User login
Anal cancer in HIV-infected patients: to screen or not?
DURBAN, SOUTH AFRICA – Screening for anal cancer in HIV-infected men or women should not be part of routine clinical practice at this time, Andrew Grulich, MBBS, PhD, declared at the 21st International AIDS Conference.
Some experts recommend anal cytologic screening or high-resolution anoscopy for HIV-positive men and women, but it’s worth noting that strategy hasn’t been incorporated into any national practice guidelines.
“And for very good reason: When we have a condition with a prevalence that’s so high and a treatment with recurrence rates that are so high, I think we need to question our approach,” said Dr. Grulich, professor of medicine and head of the HIV epidemiology and prevention program at the University of New South Wales in Sydney, Australia.
Screening proponents point to the high incidence of anal cancer in persons with HIV infection. It’s the fourth most common cancer in HIV patients in the United States, behind the AIDS-defining cancers and lung cancer. Indeed, the anal cancer rate is 10-fold greater in HIV-positive women, heterosexual men, and injection drug users than in the HIV-negative general population, and 50-fold higher in HIV-positive gay and bisexual men. Screening proponents also draw an analogy between anal cancer screening and the screening and treatment of cervical intraepithelial neoplasia (CIN), which has been enormously successful in preventing cervical cancer. But Dr. Grulich said he believes the cervical cancer screening analogy is faulty.
Colposcopy has a mean 90% specificity for diagnosis of HPV-related high-grade squamous intraepithelial lesions (HSIL) or cervical cancer, while high-resolution anoscopy as a diagnostic test has a specificity as low as 37% in HIV-positive persons. The prevalence of HSIL is 30%-40% in anal samples from HIV-infected homosexual men, compared with 1%-2% in cervical samples from HIV-negative women.
The rate of progression from CIN-3 to cervical cancer in women in the general population is about 1 in 80 per year. In contrast, the rate of progression from anal intraepithelial neoplasia (AIN)-2 or AIN-3 to anal cancer in HIV-infected homosexual men is estimated at only 1 in 400-600 per year, probably because regression of anal lesions is quite common.
Moreover, while a single treatment of high-grade CIN is typically curative and entails little morbidity, destruction of AIN by means of heat, cold, or electricity has a 70% failure rate, carries substantial morbidity, and is not supported by any evidence that it actually reduces the incidence of anal cancer, he continued.
“We’re in a bit of a quandary regarding what to do about anal cancer prevention. We really need research in order to move this field forward,” Dr. Grulich said.
He added that it’s worth keeping an eye on two ongoing studies addressing key questions surrounding anal cancer in HIV-positive persons. The U.S. National Cancer Institute–funded randomized ANCHOR trial is examining ablative therapy versus watchful waiting in HIV-infected patients with anal HSIL lesions; however, results of this large study aren’t expected until 2022 or 2023. And Dr. Grulich heads the Study of the Prevention of Anal Cancer, aimed at identifying biomarkers that predict persistence of HSIL as a marker of anal cancer risk.
A study he would very much like to see funded is a randomized, placebo-controlled, adequately powered trial of the 9-valent HPV vaccine in HIV-infected gay or bisexual men over age 26. At the 2016 meeting of the Conference on Retroviruses and Opportunistic Infections (CROI), Timothy J. Wilkin, MD, of Cornell University, New York, presented the results of the phase III ACTG A5298 trial of the quadrivalent HPV vaccine in HIV-infected adults over age 26. The vaccine group had a 27% reduction in risk of persistent anal HPV compared with placebo, which wasn’t statistically significant because of the small study size. The 9-valent vaccine would prevent a broader range of oncogenic HPV types.
Dr. Grulich reported receiving research funding from CSL Australia, Gilead Sciences, Viiv, and Hologic.
DURBAN, SOUTH AFRICA – Screening for anal cancer in HIV-infected men or women should not be part of routine clinical practice at this time, Andrew Grulich, MBBS, PhD, declared at the 21st International AIDS Conference.
Some experts recommend anal cytologic screening or high-resolution anoscopy for HIV-positive men and women, but it’s worth noting that strategy hasn’t been incorporated into any national practice guidelines.
“And for very good reason: When we have a condition with a prevalence that’s so high and a treatment with recurrence rates that are so high, I think we need to question our approach,” said Dr. Grulich, professor of medicine and head of the HIV epidemiology and prevention program at the University of New South Wales in Sydney, Australia.
Screening proponents point to the high incidence of anal cancer in persons with HIV infection. It’s the fourth most common cancer in HIV patients in the United States, behind the AIDS-defining cancers and lung cancer. Indeed, the anal cancer rate is 10-fold greater in HIV-positive women, heterosexual men, and injection drug users than in the HIV-negative general population, and 50-fold higher in HIV-positive gay and bisexual men. Screening proponents also draw an analogy between anal cancer screening and the screening and treatment of cervical intraepithelial neoplasia (CIN), which has been enormously successful in preventing cervical cancer. But Dr. Grulich said he believes the cervical cancer screening analogy is faulty.
Colposcopy has a mean 90% specificity for diagnosis of HPV-related high-grade squamous intraepithelial lesions (HSIL) or cervical cancer, while high-resolution anoscopy as a diagnostic test has a specificity as low as 37% in HIV-positive persons. The prevalence of HSIL is 30%-40% in anal samples from HIV-infected homosexual men, compared with 1%-2% in cervical samples from HIV-negative women.
The rate of progression from CIN-3 to cervical cancer in women in the general population is about 1 in 80 per year. In contrast, the rate of progression from anal intraepithelial neoplasia (AIN)-2 or AIN-3 to anal cancer in HIV-infected homosexual men is estimated at only 1 in 400-600 per year, probably because regression of anal lesions is quite common.
Moreover, while a single treatment of high-grade CIN is typically curative and entails little morbidity, destruction of AIN by means of heat, cold, or electricity has a 70% failure rate, carries substantial morbidity, and is not supported by any evidence that it actually reduces the incidence of anal cancer, he continued.
“We’re in a bit of a quandary regarding what to do about anal cancer prevention. We really need research in order to move this field forward,” Dr. Grulich said.
He added that it’s worth keeping an eye on two ongoing studies addressing key questions surrounding anal cancer in HIV-positive persons. The U.S. National Cancer Institute–funded randomized ANCHOR trial is examining ablative therapy versus watchful waiting in HIV-infected patients with anal HSIL lesions; however, results of this large study aren’t expected until 2022 or 2023. And Dr. Grulich heads the Study of the Prevention of Anal Cancer, aimed at identifying biomarkers that predict persistence of HSIL as a marker of anal cancer risk.
A study he would very much like to see funded is a randomized, placebo-controlled, adequately powered trial of the 9-valent HPV vaccine in HIV-infected gay or bisexual men over age 26. At the 2016 meeting of the Conference on Retroviruses and Opportunistic Infections (CROI), Timothy J. Wilkin, MD, of Cornell University, New York, presented the results of the phase III ACTG A5298 trial of the quadrivalent HPV vaccine in HIV-infected adults over age 26. The vaccine group had a 27% reduction in risk of persistent anal HPV compared with placebo, which wasn’t statistically significant because of the small study size. The 9-valent vaccine would prevent a broader range of oncogenic HPV types.
Dr. Grulich reported receiving research funding from CSL Australia, Gilead Sciences, Viiv, and Hologic.
DURBAN, SOUTH AFRICA – Screening for anal cancer in HIV-infected men or women should not be part of routine clinical practice at this time, Andrew Grulich, MBBS, PhD, declared at the 21st International AIDS Conference.
Some experts recommend anal cytologic screening or high-resolution anoscopy for HIV-positive men and women, but it’s worth noting that strategy hasn’t been incorporated into any national practice guidelines.
“And for very good reason: When we have a condition with a prevalence that’s so high and a treatment with recurrence rates that are so high, I think we need to question our approach,” said Dr. Grulich, professor of medicine and head of the HIV epidemiology and prevention program at the University of New South Wales in Sydney, Australia.
Screening proponents point to the high incidence of anal cancer in persons with HIV infection. It’s the fourth most common cancer in HIV patients in the United States, behind the AIDS-defining cancers and lung cancer. Indeed, the anal cancer rate is 10-fold greater in HIV-positive women, heterosexual men, and injection drug users than in the HIV-negative general population, and 50-fold higher in HIV-positive gay and bisexual men. Screening proponents also draw an analogy between anal cancer screening and the screening and treatment of cervical intraepithelial neoplasia (CIN), which has been enormously successful in preventing cervical cancer. But Dr. Grulich said he believes the cervical cancer screening analogy is faulty.
Colposcopy has a mean 90% specificity for diagnosis of HPV-related high-grade squamous intraepithelial lesions (HSIL) or cervical cancer, while high-resolution anoscopy as a diagnostic test has a specificity as low as 37% in HIV-positive persons. The prevalence of HSIL is 30%-40% in anal samples from HIV-infected homosexual men, compared with 1%-2% in cervical samples from HIV-negative women.
The rate of progression from CIN-3 to cervical cancer in women in the general population is about 1 in 80 per year. In contrast, the rate of progression from anal intraepithelial neoplasia (AIN)-2 or AIN-3 to anal cancer in HIV-infected homosexual men is estimated at only 1 in 400-600 per year, probably because regression of anal lesions is quite common.
Moreover, while a single treatment of high-grade CIN is typically curative and entails little morbidity, destruction of AIN by means of heat, cold, or electricity has a 70% failure rate, carries substantial morbidity, and is not supported by any evidence that it actually reduces the incidence of anal cancer, he continued.
“We’re in a bit of a quandary regarding what to do about anal cancer prevention. We really need research in order to move this field forward,” Dr. Grulich said.
