Dapivirine is released continuously while the ring is in place, so the less residual dapivirine contained in a used ring, the greater the adherence during that month, explained Dr. Brown, a biostatistician at the University of Washington, Seattle.
A dose-response effect was observed. In the most encouraging of the exploratory time-dependent use analyses, the rate of HIV acquisition was 4.7 cases per 100 person-years with placebo, 4.9/100 person-years in women defined as nonadherent based upon a residual dapivirine level of 23.5 mg or more, 3.1 with low use, 1.9 with moderate use, and 0.4 cases/100 person-years with consistent use as directed, meaning a patient left the device in place continuously for a month as reflected in a residual dapivirine rate below 22 mg.
This translates into a 91% reduction in risk, compared with placebo, in the most consistent users, a 58% relative risk reduction with moderate use, and a 29% reduction with low use, she continued.
The new results were hailed as a major advance in preventing HIV infection.
“We’re meeting here at AIDS 2016 in Durban, capital of the KwaZulu-Natal province of South Africa, which is one of the hardest-hit areas of the world for HIV. Over the course of their lifetime, women in Southern Africa have more than a 50% chance of HIV infection. So a new prevention tool that women can use on their own discretely to protect themselves against HIV would be a game changer,” Jared Baeten, MD, PhD, said in an interview.
“I see this as having an important potential role in the [United States] as well for women who face an increased risk of HIV. It’s extremely valuable for them to have a method of protection that they can control and feel good about and safely use for an extended period of time,” added Dr. Baeten, professor and vice chair of global health, as well as professor of allergy and infectious diseases at the University of Washington, Seattle.
The challenges involved in consistent use of the dapivirine ring are less formidable than are those posed by consistent use of a daily oral medication for preexposure prevention, he noted.
Dr. Baeten was a leader of ASPIRE and is codirector of the HOPE (HIV Open-label Prevention Extension) study, which is now underway. The expectation is that adherence to the dapivirine vaginal ring will be better in HOPE than in ASPIRE because participants can now be counseled that the ring works, it’s safe, and there is no chance of getting a placebo device, he said.
“There is an urgent unmet need to expand long-acting HIV prevention options for women and girls,” Zeda Rosenberg, ScD, founder and chief executive officer of the nonprofit International Partnership for Microbicides (IPM) in Silver Spring, Md., which is the sponsor and developer of the ring.
Toward that end, in the next several months the ASPIRE data will be combined with those from the IPM-sponsored Ring Study, another completed large phase III trial of the dapivirine ring conducted in Africa. The goals will be to try to identify potentially modifiable sociodemographic and behavioral correlates of adherence and to further strengthen the safety and efficacy findings. The plan is to submit the full data package to the Food and Drug Administration and regulat ory agencies in other countries in the spring of 2017.
“Hopefully, the product will receive marketing approval in 2018,” Dr. Rosenberg said.
In addition to sponsoring HOPE, IPM is also sponsoring the ongoing open-label DREAM (Dapivirine Ring Extended Access and Monitoring) study. IPM is also partnering with the Microbicides Trial Network to conduct a separate study of the vaginal ring in adolescents and young women in Africa.
A dapivirine vaginal ring that’s active for 3 months rather than 1 month will begin safety studies later in 2016. The potential advantages of this product are reduced annual cost and fewer clinic visits. Also, a 3-month version of the ring with an added contraceptive has been developed and will begin safety studies in the United States in fall 2016. The possibility that suboptimal adherence can be overcome by a ring containing a higher dose of dapivirine will also be explored, according to Dr. Rosenberg.
Dr. Brown, Dr. Baeten, and Dr. Rosenberg reported having no financial conflicts of interest.
AT AIDS 2016
Key clinical point: Consistent use of monthly vaginal ring containing dapivirine resulted in significant risk reductions in HIV acquisition, compared with placebo.
Major finding: Women at increased risk for HIV infection who used a dapivirine-impregnated silicone vaginal ring as directed – leaving it in place for a full month and then exchanging it for a new one – had 75%-91% relative risk reductions in HIV acquisition.