He added that it’s worth keeping an eye on two ongoing studies addressing key questions surrounding anal cancer in HIV-positive persons. The U.S. National Cancer Institute–funded randomized ANCHOR trial is examining ablative therapy versus watchful waiting in HIV-infected patients with anal HSIL lesions; however, results of this large study aren’t expected until 2022 or 2023. And Dr. Grulich heads the Study of the Prevention of Anal Cancer, aimed at identifying biomarkers that predict persistence of HSIL as a marker of anal cancer risk.
A study he would very much like to see funded is a randomized, placebo-controlled, adequately powered trial of the 9-valent HPV vaccine in HIV-infected gay or bisexual men over age 26. At the 2016 meeting of the Conference on Retroviruses and Opportunistic Infections (CROI), Timothy J. Wilkin, MD, of Cornell University, New York, presented the results of the phase III ACTG A5298 trial of the quadrivalent HPV vaccine in HIV-infected adults over age 26. The vaccine group had a 27% reduction in risk of persistent anal HPV compared with placebo, which wasn’t statistically significant because of the small study size. The 9-valent vaccine would prevent a broader range of oncogenic HPV types.
Dr. Grulich reported receiving research funding from CSL Australia, Gilead Sciences, Viiv, and Hologic.
EXPERT ANALYSIS FROM AIDS 2016
Cancer trends shifting in HIV-positive patients
DURBAN, SOUTH AFRICA – The rates of the AIDS-defining cancers Kaposi’s sarcoma and non-Hodgkin’s lymphoma have plummeted in the antiretroviral era, yet they are still the two top cancers in terms of cumulative incidence in HIV-infected patients by age 75, Benigno Rodriguez, MD, said at the 21st International AIDS Conference.
Lung cancer also remains a major concern in the HIV-infected population. Each of these three cancers carries about a 1 in 25 lifetime risk to age 75, the estimated lifespan of HIV-positive patients on combination antiretroviral therapy (ART), according to Dr. Rodriguez of Case Western Reserve University in Cleveland.
ART has resulted in a marked change in cancer trends among HIV-infected patients. The incidence of AIDS-defining cancers has decreased “massively,” Dr. Rodriguez observed; but because ART has extended the lifespan, patients are now living long enough to get other cancers. And they do so at a higher rate than that of the general population because of their impaired immune function, higher rate of risk factors such as smoking, and greater prevalence of oncogenic viral coinfections such as hepatitis B and C and Epstein-Barr virus.
The increase in the incidence and risk of colorectal, liver, and anal cancers among HIV-positive individuals since the introduction of combination ART can largely be explained by the population’s longer exposure to risk due to increasing survival, he said.
Dr. Rodriguez presented highlights of an analysis of cancer trends in North America during 1996-2009. The study was based on 86,620 HIV-infected persons with 475,660 person-years of follow-up and 196,987 subjects without HIV infection and with more than 1.8 million person-years of follow-up. Trends over time were assessed for nine cancers: Kaposi’s sarcoma, non-Hodgkin’s lymphoma, Hodgkin’s lymphoma, and melanoma, as well as anal, lung, colorectal, liver, and oropharyngeal cancers (Ann Intern Med. 2015;163:507-18).
Examining trends in three periods – 1996-1999, 2000-2004, and 2005-2009 – the investigators looked at the impact of ART over time on rates of AIDS-related and non–AIDS-related cancers in HIV-infected patients and compared them to results in the general HIV-uninfected population.
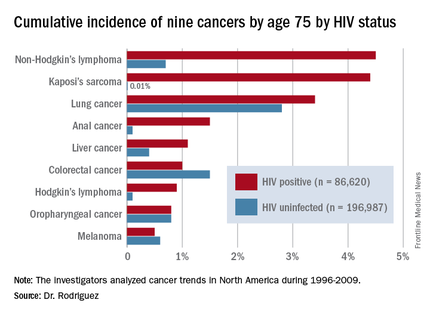
By conducting a competing risk analysis, Dr. Rodriguez and his coworkers were able to estimate the cumulative lifetime risk of the various cancers by age 75, a metric that provides readily understandable information for counseling HIV-infected patients about their long-term cancer risk.
The measure “is more intuitive than using incidence rates,” Dr. Rodriguez said. In a study of 1,578 HIV-infected patients who received the hepatitis B vaccine, for example, those patients whose immune function did not to respond to the vaccine were more likely to be among the 6% of patients who subsequently developed cancer during up to 20 years of follow-up.
The findings on cancer trends in the ART era have clinical implications for cancer screening and prevention in HIV-infected patients. The high rates of smoking and lung cancer in this population make HIV-positive smokers a logical target for lung cancer screening. The rising risk of colorectal cancer – the cumulative lifetime risk to age 75 was 0.4% in 1996-1999, 0.7% in 2000-2004, and 1.3% in 2005-2009 – suggests a need for increased colorectal cancer screening in the older HIV-positive population.
Early and sustained HIV suppression with combination ART remains the only known method of preventing AIDS-defining cancers. Dr. Rodriguez and his coinvestigators in the Centers for AIDS Research Network of Integrated Clinical Systems demonstrated the crucial role of suppressing HIV in a study of 6,036 HIV-infected patients who started on ART and were followed for more than 21,000 person-years. Compared with HIV-infected patients with a 3-month lagged HIV viremia of no more than 50 copies/mL, patients’ risk of developing non-Hodgkin’s lymphoma was 2.1-fold greater if their 3-month lagged HIV viremia was 51-500 copies/mL and 3.56-fold greater if it exceeded 500 copies/mL (Clin Infect Dis. 2014 Jun;58[11]:1599-606).
The study on cancer trends over time was funded by the National Institutes of Health. Dr. Rodriguez reported receiving honoraria from Gilead Sciences.
DURBAN, SOUTH AFRICA – The rates of the AIDS-defining cancers Kaposi’s sarcoma and non-Hodgkin’s lymphoma have plummeted in the antiretroviral era, yet they are still the two top cancers in terms of cumulative incidence in HIV-infected patients by age 75, Benigno Rodriguez, MD, said at the 21st International AIDS Conference.
Lung cancer also remains a major concern in the HIV-infected population. Each of these three cancers carries about a 1 in 25 lifetime risk to age 75, the estimated lifespan of HIV-positive patients on combination antiretroviral therapy (ART), according to Dr. Rodriguez of Case Western Reserve University in Cleveland.
ART has resulted in a marked change in cancer trends among HIV-infected patients. The incidence of AIDS-defining cancers has decreased “massively,” Dr. Rodriguez observed; but because ART has extended the lifespan, patients are now living long enough to get other cancers. And they do so at a higher rate than that of the general population because of their impaired immune function, higher rate of risk factors such as smoking, and greater prevalence of oncogenic viral coinfections such as hepatitis B and C and Epstein-Barr virus.
The increase in the incidence and risk of colorectal, liver, and anal cancers among HIV-positive individuals since the introduction of combination ART can largely be explained by the population’s longer exposure to risk due to increasing survival, he said.
Dr. Rodriguez presented highlights of an analysis of cancer trends in North America during 1996-2009. The study was based on 86,620 HIV-infected persons with 475,660 person-years of follow-up and 196,987 subjects without HIV infection and with more than 1.8 million person-years of follow-up. Trends over time were assessed for nine cancers: Kaposi’s sarcoma, non-Hodgkin’s lymphoma, Hodgkin’s lymphoma, and melanoma, as well as anal, lung, colorectal, liver, and oropharyngeal cancers (Ann Intern Med. 2015;163:507-18).
Examining trends in three periods – 1996-1999, 2000-2004, and 2005-2009 – the investigators looked at the impact of ART over time on rates of AIDS-related and non–AIDS-related cancers in HIV-infected patients and compared them to results in the general HIV-uninfected population.

By conducting a competing risk analysis, Dr. Rodriguez and his coworkers were able to estimate the cumulative lifetime risk of the various cancers by age 75, a metric that provides readily understandable information for counseling HIV-infected patients about their long-term cancer risk.
The measure “is more intuitive than using incidence rates,” Dr. Rodriguez said. In a study of 1,578 HIV-infected patients who received the hepatitis B vaccine, for example, those patients whose immune function did not to respond to the vaccine were more likely to be among the 6% of patients who subsequently developed cancer during up to 20 years of follow-up.
The findings on cancer trends in the ART era have clinical implications for cancer screening and prevention in HIV-infected patients. The high rates of smoking and lung cancer in this population make HIV-positive smokers a logical target for lung cancer screening. The rising risk of colorectal cancer – the cumulative lifetime risk to age 75 was 0.4% in 1996-1999, 0.7% in 2000-2004, and 1.3% in 2005-2009 – suggests a need for increased colorectal cancer screening in the older HIV-positive population.
Early and sustained HIV suppression with combination ART remains the only known method of preventing AIDS-defining cancers. Dr. Rodriguez and his coinvestigators in the Centers for AIDS Research Network of Integrated Clinical Systems demonstrated the crucial role of suppressing HIV in a study of 6,036 HIV-infected patients who started on ART and were followed for more than 21,000 person-years. Compared with HIV-infected patients with a 3-month lagged HIV viremia of no more than 50 copies/mL, patients’ risk of developing non-Hodgkin’s lymphoma was 2.1-fold greater if their 3-month lagged HIV viremia was 51-500 copies/mL and 3.56-fold greater if it exceeded 500 copies/mL (Clin Infect Dis. 2014 Jun;58[11]:1599-606).
The study on cancer trends over time was funded by the National Institutes of Health. Dr. Rodriguez reported receiving honoraria from Gilead Sciences.
DURBAN, SOUTH AFRICA – The rates of the AIDS-defining cancers Kaposi’s sarcoma and non-Hodgkin’s lymphoma have plummeted in the antiretroviral era, yet they are still the two top cancers in terms of cumulative incidence in HIV-infected patients by age 75, Benigno Rodriguez, MD, said at the 21st International AIDS Conference.
Lung cancer also remains a major concern in the HIV-infected population. Each of these three cancers carries about a 1 in 25 lifetime risk to age 75, the estimated lifespan of HIV-positive patients on combination antiretroviral therapy (ART), according to Dr. Rodriguez of Case Western Reserve University in Cleveland.
ART has resulted in a marked change in cancer trends among HIV-infected patients. The incidence of AIDS-defining cancers has decreased “massively,” Dr. Rodriguez observed; but because ART has extended the lifespan, patients are now living long enough to get other cancers. And they do so at a higher rate than that of the general population because of their impaired immune function, higher rate of risk factors such as smoking, and greater prevalence of oncogenic viral coinfections such as hepatitis B and C and Epstein-Barr virus.
The increase in the incidence and risk of colorectal, liver, and anal cancers among HIV-positive individuals since the introduction of combination ART can largely be explained by the population’s longer exposure to risk due to increasing survival, he said.
Dr. Rodriguez presented highlights of an analysis of cancer trends in North America during 1996-2009. The study was based on 86,620 HIV-infected persons with 475,660 person-years of follow-up and 196,987 subjects without HIV infection and with more than 1.8 million person-years of follow-up. Trends over time were assessed for nine cancers: Kaposi’s sarcoma, non-Hodgkin’s lymphoma, Hodgkin’s lymphoma, and melanoma, as well as anal, lung, colorectal, liver, and oropharyngeal cancers (Ann Intern Med. 2015;163:507-18).
Examining trends in three periods – 1996-1999, 2000-2004, and 2005-2009 – the investigators looked at the impact of ART over time on rates of AIDS-related and non–AIDS-related cancers in HIV-infected patients and compared them to results in the general HIV-uninfected population.

By conducting a competing risk analysis, Dr. Rodriguez and his coworkers were able to estimate the cumulative lifetime risk of the various cancers by age 75, a metric that provides readily understandable information for counseling HIV-infected patients about their long-term cancer risk.
The measure “is more intuitive than using incidence rates,” Dr. Rodriguez said. In a study of 1,578 HIV-infected patients who received the hepatitis B vaccine, for example, those patients whose immune function did not to respond to the vaccine were more likely to be among the 6% of patients who subsequently developed cancer during up to 20 years of follow-up.
The findings on cancer trends in the ART era have clinical implications for cancer screening and prevention in HIV-infected patients. The high rates of smoking and lung cancer in this population make HIV-positive smokers a logical target for lung cancer screening. The rising risk of colorectal cancer – the cumulative lifetime risk to age 75 was 0.4% in 1996-1999, 0.7% in 2000-2004, and 1.3% in 2005-2009 – suggests a need for increased colorectal cancer screening in the older HIV-positive population.
Early and sustained HIV suppression with combination ART remains the only known method of preventing AIDS-defining cancers. Dr. Rodriguez and his coinvestigators in the Centers for AIDS Research Network of Integrated Clinical Systems demonstrated the crucial role of suppressing HIV in a study of 6,036 HIV-infected patients who started on ART and were followed for more than 21,000 person-years. Compared with HIV-infected patients with a 3-month lagged HIV viremia of no more than 50 copies/mL, patients’ risk of developing non-Hodgkin’s lymphoma was 2.1-fold greater if their 3-month lagged HIV viremia was 51-500 copies/mL and 3.56-fold greater if it exceeded 500 copies/mL (Clin Infect Dis. 2014 Jun;58[11]:1599-606).
The study on cancer trends over time was funded by the National Institutes of Health. Dr. Rodriguez reported receiving honoraria from Gilead Sciences.
AT AIDS 2016
Key clinical point: HIV-infected persons in North America have roughly a 1 in 25 cumulative lifetime risk of developing lung cancer, Kaposi’s sarcoma, or non-Hodgkin’s lymphoma.
Major finding: The cumulative lifetime risk of developing non-Hodgkin’s lymphoma in HIV-infected patients on combination antiretroviral therapy is sevenfold greater than the risk in the general population.
Data source: A competing risk analysis for nine cancers based upon 86,620 HIV-infected persons followed for 475,660 person-years and 196,987 subjects not infected with HIV and with more than 1.8 million person-years of follow-up.
Disclosures: The National Institutes of Health funded the study. The presenter reported receiving honoraria from Gilead Sciences.
Extreme alcohol use worsens HIV disease
DURBAN, SOUTH AFRICA – A large, longitudinal study of alcohol consumption patterns among HIV-infected U.S. military veterans indicates that only the highest level of persistent heavy drinking is associated with more advanced HIV disease severity over time.
In this study of 3,539 veterans receiving care for HIV infection for 15,354 person-years of follow-up at 8 VA centers, only those scoring in the top 8% on a validated measure of unhealthy drinking showed significant worsening of HIV disease over the 8-year study period, Brandon D.L. Marshall, PhD, reported at the 21st International AIDS Conference.
“The relationship between persistent unhealthy alcohol use and greater HIV disease severity is perhaps not as strong as we would have hypothesized. This suggests that, given the relatively small number of people reporting consistent unhealthy alcohol use, targeted risk reduction and treatment strategies are needed only in those consistent unhealthy drinkers,” said Dr. Marshall, an epidemiologist at Brown University in Providence, R.I.
The subjects’ median age was 49 years; 98% were men, and 68% were African American.
Alcohol use patterns were evaluated annually using the Alcohol Use Disorders Identification Test (AUDIT-C), a validated 3-question screening tool measuring self-reported frequency, quantity, and binge alcohol use. Alcohol use trajectories were linear and relatively stable over time. Eight percent of subjects were classified as high-risk drinkers on the basis of an AUDIT-C score of 8-12; 24% were deemed at moderate risk, with a score of 6-7; the 44% with a score of 4-5 were categorized as lower risk; and 24% of participants were abstainers. The abstainers fell into two distinct groups: sick quitters with worsening HIV disease and healthy abstainers.
Of note, this was the first large study to utilize an objective biomarker in order to validate long-term self-reported alcohol use patterns as assessed by the AUDIT-C test. Nearly 1,500 subjects had a blood test for phosphatidylethanol, a reliable indicator of exposure to alcohol within the previous 21 days. The biomarker has high specificity for alcohol abstinence and showed good correlation with AUDIT-C results across the board, according to Dr. Marshall.
Subjects’ HIV disease severity trajectory was determined annually using the Veterans Aging Cohort Study (VACS) Index, a weighted score that estimates an individual’s risk of all-cause mortality based upon age, HIV RNA viral load, CD4 count, and general indicators of organ system injury including hemoglobin, platelets, glomerular filtration rate, and hepatitis C infection. As was the case for AUDIT-C scores, VACS scores remained relatively stable over 8 years of follow-up. The HIV disease trajectory was categorized as low risk in 2% of subjects, moderate in 46%, high risk in 36%, and extreme in 16%.
To plot the joint trajectories of alcohol use and HIV disease severity, the investigators employed a statistical technique called group-based finite mixture modeling and performed a multivariate logistic regression analysis in which the moderate-risk drinkers and moderate VACS subgroups served as reference standards. Only two significant associations emerged: the highest-risk subgroup of drinkers were at 1.83-fold increased risk of extremely poor VACS trajectory, and the abstainers were at 1.9-fold increased risk for both the most favorable VACS trajectory and an extremely-high-mortality VACS trajectory, reflecting the split in prognosis between the healthy abstainer and sick quitter subgroups. No high-risk drinkers were in the low VACS group.
Unhealthy alcohol use is hypothesized to accelerate HIV disease progression through two mechanisms: Heavy drinkers are less likely to adhere to antiretroviral therapy and remain in care, and the heavy drinking itself has direct negative immunologic effects, Dr. Marshall said.
He reported having no financial conflicts of interest regarding his study, funded by the National Institute on Alcohol Abuse and Alcoholism and the National Institute of Allergy and Infectious Diseases.
DURBAN, SOUTH AFRICA – A large, longitudinal study of alcohol consumption patterns among HIV-infected U.S. military veterans indicates that only the highest level of persistent heavy drinking is associated with more advanced HIV disease severity over time.
In this study of 3,539 veterans receiving care for HIV infection for 15,354 person-years of follow-up at 8 VA centers, only those scoring in the top 8% on a validated measure of unhealthy drinking showed significant worsening of HIV disease over the 8-year study period, Brandon D.L. Marshall, PhD, reported at the 21st International AIDS Conference.
“The relationship between persistent unhealthy alcohol use and greater HIV disease severity is perhaps not as strong as we would have hypothesized. This suggests that, given the relatively small number of people reporting consistent unhealthy alcohol use, targeted risk reduction and treatment strategies are needed only in those consistent unhealthy drinkers,” said Dr. Marshall, an epidemiologist at Brown University in Providence, R.I.
The subjects’ median age was 49 years; 98% were men, and 68% were African American.
Alcohol use patterns were evaluated annually using the Alcohol Use Disorders Identification Test (AUDIT-C), a validated 3-question screening tool measuring self-reported frequency, quantity, and binge alcohol use. Alcohol use trajectories were linear and relatively stable over time. Eight percent of subjects were classified as high-risk drinkers on the basis of an AUDIT-C score of 8-12; 24% were deemed at moderate risk, with a score of 6-7; the 44% with a score of 4-5 were categorized as lower risk; and 24% of participants were abstainers. The abstainers fell into two distinct groups: sick quitters with worsening HIV disease and healthy abstainers.
Of note, this was the first large study to utilize an objective biomarker in order to validate long-term self-reported alcohol use patterns as assessed by the AUDIT-C test. Nearly 1,500 subjects had a blood test for phosphatidylethanol, a reliable indicator of exposure to alcohol within the previous 21 days. The biomarker has high specificity for alcohol abstinence and showed good correlation with AUDIT-C results across the board, according to Dr. Marshall.