Data source: Exploratory analyses of data from ASPIRE, a randomized, placebo-controlled, phase III multicenter clinical trial in which 2,629 women in four Southern African countries were followed monthly for 12-33 months.
Disclosures: The ASPIRE study was supported by the National Institutes of Health–funded Microbicide Trials Network. The presenter reported having no financial conflicts of interest.
Early ART prevents HIV transmission to serodiscordant partner
Suppression of HIV-1 infection with antiretroviral therapy reduced by 93% the risk of transmission of the virus between serodiscordant partners, according to data from the HIV Prevention Trials Network presented at the 21st International AIDS Conference.
“We hope that the newly emphasized importance of early initiation of ART will encourage patients with HIV-1 infection to start such therapy without delay,” the investigators reported in their paper, which was published simultaneously online July 18 in the New England Journal of Medicine.
The international 5-year study in serodiscordant couples randomized 886 HIV-1 infected individuals to antiretroviral therapy as soon as they were enrolled in the study, while the other 877 participants initiated antiretroviral therapy only if their CD4+ cell count showed a sustained decline or they developed an illness suggestive of AIDS.
Overall, 46 genetically-linked new HIV infections were observed during the trial – 3 in the early ART group and 43 in the delayed therapy group – with early antiretroviral therapy therefore showing a 93% lower risk of linked partner infection. Eight of the linked-partner transmissions were diagnosed after the infected partner had begun ART; four of these were diagnosed less than 90 days after they had started treatment (N Engl J Med 2016 July 18. doi: 10.1056/NEJMoa1600693).
“In these cases, further analysis suggested that all four of these infections probably occurred before the virus was virally suppressed in the index participant,” wrote Dr. Myron S. Cohen of the University of North Carolina at Chapel Hill and his coauthors. “In the other four cases, partner infection occurred after ART failed in the index participant.”
While the study was obviously not able to measure viral load at the time of the transmission event, the authors said the observed relationship between viremia and HIV transmission signaled the importance of counseling serodiscordant couples about the risks of transmission before viral suppression is achieved, as well as the need to closely monitor viral load during treatment.
They also noted that they did not observe a single case of transmission occurring when the index case had stable viral suppression on antiretroviral therapy.
Even after the interim results of the HPTN trial were released and study participants were informed of the benefits of early antiretroviral therapy, 17% of individuals in the delayed initiation group still chose not to begin therapy. The authors suggested this may be the result of the previous recommendations that therapy is not required unless there is a drop in CD4+ cell count or decline in health.
The study was supported by grants from the National Institutes of Health, and study drugs were donated by Abbott Laboratories, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline/Viiv Healthcare, and Merck. Several authors declared grants from the NIH, and several declared support, grants, and fees from private companies, including those who donated study drugs.
Suppression of HIV-1 infection with antiretroviral therapy reduced by 93% the risk of transmission of the virus between serodiscordant partners, according to data from the HIV Prevention Trials Network presented at the 21st International AIDS Conference.
“We hope that the newly emphasized importance of early initiation of ART will encourage patients with HIV-1 infection to start such therapy without delay,” the investigators reported in their paper, which was published simultaneously online July 18 in the New England Journal of Medicine.
The international 5-year study in serodiscordant couples randomized 886 HIV-1 infected individuals to antiretroviral therapy as soon as they were enrolled in the study, while the other 877 participants initiated antiretroviral therapy only if their CD4+ cell count showed a sustained decline or they developed an illness suggestive of AIDS.
Overall, 46 genetically-linked new HIV infections were observed during the trial – 3 in the early ART group and 43 in the delayed therapy group – with early antiretroviral therapy therefore showing a 93% lower risk of linked partner infection. Eight of the linked-partner transmissions were diagnosed after the infected partner had begun ART; four of these were diagnosed less than 90 days after they had started treatment (N Engl J Med 2016 July 18. doi: 10.1056/NEJMoa1600693).
“In these cases, further analysis suggested that all four of these infections probably occurred before the virus was virally suppressed in the index participant,” wrote Dr. Myron S. Cohen of the University of North Carolina at Chapel Hill and his coauthors. “In the other four cases, partner infection occurred after ART failed in the index participant.”