Subjects’ HIV disease severity trajectory was determined annually using the Veterans Aging Cohort Study (VACS) Index, a weighted score that estimates an individual’s risk of all-cause mortality based upon age, HIV RNA viral load, CD4 count, and general indicators of organ system injury including hemoglobin, platelets, glomerular filtration rate, and hepatitis C infection. As was the case for AUDIT-C scores, VACS scores remained relatively stable over 8 years of follow-up. The HIV disease trajectory was categorized as low risk in 2% of subjects, moderate in 46%, high risk in 36%, and extreme in 16%.
To plot the joint trajectories of alcohol use and HIV disease severity, the investigators employed a statistical technique called group-based finite mixture modeling and performed a multivariate logistic regression analysis in which the moderate-risk drinkers and moderate VACS subgroups served as reference standards. Only two significant associations emerged: the highest-risk subgroup of drinkers were at 1.83-fold increased risk of extremely poor VACS trajectory, and the abstainers were at 1.9-fold increased risk for both the most favorable VACS trajectory and an extremely-high-mortality VACS trajectory, reflecting the split in prognosis between the healthy abstainer and sick quitter subgroups. No high-risk drinkers were in the low VACS group.
Unhealthy alcohol use is hypothesized to accelerate HIV disease progression through two mechanisms: Heavy drinkers are less likely to adhere to antiretroviral therapy and remain in care, and the heavy drinking itself has direct negative immunologic effects, Dr. Marshall said.
He reported having no financial conflicts of interest regarding his study, funded by the National Institute on Alcohol Abuse and Alcoholism and the National Institute of Allergy and Infectious Diseases.
DURBAN, SOUTH AFRICA – A large, longitudinal study of alcohol consumption patterns among HIV-infected U.S. military veterans indicates that only the highest level of persistent heavy drinking is associated with more advanced HIV disease severity over time.
In this study of 3,539 veterans receiving care for HIV infection for 15,354 person-years of follow-up at 8 VA centers, only those scoring in the top 8% on a validated measure of unhealthy drinking showed significant worsening of HIV disease over the 8-year study period, Brandon D.L. Marshall, PhD, reported at the 21st International AIDS Conference.
“The relationship between persistent unhealthy alcohol use and greater HIV disease severity is perhaps not as strong as we would have hypothesized. This suggests that, given the relatively small number of people reporting consistent unhealthy alcohol use, targeted risk reduction and treatment strategies are needed only in those consistent unhealthy drinkers,” said Dr. Marshall, an epidemiologist at Brown University in Providence, R.I.
The subjects’ median age was 49 years; 98% were men, and 68% were African American.
Alcohol use patterns were evaluated annually using the Alcohol Use Disorders Identification Test (AUDIT-C), a validated 3-question screening tool measuring self-reported frequency, quantity, and binge alcohol use. Alcohol use trajectories were linear and relatively stable over time. Eight percent of subjects were classified as high-risk drinkers on the basis of an AUDIT-C score of 8-12; 24% were deemed at moderate risk, with a score of 6-7; the 44% with a score of 4-5 were categorized as lower risk; and 24% of participants were abstainers. The abstainers fell into two distinct groups: sick quitters with worsening HIV disease and healthy abstainers.
Of note, this was the first large study to utilize an objective biomarker in order to validate long-term self-reported alcohol use patterns as assessed by the AUDIT-C test. Nearly 1,500 subjects had a blood test for phosphatidylethanol, a reliable indicator of exposure to alcohol within the previous 21 days. The biomarker has high specificity for alcohol abstinence and showed good correlation with AUDIT-C results across the board, according to Dr. Marshall.
Subjects’ HIV disease severity trajectory was determined annually using the Veterans Aging Cohort Study (VACS) Index, a weighted score that estimates an individual’s risk of all-cause mortality based upon age, HIV RNA viral load, CD4 count, and general indicators of organ system injury including hemoglobin, platelets, glomerular filtration rate, and hepatitis C infection. As was the case for AUDIT-C scores, VACS scores remained relatively stable over 8 years of follow-up. The HIV disease trajectory was categorized as low risk in 2% of subjects, moderate in 46%, high risk in 36%, and extreme in 16%.
To plot the joint trajectories of alcohol use and HIV disease severity, the investigators employed a statistical technique called group-based finite mixture modeling and performed a multivariate logistic regression analysis in which the moderate-risk drinkers and moderate VACS subgroups served as reference standards. Only two significant associations emerged: the highest-risk subgroup of drinkers were at 1.83-fold increased risk of extremely poor VACS trajectory, and the abstainers were at 1.9-fold increased risk for both the most favorable VACS trajectory and an extremely-high-mortality VACS trajectory, reflecting the split in prognosis between the healthy abstainer and sick quitter subgroups. No high-risk drinkers were in the low VACS group.
Unhealthy alcohol use is hypothesized to accelerate HIV disease progression through two mechanisms: Heavy drinkers are less likely to adhere to antiretroviral therapy and remain in care, and the heavy drinking itself has direct negative immunologic effects, Dr. Marshall said.
He reported having no financial conflicts of interest regarding his study, funded by the National Institute on Alcohol Abuse and Alcoholism and the National Institute of Allergy and Infectious Diseases.
AT AIDS 2016
Key clinical point: A pattern of heavy alcohol use over time in HIV-infected patients was associated with accelerated HIV disease progression.
Major finding: Long-term heavy alcohol use by middle-aged, HIV-infected military veterans was associated with a 1.83-fold increased likelihood of also being in the highest-risk group for accelerated progression of HIV disease.
Data source: This study included 3,539 U.S. military veterans receiving care for HIV infection at eight VA centers. The impact of their long-term pattern of alcohol use on HIV disease progression was assessed over an 8-year period by annual assessments using validated instruments.
Disclosures: The presenter reported having no financial conflicts of interest regarding the study, funded by the National Institute on Alcohol Abuse and Alcoholism and the National Institute of Allergy and Infectious Diseases.
Antiretroviral efavirenz linked to increased suicidality
DURBAN, SOUTH AFRICA – The use of efavirenz in HIV-infected participants in the landmark START trial was associated with significantly increased risk of suicidal behavior, Alejandro Arenas-Pinto, MD, reported at the 21st International AIDS Conference.
“The impact of efavirenz exposure was particularly high in those with a prior psychiatric diagnosis,” according to the infectious diseases specialist. “The message here is that it’s probably just those patients with preexisting neuropsychiatric conditions who are at higher risk of suicidal behavior, according to our data. This supports a recommendation to screen for preexisting depression and other neuropsychiatric conditions before efavirenz initiation.”
START was a clinical practice-transforming randomized trial that established a clear clinical benefit for a strategy of initiating antiretroviral therapy immediately upon diagnosis of HIV infection in asymptomatic adults instead of waiting until their CD4+ count drops below the level of 350 cells/mm3 (N Engl J Med. 2015 Aug 27;373[9]:795-807). Immediate antiretroviral therapy (ART) was associated with a 57% reduction in the risk of the primary outcome, a composite comprised of serious AIDS-defining events, serious non-AIDS adverse events, and all-cause mortality.
Once the results were in, however, Dr. Arenas-Pinto and the other START investigators quickly noticed that suicidal behavior was the second-most-common serious non-AIDS event observed in the study. A total of 57 patients were affected, for a rate of 0.36 events per 100 person-years of follow-up. There were 30 suicide attempts, 16 cases of suicidal ideation, 3 completed suicides, and 1 case each classified as intentional self-injury or self-injurious ideation. A closer look was warranted, said Dr. Arenas-Pinto of University College London.
The investigators next determined that the risk of suicidal behavior was significantly higher in the 3,516 START participants on efavirenz than in the 1,169 subjects on other ART drugs. The risk was 4.2-fold greater in patients who started on efavirenz immediately than in those randomized to begin the drug on a deferred basis. In contrast, suicidal behavior was no more frequent in patients who started on other ART medications immediately than if treatment was deferred.
In a multivariate Cox proportional hazards analysis of predictors of suicidal behavior in patients on efavirenz, the stand-out predictor was prior diagnosis of major depression, bipolar disorder, or schizophrenia or another psychotic disorder, with an associated 12.8-fold increased risk. Heavy alcohol use was associated with a 6.1-fold increased risk and ever having used recreational drugs conferred a 2.9-fold increased risk. In contrast, the risk of suicidal behavior in patients on efavirenz dropped sharply with advancing age.
Efavirenz is a non-nucleoside transcriptase inhibitor marketed as Sustiva, or when incorporated into once-daily, fixed-dose, triple-drug therapy with tenofovir/emtricitabine, as Atripla.
The labeling for efavirenz states that many of the drug’s more common side effects involve the brain. They include dizziness, insomnia, depression, anxiety, nightmares, hallucinations, delusions, and confusion.
The START study was funded by the National Institutes of Health. Dr. Arenas-Pinto reported having no financial conflicts of interest.
DURBAN, SOUTH AFRICA – The use of efavirenz in HIV-infected participants in the landmark START trial was associated with significantly increased risk of suicidal behavior, Alejandro Arenas-Pinto, MD, reported at the 21st International AIDS Conference.
“The impact of efavirenz exposure was particularly high in those with a prior psychiatric diagnosis,” according to the infectious diseases specialist. “The message here is that it’s probably just those patients with preexisting neuropsychiatric conditions who are at higher risk of suicidal behavior, according to our data. This supports a recommendation to screen for preexisting depression and other neuropsychiatric conditions before efavirenz initiation.”