While the study was obviously not able to measure viral load at the time of the transmission event, the authors said the observed relationship between viremia and HIV transmission signaled the importance of counseling serodiscordant couples about the risks of transmission before viral suppression is achieved, as well as the need to closely monitor viral load during treatment.
They also noted that they did not observe a single case of transmission occurring when the index case had stable viral suppression on antiretroviral therapy.
Even after the interim results of the HPTN trial were released and study participants were informed of the benefits of early antiretroviral therapy, 17% of individuals in the delayed initiation group still chose not to begin therapy. The authors suggested this may be the result of the previous recommendations that therapy is not required unless there is a drop in CD4+ cell count or decline in health.
The study was supported by grants from the National Institutes of Health, and study drugs were donated by Abbott Laboratories, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline/Viiv Healthcare, and Merck. Several authors declared grants from the NIH, and several declared support, grants, and fees from private companies, including those who donated study drugs.
Suppression of HIV-1 infection with antiretroviral therapy reduced by 93% the risk of transmission of the virus between serodiscordant partners, according to data from the HIV Prevention Trials Network presented at the 21st International AIDS Conference.
“We hope that the newly emphasized importance of early initiation of ART will encourage patients with HIV-1 infection to start such therapy without delay,” the investigators reported in their paper, which was published simultaneously online July 18 in the New England Journal of Medicine.
The international 5-year study in serodiscordant couples randomized 886 HIV-1 infected individuals to antiretroviral therapy as soon as they were enrolled in the study, while the other 877 participants initiated antiretroviral therapy only if their CD4+ cell count showed a sustained decline or they developed an illness suggestive of AIDS.
Overall, 46 genetically-linked new HIV infections were observed during the trial – 3 in the early ART group and 43 in the delayed therapy group – with early antiretroviral therapy therefore showing a 93% lower risk of linked partner infection. Eight of the linked-partner transmissions were diagnosed after the infected partner had begun ART; four of these were diagnosed less than 90 days after they had started treatment (N Engl J Med 2016 July 18. doi: 10.1056/NEJMoa1600693).
“In these cases, further analysis suggested that all four of these infections probably occurred before the virus was virally suppressed in the index participant,” wrote Dr. Myron S. Cohen of the University of North Carolina at Chapel Hill and his coauthors. “In the other four cases, partner infection occurred after ART failed in the index participant.”
While the study was obviously not able to measure viral load at the time of the transmission event, the authors said the observed relationship between viremia and HIV transmission signaled the importance of counseling serodiscordant couples about the risks of transmission before viral suppression is achieved, as well as the need to closely monitor viral load during treatment.
They also noted that they did not observe a single case of transmission occurring when the index case had stable viral suppression on antiretroviral therapy.
Even after the interim results of the HPTN trial were released and study participants were informed of the benefits of early antiretroviral therapy, 17% of individuals in the delayed initiation group still chose not to begin therapy. The authors suggested this may be the result of the previous recommendations that therapy is not required unless there is a drop in CD4+ cell count or decline in health.
The study was supported by grants from the National Institutes of Health, and study drugs were donated by Abbott Laboratories, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline/Viiv Healthcare, and Merck. Several authors declared grants from the NIH, and several declared support, grants, and fees from private companies, including those who donated study drugs.
FROM AIDS 2016
Key clinical point: Suppression of HIV-1 infection with antiretroviral therapy can drastically reduce the risk of transmission of the virus between serodiscordant partners.
Major finding: Early antiretroviral therapy was associated with a 93% lower risk of transmission of HIV-1 between serodiscordant couples compared to therapy initiated only after a decline in CD4+ cell count or the onset of AIDS-related illness.
Data source: The 5-year prospective randomized HIV Prevention Trials Network study in 1,763 index individuals with HIV-1 infection.
Disclosures: The study was supported by grants from the National Institutes of Health, and study drugs were donated by Abbott Laboratories, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline/Viiv Healthcare, and Merck. Several authors declared grants from the NIH, and several declared support, grants, and fees from private companies, including those who donated study drugs.