START was a clinical practice-transforming randomized trial that established a clear clinical benefit for a strategy of initiating antiretroviral therapy immediately upon diagnosis of HIV infection in asymptomatic adults instead of waiting until their CD4+ count drops below the level of 350 cells/mm3 (N Engl J Med. 2015 Aug 27;373[9]:795-807). Immediate antiretroviral therapy (ART) was associated with a 57% reduction in the risk of the primary outcome, a composite comprised of serious AIDS-defining events, serious non-AIDS adverse events, and all-cause mortality.
Once the results were in, however, Dr. Arenas-Pinto and the other START investigators quickly noticed that suicidal behavior was the second-most-common serious non-AIDS event observed in the study. A total of 57 patients were affected, for a rate of 0.36 events per 100 person-years of follow-up. There were 30 suicide attempts, 16 cases of suicidal ideation, 3 completed suicides, and 1 case each classified as intentional self-injury or self-injurious ideation. A closer look was warranted, said Dr. Arenas-Pinto of University College London.
The investigators next determined that the risk of suicidal behavior was significantly higher in the 3,516 START participants on efavirenz than in the 1,169 subjects on other ART drugs. The risk was 4.2-fold greater in patients who started on efavirenz immediately than in those randomized to begin the drug on a deferred basis. In contrast, suicidal behavior was no more frequent in patients who started on other ART medications immediately than if treatment was deferred.
In a multivariate Cox proportional hazards analysis of predictors of suicidal behavior in patients on efavirenz, the stand-out predictor was prior diagnosis of major depression, bipolar disorder, or schizophrenia or another psychotic disorder, with an associated 12.8-fold increased risk. Heavy alcohol use was associated with a 6.1-fold increased risk and ever having used recreational drugs conferred a 2.9-fold increased risk. In contrast, the risk of suicidal behavior in patients on efavirenz dropped sharply with advancing age.
Efavirenz is a non-nucleoside transcriptase inhibitor marketed as Sustiva, or when incorporated into once-daily, fixed-dose, triple-drug therapy with tenofovir/emtricitabine, as Atripla.
The labeling for efavirenz states that many of the drug’s more common side effects involve the brain. They include dizziness, insomnia, depression, anxiety, nightmares, hallucinations, delusions, and confusion.
The START study was funded by the National Institutes of Health. Dr. Arenas-Pinto reported having no financial conflicts of interest.
DURBAN, SOUTH AFRICA – The use of efavirenz in HIV-infected participants in the landmark START trial was associated with significantly increased risk of suicidal behavior, Alejandro Arenas-Pinto, MD, reported at the 21st International AIDS Conference.
“The impact of efavirenz exposure was particularly high in those with a prior psychiatric diagnosis,” according to the infectious diseases specialist. “The message here is that it’s probably just those patients with preexisting neuropsychiatric conditions who are at higher risk of suicidal behavior, according to our data. This supports a recommendation to screen for preexisting depression and other neuropsychiatric conditions before efavirenz initiation.”
START was a clinical practice-transforming randomized trial that established a clear clinical benefit for a strategy of initiating antiretroviral therapy immediately upon diagnosis of HIV infection in asymptomatic adults instead of waiting until their CD4+ count drops below the level of 350 cells/mm3 (N Engl J Med. 2015 Aug 27;373[9]:795-807). Immediate antiretroviral therapy (ART) was associated with a 57% reduction in the risk of the primary outcome, a composite comprised of serious AIDS-defining events, serious non-AIDS adverse events, and all-cause mortality.
Once the results were in, however, Dr. Arenas-Pinto and the other START investigators quickly noticed that suicidal behavior was the second-most-common serious non-AIDS event observed in the study. A total of 57 patients were affected, for a rate of 0.36 events per 100 person-years of follow-up. There were 30 suicide attempts, 16 cases of suicidal ideation, 3 completed suicides, and 1 case each classified as intentional self-injury or self-injurious ideation. A closer look was warranted, said Dr. Arenas-Pinto of University College London.
The investigators next determined that the risk of suicidal behavior was significantly higher in the 3,516 START participants on efavirenz than in the 1,169 subjects on other ART drugs. The risk was 4.2-fold greater in patients who started on efavirenz immediately than in those randomized to begin the drug on a deferred basis. In contrast, suicidal behavior was no more frequent in patients who started on other ART medications immediately than if treatment was deferred.
In a multivariate Cox proportional hazards analysis of predictors of suicidal behavior in patients on efavirenz, the stand-out predictor was prior diagnosis of major depression, bipolar disorder, or schizophrenia or another psychotic disorder, with an associated 12.8-fold increased risk. Heavy alcohol use was associated with a 6.1-fold increased risk and ever having used recreational drugs conferred a 2.9-fold increased risk. In contrast, the risk of suicidal behavior in patients on efavirenz dropped sharply with advancing age.
Efavirenz is a non-nucleoside transcriptase inhibitor marketed as Sustiva, or when incorporated into once-daily, fixed-dose, triple-drug therapy with tenofovir/emtricitabine, as Atripla.
The labeling for efavirenz states that many of the drug’s more common side effects involve the brain. They include dizziness, insomnia, depression, anxiety, nightmares, hallucinations, delusions, and confusion.
The START study was funded by the National Institutes of Health. Dr. Arenas-Pinto reported having no financial conflicts of interest.
AT AIDS 2016
Key clinical point: Screen for depression and other psychiatric disorders before placing an HIV-positive patient on efavirenz.
Major finding: The risk of suicidal behavior was increased 12.8-fold in HIV-infected patients on efavirenz who had a prior psychiatric diagnosis.
Data source: This was a secondary analysis of suicidal behavior in 4,685 HIV-infected participants in the randomized START trial.
Disclosures: The National Institutes of Health sponsored the study. The presenter reported having no financial conflicts of interest.
Intensified rifampicin boosts outcomes in TB/HIV coinfection
DURBAN, SOUTH AFRICA – Prescribing high-dose rifampicin in addition to antiretroviral therapy reduces 12-month all-cause mortality in patients who are coinfected with tuberculosis and HIV and who have a low CD4 cell count, Corinne S. Merle, MD, reported at the 21st International AIDS Conference.
“Current strategies to reduce TB/HIV mortality rely largely on optimal management of HIV disease with early ART [antiretroviral therapy]. We wanted to look at whether there is value in focusing on the TB side of the problem. This is the first study to look at more intensive TB therapy for reducing mortality; and we think that, at least in patients who are immunosuppressed, there might be some benefit in a more aggressive TB treatment from the start,” said Dr. Merle of the London School of Hygiene and Tropical Medicine.
She presented the results of the open-label, multicenter trial of 747 ART-naive adults from West Africa. All were coinfected with TB/HIV and had a CD4 count of at least 50 cells/mm3 at enrollment. They were randomized to one of three treatment arms: ART starting at 2 weeks combined with standard TB treatment; ART starting at 8 weeks plus standard TB therapy; or ART initiation at 8 weeks coupled with 2 months of high-dose rifampicin (Rifadin) at 15 mg/kg daily, followed by standard TB therapy. None of the participants had multidrug-resistant TB. More than one-quarter of them were undernourished as evidenced by a baseline body mass index below 16 kg/m2.
The primary outcome was all-cause mortality at 12 months. There was no significant difference between the study arms, with a 10% rate in the intensified TB treatment arm and mortality rates of 11% and 14% with standard TB therapy and ART starting after 2 and 8 weeks, respectively.
However, a prespecified secondary analysis restricted to the 159 subjects with a baseline CD4 count below 100 cells/mm3 struck gold. Overall 12-month mortality was 4% in the intensified TB treatment subgroup, compared with 19% in patients on standard TB therapy with ART starting at 2 weeks and 28% with ART starting at 8 weeks. In a Cox regression analysis, severely immunosuppressed patients in the high-dose rifampicin group were an adjusted 88% less likely to die within 12 months than those on standard TB treatment with ART starting at 8 weeks and 80% less likely to die than those starting ART at 2 weeks.
At 18 months after randomization, roughly three-quarters of patients in each study arm had undetectable HIV viral loads.
There was no evidence of an increased risk of hepatotoxicity with 2 months of high-dose rifampicin. Only 4 of nearly 3,800 aspartate aminotransferase measurements obtained during the trial showed grade 3 or 4 hepatotoxicity, Dr. Merle noted.
In a plenary lecture on TB/HIV coinfection at the AIDS 2016 conference, Anton Pozniak, MD, singled out the trial as a sterling example of how to optimize available clinical management tools while awaiting a desperately needed new TB vaccine and better drugs.
More than 1 million new TB cases occur annually in HIV-infected persons, roughly 80% of them in sub-Saharan Africa. There are now 400,000 deaths per year worldwide in coinfected TB/HIV patients. Indeed, TB has become the No. 1 cause of death among people living with HIV infection, said Dr. Pozniak, director of HIV services at Chelsea and Westminster Hospital in London.
He offered a road map to eliminating TB by the year 2035. At present, the global trend is a 2% per year decline in new cases. Optimizing TB case finding, treatment, and preventive therapy could achieve a 10% per year decrease in new cases. That rate still wouldn’t reach the goal by 2035. But more than a dozen candidate TB vaccines are in the developmental pipeline, including a mycobacterial whole cell extract in phase III testing in China. If a new vaccine can be introduced by 2025, that would be a game changer.
“A new vaccine that could prevent adolescents and adults from developing and transmitting TB would be the single most cost-effective tool in mitigating the epidemic,” he said. “Even if we had only a 60% efficacious vaccine and delivered it to 20% of the target population, it could potentially avert 30-50 million incident cases of TB by 2050.”
A new vaccine plus effective alternatives to the standard 6 months of isoniazid for latency prophylaxis by 2025 are estimated to reduce new cases of TB by an average of 17% per year. That circumstance would mean the end of TB by 2035, Dr. Pozniak declared.
The trial was funded by the European and Developing Countries Clinical Trials Partnership. Dr. Merle reported having no financial conflicts of interest.
DURBAN, SOUTH AFRICA – Prescribing high-dose rifampicin in addition to antiretroviral therapy reduces 12-month all-cause mortality in patients who are coinfected with tuberculosis and HIV and who have a low CD4 cell count, Corinne S. Merle, MD, reported at the 21st International AIDS Conference.
“Current strategies to reduce TB/HIV mortality rely largely on optimal management of HIV disease with early ART [antiretroviral therapy]. We wanted to look at whether there is value in focusing on the TB side of the problem. This is the first study to look at more intensive TB therapy for reducing mortality; and we think that, at least in patients who are immunosuppressed, there might be some benefit in a more aggressive TB treatment from the start,” said Dr. Merle of the London School of Hygiene and Tropical Medicine.
She presented the results of the open-label, multicenter trial of 747 ART-naive adults from West Africa. All were coinfected with TB/HIV and had a CD4 count of at least 50 cells/mm3 at enrollment. They were randomized to one of three treatment arms: ART starting at 2 weeks combined with standard TB treatment; ART starting at 8 weeks plus standard TB therapy; or ART initiation at 8 weeks coupled with 2 months of high-dose rifampicin (Rifadin) at 15 mg/kg daily, followed by standard TB therapy. None of the participants had multidrug-resistant TB. More than one-quarter of them were undernourished as evidenced by a baseline body mass index below 16 kg/m2.
The primary outcome was all-cause mortality at 12 months. There was no significant difference between the study arms, with a 10% rate in the intensified TB treatment arm and mortality rates of 11% and 14% with standard TB therapy and ART starting after 2 and 8 weeks, respectively.
However, a prespecified secondary analysis restricted to the 159 subjects with a baseline CD4 count below 100 cells/mm3 struck gold. Overall 12-month mortality was 4% in the intensified TB treatment subgroup, compared with 19% in patients on standard TB therapy with ART starting at 2 weeks and 28% with ART starting at 8 weeks. In a Cox regression analysis, severely immunosuppressed patients in the high-dose rifampicin group were an adjusted 88% less likely to die within 12 months than those on standard TB treatment with ART starting at 8 weeks and 80% less likely to die than those starting ART at 2 weeks.
At 18 months after randomization, roughly three-quarters of patients in each study arm had undetectable HIV viral loads.
There was no evidence of an increased risk of hepatotoxicity with 2 months of high-dose rifampicin. Only 4 of nearly 3,800 aspartate aminotransferase measurements obtained during the trial showed grade 3 or 4 hepatotoxicity, Dr. Merle noted.
In a plenary lecture on TB/HIV coinfection at the AIDS 2016 conference, Anton Pozniak, MD, singled out the trial as a sterling example of how to optimize available clinical management tools while awaiting a desperately needed new TB vaccine and better drugs.
More than 1 million new TB cases occur annually in HIV-infected persons, roughly 80% of them in sub-Saharan Africa. There are now 400,000 deaths per year worldwide in coinfected TB/HIV patients. Indeed, TB has become the No. 1 cause of death among people living with HIV infection, said Dr. Pozniak, director of HIV services at Chelsea and Westminster Hospital in London.
He offered a road map to eliminating TB by the year 2035. At present, the global trend is a 2% per year decline in new cases. Optimizing TB case finding, treatment, and preventive therapy could achieve a 10% per year decrease in new cases. That rate still wouldn’t reach the goal by 2035. But more than a dozen candidate TB vaccines are in the developmental pipeline, including a mycobacterial whole cell extract in phase III testing in China. If a new vaccine can be introduced by 2025, that would be a game changer.
“A new vaccine that could prevent adolescents and adults from developing and transmitting TB would be the single most cost-effective tool in mitigating the epidemic,” he said. “Even if we had only a 60% efficacious vaccine and delivered it to 20% of the target population, it could potentially avert 30-50 million incident cases of TB by 2050.”
A new vaccine plus effective alternatives to the standard 6 months of isoniazid for latency prophylaxis by 2025 are estimated to reduce new cases of TB by an average of 17% per year. That circumstance would mean the end of TB by 2035, Dr. Pozniak declared.
The trial was funded by the European and Developing Countries Clinical Trials Partnership. Dr. Merle reported having no financial conflicts of interest.
DURBAN, SOUTH AFRICA – Prescribing high-dose rifampicin in addition to antiretroviral therapy reduces 12-month all-cause mortality in patients who are coinfected with tuberculosis and HIV and who have a low CD4 cell count, Corinne S. Merle, MD, reported at the 21st International AIDS Conference.
“Current strategies to reduce TB/HIV mortality rely largely on optimal management of HIV disease with early ART [antiretroviral therapy]. We wanted to look at whether there is value in focusing on the TB side of the problem. This is the first study to look at more intensive TB therapy for reducing mortality; and we think that, at least in patients who are immunosuppressed, there might be some benefit in a more aggressive TB treatment from the start,” said Dr. Merle of the London School of Hygiene and Tropical Medicine.
She presented the results of the open-label, multicenter trial of 747 ART-naive adults from West Africa. All were coinfected with TB/HIV and had a CD4 count of at least 50 cells/mm3 at enrollment. They were randomized to one of three treatment arms: ART starting at 2 weeks combined with standard TB treatment; ART starting at 8 weeks plus standard TB therapy; or ART initiation at 8 weeks coupled with 2 months of high-dose rifampicin (Rifadin) at 15 mg/kg daily, followed by standard TB therapy. None of the participants had multidrug-resistant TB. More than one-quarter of them were undernourished as evidenced by a baseline body mass index below 16 kg/m2.
The primary outcome was all-cause mortality at 12 months. There was no significant difference between the study arms, with a 10% rate in the intensified TB treatment arm and mortality rates of 11% and 14% with standard TB therapy and ART starting after 2 and 8 weeks, respectively.
However, a prespecified secondary analysis restricted to the 159 subjects with a baseline CD4 count below 100 cells/mm3 struck gold. Overall 12-month mortality was 4% in the intensified TB treatment subgroup, compared with 19% in patients on standard TB therapy with ART starting at 2 weeks and 28% with ART starting at 8 weeks. In a Cox regression analysis, severely immunosuppressed patients in the high-dose rifampicin group were an adjusted 88% less likely to die within 12 months than those on standard TB treatment with ART starting at 8 weeks and 80% less likely to die than those starting ART at 2 weeks.
At 18 months after randomization, roughly three-quarters of patients in each study arm had undetectable HIV viral loads.
There was no evidence of an increased risk of hepatotoxicity with 2 months of high-dose rifampicin. Only 4 of nearly 3,800 aspartate aminotransferase measurements obtained during the trial showed grade 3 or 4 hepatotoxicity, Dr. Merle noted.
In a plenary lecture on TB/HIV coinfection at the AIDS 2016 conference, Anton Pozniak, MD, singled out the trial as a sterling example of how to optimize available clinical management tools while awaiting a desperately needed new TB vaccine and better drugs.
More than 1 million new TB cases occur annually in HIV-infected persons, roughly 80% of them in sub-Saharan Africa. There are now 400,000 deaths per year worldwide in coinfected TB/HIV patients. Indeed, TB has become the No. 1 cause of death among people living with HIV infection, said Dr. Pozniak, director of HIV services at Chelsea and Westminster Hospital in London.
He offered a road map to eliminating TB by the year 2035. At present, the global trend is a 2% per year decline in new cases. Optimizing TB case finding, treatment, and preventive therapy could achieve a 10% per year decrease in new cases. That rate still wouldn’t reach the goal by 2035. But more than a dozen candidate TB vaccines are in the developmental pipeline, including a mycobacterial whole cell extract in phase III testing in China. If a new vaccine can be introduced by 2025, that would be a game changer.
“A new vaccine that could prevent adolescents and adults from developing and transmitting TB would be the single most cost-effective tool in mitigating the epidemic,” he said. “Even if we had only a 60% efficacious vaccine and delivered it to 20% of the target population, it could potentially avert 30-50 million incident cases of TB by 2050.”
A new vaccine plus effective alternatives to the standard 6 months of isoniazid for latency prophylaxis by 2025 are estimated to reduce new cases of TB by an average of 17% per year. That circumstance would mean the end of TB by 2035, Dr. Pozniak declared.
The trial was funded by the European and Developing Countries Clinical Trials Partnership. Dr. Merle reported having no financial conflicts of interest.
AT AIDS 2016
Key clinical point: High-dose rifampicin improves survival in patients who are coinfected with tuberculosis and HIV and have low CD4 counts.
Major finding: Overall 12-month mortality was 4% in the intensified TB treatment subgroup, 19% in patients on standard TB therapy with ART starting at 2 weeks, and 28% with standard TB therapy and ART starting at 8 weeks.
Data source: This was a randomized, prospective, three-arm, open-label trial including 747 patients coinfected with tuberculosis and HIV.
Disclosures: The trial was funded by the European and Developing Countries Clinical Trials Partnership. The presenter reported having no financial conflicts of interest.
HIV-related lymphoma rate remains sky-high despite ART
DURBAN, SOUTH AFRICA – The good news about non-Hodgkin lymphoma in the setting of HIV infection is that the risk drops dramatically after several years of antiretroviral therapy. The bad news? The risk still remains extraordinarily high, compared with the risk seen in the general population, Mathias Egger, MD, reported at the 21st International AIDS Conference.
That was a key finding in a new analysis of lymphoma trends in more than 210,000 HIV-positive adults on combination antiretroviral therapy (ART) during more than 1.1 million person-years of follow-up in North America, Europe, Latin America, and South Africa.
The non-Hodgkin lymphoma (NHL) incidence rate standardized to 40 years of age was 287 cases per 100,000 person-years. From a pre-ART baseline of about 500 cases per 100,000 person-years, it dropped “massively” within a year after going on ART. Even after 5 years of ART, however, the rate remained in the range of 60-200 cases per 100,000 person-years, depending upon geographic location and HIV transmission route. In contrast, the incidence rate among the general population of the U.S. and Canada, which is among the world’s highest, is less than 10 per 100,000 person-years, according to Dr. Egger, professor of epidemiology and public health at the University of Bern, Switzerland.
The risk of developing NHL in the setting of HIV infection varied by continent. It was slightly higher in HIV-infected patients in North America than in Europe or South Africa, although the South African data are considered unreliable due to underascertainment of cancers.
In Latin American HIV-infected adults the NHL rate was lowest of all, fully 54% lower than in Europe after adjustment for current CD4 cell count, ART regimen and duration, and transmission risk group. The low NHL rate in Latin America was driven by a very low risk in HIV-infected women.
Across the world, NHL rates in patients on ART were consistently lowest in women, intermediate in heterosexual men, and highest in men who have sex with men.
The explanation for the regional variation in NHL trends might plausibly involve differing prevalences of Epstein-Barr virus–2 and other oncogenic viruses as well as differences in the completeness of cancer ascertainment, Dr. Egger said.
While NHL is categorized as an AIDS-defining condition, Hodgkin lymphoma is not. Nonetheless, the risk of Hodgkin lymphoma is markedly increased in the setting of HIV infection. In one classic meta-analysis, it was increased by 11-fold, compared with that seen in the general population (Lancet. 2007 Jul 7;370(9581):59-67).
In a study of more than 41,000 HIV-infected European adults by Dr. Egger and his coworkers, the incidence of Hodgkin lymphoma was 49 cases per 100,000 person-years. Importantly, unlike in NHL, the cumulative incidence and mortality of Hodgkin lymphoma were unaffected by ART (Blood. 2011 Jun 9;117(23):6100-8).
The clinical implication of these trends in HIV-related lymphomas is clear: with more than 2 million new cases of HIV infection occurring annually worldwide, and with infected patients living far longer as ART transforms HIV infection into a chronic manageable condition, physicians can anticipate encountering a steadily growing number of patients with NHL and Hodgkin lymphoma, he said.
The NHL study was funded by the European Union and the U.S. National Institutes of Health. The analysis utilized data collected by the Collaboration of Observational HIV Epidemiology and Research Europe (COHERE) and the International Epidemiologic Databases to Evaluate AIDS (IeDEA). Dr. Egger reported having no financial conflicts of interest.
DURBAN, SOUTH AFRICA – The good news about non-Hodgkin lymphoma in the setting of HIV infection is that the risk drops dramatically after several years of antiretroviral therapy. The bad news? The risk still remains extraordinarily high, compared with the risk seen in the general population, Mathias Egger, MD, reported at the 21st International AIDS Conference.
That was a key finding in a new analysis of lymphoma trends in more than 210,000 HIV-positive adults on combination antiretroviral therapy (ART) during more than 1.1 million person-years of follow-up in North America, Europe, Latin America, and South Africa.
The non-Hodgkin lymphoma (NHL) incidence rate standardized to 40 years of age was 287 cases per 100,000 person-years. From a pre-ART baseline of about 500 cases per 100,000 person-years, it dropped “massively” within a year after going on ART. Even after 5 years of ART, however, the rate remained in the range of 60-200 cases per 100,000 person-years, depending upon geographic location and HIV transmission route. In contrast, the incidence rate among the general population of the U.S. and Canada, which is among the world’s highest, is less than 10 per 100,000 person-years, according to Dr. Egger, professor of epidemiology and public health at the University of Bern, Switzerland.
The risk of developing NHL in the setting of HIV infection varied by continent. It was slightly higher in HIV-infected patients in North America than in Europe or South Africa, although the South African data are considered unreliable due to underascertainment of cancers.
In Latin American HIV-infected adults the NHL rate was lowest of all, fully 54% lower than in Europe after adjustment for current CD4 cell count, ART regimen and duration, and transmission risk group. The low NHL rate in Latin America was driven by a very low risk in HIV-infected women.
Across the world, NHL rates in patients on ART were consistently lowest in women, intermediate in heterosexual men, and highest in men who have sex with men.
The explanation for the regional variation in NHL trends might plausibly involve differing prevalences of Epstein-Barr virus–2 and other oncogenic viruses as well as differences in the completeness of cancer ascertainment, Dr. Egger said.
While NHL is categorized as an AIDS-defining condition, Hodgkin lymphoma is not. Nonetheless, the risk of Hodgkin lymphoma is markedly increased in the setting of HIV infection. In one classic meta-analysis, it was increased by 11-fold, compared with that seen in the general population (Lancet. 2007 Jul 7;370(9581):59-67).
In a study of more than 41,000 HIV-infected European adults by Dr. Egger and his coworkers, the incidence of Hodgkin lymphoma was 49 cases per 100,000 person-years. Importantly, unlike in NHL, the cumulative incidence and mortality of Hodgkin lymphoma were unaffected by ART (Blood. 2011 Jun 9;117(23):6100-8).
The clinical implication of these trends in HIV-related lymphomas is clear: with more than 2 million new cases of HIV infection occurring annually worldwide, and with infected patients living far longer as ART transforms HIV infection into a chronic manageable condition, physicians can anticipate encountering a steadily growing number of patients with NHL and Hodgkin lymphoma, he said.
The NHL study was funded by the European Union and the U.S. National Institutes of Health. The analysis utilized data collected by the Collaboration of Observational HIV Epidemiology and Research Europe (COHERE) and the International Epidemiologic Databases to Evaluate AIDS (IeDEA). Dr. Egger reported having no financial conflicts of interest.
DURBAN, SOUTH AFRICA – The good news about non-Hodgkin lymphoma in the setting of HIV infection is that the risk drops dramatically after several years of antiretroviral therapy. The bad news? The risk still remains extraordinarily high, compared with the risk seen in the general population, Mathias Egger, MD, reported at the 21st International AIDS Conference.
That was a key finding in a new analysis of lymphoma trends in more than 210,000 HIV-positive adults on combination antiretroviral therapy (ART) during more than 1.1 million person-years of follow-up in North America, Europe, Latin America, and South Africa.
The non-Hodgkin lymphoma (NHL) incidence rate standardized to 40 years of age was 287 cases per 100,000 person-years. From a pre-ART baseline of about 500 cases per 100,000 person-years, it dropped “massively” within a year after going on ART. Even after 5 years of ART, however, the rate remained in the range of 60-200 cases per 100,000 person-years, depending upon geographic location and HIV transmission route. In contrast, the incidence rate among the general population of the U.S. and Canada, which is among the world’s highest, is less than 10 per 100,000 person-years, according to Dr. Egger, professor of epidemiology and public health at the University of Bern, Switzerland.
The risk of developing NHL in the setting of HIV infection varied by continent. It was slightly higher in HIV-infected patients in North America than in Europe or South Africa, although the South African data are considered unreliable due to underascertainment of cancers.
In Latin American HIV-infected adults the NHL rate was lowest of all, fully 54% lower than in Europe after adjustment for current CD4 cell count, ART regimen and duration, and transmission risk group. The low NHL rate in Latin America was driven by a very low risk in HIV-infected women.
Across the world, NHL rates in patients on ART were consistently lowest in women, intermediate in heterosexual men, and highest in men who have sex with men.
The explanation for the regional variation in NHL trends might plausibly involve differing prevalences of Epstein-Barr virus–2 and other oncogenic viruses as well as differences in the completeness of cancer ascertainment, Dr. Egger said.
While NHL is categorized as an AIDS-defining condition, Hodgkin lymphoma is not. Nonetheless, the risk of Hodgkin lymphoma is markedly increased in the setting of HIV infection. In one classic meta-analysis, it was increased by 11-fold, compared with that seen in the general population (Lancet. 2007 Jul 7;370(9581):59-67).
In a study of more than 41,000 HIV-infected European adults by Dr. Egger and his coworkers, the incidence of Hodgkin lymphoma was 49 cases per 100,000 person-years. Importantly, unlike in NHL, the cumulative incidence and mortality of Hodgkin lymphoma were unaffected by ART (Blood. 2011 Jun 9;117(23):6100-8).
The clinical implication of these trends in HIV-related lymphomas is clear: with more than 2 million new cases of HIV infection occurring annually worldwide, and with infected patients living far longer as ART transforms HIV infection into a chronic manageable condition, physicians can anticipate encountering a steadily growing number of patients with NHL and Hodgkin lymphoma, he said.
The NHL study was funded by the European Union and the U.S. National Institutes of Health. The analysis utilized data collected by the Collaboration of Observational HIV Epidemiology and Research Europe (COHERE) and the International Epidemiologic Databases to Evaluate AIDS (IeDEA). Dr. Egger reported having no financial conflicts of interest.
AT AIDS 2016
Key clinical point: Antiretroviral therapy has had a major impact upon the incidence of HIV-related non-Hodgkin lymphoma but no effect on Hodgkin lymphoma.
Major finding: The overall incidence of non-Hodgkin lymphoma in HIV-positive adults on antiretroviral therapy is 287 cases per 100,000 person-years, varying by location and route of HIV acquisition.
Data source: This was a longitudinal analysis of non-Hodgkin lymphoma incidence in more than 210,000 HIV-infected adults on combination antiretroviral therapy on four continents.
Disclosures: The study was funded by the European Union and the U.S. National Institutes of Health. The presenter reported having no financial conflicts of interest.
Monitoring renal function during daily oral HIV PrEP
DURBAN, SOUTH AFRICA – The optimal frequency of kidney safety monitoring in patients using oral daily tenofovir/emtricitabine for pre-exposure prophylaxis against HIV infection is every 6 months, but less frequent monitoring may be reasonable in most low-risk patients, Renee Heffron, PhD, said at the 21st International AIDS Conference.
The occurrence and pattern of detection of a drop in creatinine clearance to less than 60 mL/min during the first 12 months of therapy didn’t differ significantly regardless of whether monitoring was done at 3- or 6-month intervals. The risk of a clinically relevant decline in creatinine clearance during the first 12 months of therapy appears to be largely confined to the subgroup of patients on tenofovir/emtricitabine (Truvada) for pre-exposure prophylaxis (PrEP) who weigh 55 kg or less, have a baseline creatinine clearance rate of 60-90 mL/min, or are at least 45 years old, according to Dr. Heffron of the University of Washington, Seattle.
The question of how frequently to monitor renal function is a key issue as PrEP with tenofovir/emtricitabine is ramped up to scale in sub-Saharan Africa and other parts of the developing world where the majority of new HIV infections occur – and where laboratory resources are often limited. The randomized clinical trials that led to marketing approval of tenofovir/emtricitabine for PrEP in the United States and elsewhere monitored creatinine clearance every 3 months. But the confirmatory demonstration projects used a range of kidney monitoring schedules, she explained.
She presented an analysis of clinically relevant kidney toxicity in 4,404 initially HIV-negative subjects on tenofovir/emtricitabine in the Partners PrEP Study, in which creatinine clearance was measured every 3 months, and in 955 participants in the Partners Demonstration Study, in which monitoring was performed every 6 months. All participants were at high risk for HIV acquisition because they were members of serodiscordant couples.
The occurrence and pattern of detection of a drop in creatinine clearance to less than 60 mL/min during the first 12 months of therapy didn’t differ significantly regardless of whether monitoring was done at 3- or 6-month intervals. The cumulative rate in the randomized trial was 0.4%, 0.5%, and 0.7% at 3, 6, and 12 months, and it was 0.2% at both 6 and 12 months in the demonstration project, Dr. Heffron reported.
These renal events were not only rare, they were reassuringly nonprogressive and resolved within a few weeks of PrEP discontinuation, she added.
Her analysis of the combined 5,359 subjects in the two Partners studies identified three independent predictors of a fall in creatinine clearance to below 60 mL/min during the first 12 months of therapy. A baseline age of 45 years or more was associated with an adjusted 2.5-fold increase, compared with younger patients. Subjects with a creatinine clearance of 60-90 mL/min at enrollment were 74 times more likely to experience a significant drop in creatinine clearance than those who started on PrEP with a creatinine clearance rate in excess of 90 mL/min. And patients weighing 55 kg or less had a 2.7-fold greater risk than those weighing more. But fewer than 5% of patients with any of these three predictors actually experienced a drop in creatinine clearance to below 60 mL/min.
The data from the two Partners studies support guidelines from the Centers for Disease Control and Prevention recommending creatinine monitoring every 6 months for people on oral daily PrEP. Still, patients with one of the defined risk factors might logically be candidates for targeted monitoring, Dr. Heffron observed.
The Partners studies were funded by the National Institutes of Health, the Bill and Melinda Gates Foundation, and the U.S. Agency for International Development. Dr. Heffron reported having no financial conflicts of interest.
DURBAN, SOUTH AFRICA – The optimal frequency of kidney safety monitoring in patients using oral daily tenofovir/emtricitabine for pre-exposure prophylaxis against HIV infection is every 6 months, but less frequent monitoring may be reasonable in most low-risk patients, Renee Heffron, PhD, said at the 21st International AIDS Conference.
The occurrence and pattern of detection of a drop in creatinine clearance to less than 60 mL/min during the first 12 months of therapy didn’t differ significantly regardless of whether monitoring was done at 3- or 6-month intervals. The risk of a clinically relevant decline in creatinine clearance during the first 12 months of therapy appears to be largely confined to the subgroup of patients on tenofovir/emtricitabine (Truvada) for pre-exposure prophylaxis (PrEP) who weigh 55 kg or less, have a baseline creatinine clearance rate of 60-90 mL/min, or are at least 45 years old, according to Dr. Heffron of the University of Washington, Seattle.
The question of how frequently to monitor renal function is a key issue as PrEP with tenofovir/emtricitabine is ramped up to scale in sub-Saharan Africa and other parts of the developing world where the majority of new HIV infections occur – and where laboratory resources are often limited. The randomized clinical trials that led to marketing approval of tenofovir/emtricitabine for PrEP in the United States and elsewhere monitored creatinine clearance every 3 months. But the confirmatory demonstration projects used a range of kidney monitoring schedules, she explained.
She presented an analysis of clinically relevant kidney toxicity in 4,404 initially HIV-negative subjects on tenofovir/emtricitabine in the Partners PrEP Study, in which creatinine clearance was measured every 3 months, and in 955 participants in the Partners Demonstration Study, in which monitoring was performed every 6 months. All participants were at high risk for HIV acquisition because they were members of serodiscordant couples.
The occurrence and pattern of detection of a drop in creatinine clearance to less than 60 mL/min during the first 12 months of therapy didn’t differ significantly regardless of whether monitoring was done at 3- or 6-month intervals. The cumulative rate in the randomized trial was 0.4%, 0.5%, and 0.7% at 3, 6, and 12 months, and it was 0.2% at both 6 and 12 months in the demonstration project, Dr. Heffron reported.
These renal events were not only rare, they were reassuringly nonprogressive and resolved within a few weeks of PrEP discontinuation, she added.
Her analysis of the combined 5,359 subjects in the two Partners studies identified three independent predictors of a fall in creatinine clearance to below 60 mL/min during the first 12 months of therapy. A baseline age of 45 years or more was associated with an adjusted 2.5-fold increase, compared with younger patients. Subjects with a creatinine clearance of 60-90 mL/min at enrollment were 74 times more likely to experience a significant drop in creatinine clearance than those who started on PrEP with a creatinine clearance rate in excess of 90 mL/min. And patients weighing 55 kg or less had a 2.7-fold greater risk than those weighing more. But fewer than 5% of patients with any of these three predictors actually experienced a drop in creatinine clearance to below 60 mL/min.
The data from the two Partners studies support guidelines from the Centers for Disease Control and Prevention recommending creatinine monitoring every 6 months for people on oral daily PrEP. Still, patients with one of the defined risk factors might logically be candidates for targeted monitoring, Dr. Heffron observed.
The Partners studies were funded by the National Institutes of Health, the Bill and Melinda Gates Foundation, and the U.S. Agency for International Development. Dr. Heffron reported having no financial conflicts of interest.
DURBAN, SOUTH AFRICA – The optimal frequency of kidney safety monitoring in patients using oral daily tenofovir/emtricitabine for pre-exposure prophylaxis against HIV infection is every 6 months, but less frequent monitoring may be reasonable in most low-risk patients, Renee Heffron, PhD, said at the 21st International AIDS Conference.
The occurrence and pattern of detection of a drop in creatinine clearance to less than 60 mL/min during the first 12 months of therapy didn’t differ significantly regardless of whether monitoring was done at 3- or 6-month intervals. The risk of a clinically relevant decline in creatinine clearance during the first 12 months of therapy appears to be largely confined to the subgroup of patients on tenofovir/emtricitabine (Truvada) for pre-exposure prophylaxis (PrEP) who weigh 55 kg or less, have a baseline creatinine clearance rate of 60-90 mL/min, or are at least 45 years old, according to Dr. Heffron of the University of Washington, Seattle.
The question of how frequently to monitor renal function is a key issue as PrEP with tenofovir/emtricitabine is ramped up to scale in sub-Saharan Africa and other parts of the developing world where the majority of new HIV infections occur – and where laboratory resources are often limited. The randomized clinical trials that led to marketing approval of tenofovir/emtricitabine for PrEP in the United States and elsewhere monitored creatinine clearance every 3 months. But the confirmatory demonstration projects used a range of kidney monitoring schedules, she explained.
She presented an analysis of clinically relevant kidney toxicity in 4,404 initially HIV-negative subjects on tenofovir/emtricitabine in the Partners PrEP Study, in which creatinine clearance was measured every 3 months, and in 955 participants in the Partners Demonstration Study, in which monitoring was performed every 6 months. All participants were at high risk for HIV acquisition because they were members of serodiscordant couples.
The occurrence and pattern of detection of a drop in creatinine clearance to less than 60 mL/min during the first 12 months of therapy didn’t differ significantly regardless of whether monitoring was done at 3- or 6-month intervals. The cumulative rate in the randomized trial was 0.4%, 0.5%, and 0.7% at 3, 6, and 12 months, and it was 0.2% at both 6 and 12 months in the demonstration project, Dr. Heffron reported.
These renal events were not only rare, they were reassuringly nonprogressive and resolved within a few weeks of PrEP discontinuation, she added.
Her analysis of the combined 5,359 subjects in the two Partners studies identified three independent predictors of a fall in creatinine clearance to below 60 mL/min during the first 12 months of therapy. A baseline age of 45 years or more was associated with an adjusted 2.5-fold increase, compared with younger patients. Subjects with a creatinine clearance of 60-90 mL/min at enrollment were 74 times more likely to experience a significant drop in creatinine clearance than those who started on PrEP with a creatinine clearance rate in excess of 90 mL/min. And patients weighing 55 kg or less had a 2.7-fold greater risk than those weighing more. But fewer than 5% of patients with any of these three predictors actually experienced a drop in creatinine clearance to below 60 mL/min.
The data from the two Partners studies support guidelines from the Centers for Disease Control and Prevention recommending creatinine monitoring every 6 months for people on oral daily PrEP. Still, patients with one of the defined risk factors might logically be candidates for targeted monitoring, Dr. Heffron observed.
The Partners studies were funded by the National Institutes of Health, the Bill and Melinda Gates Foundation, and the U.S. Agency for International Development. Dr. Heffron reported having no financial conflicts of interest.
AT AIDS 2016
Key clinical point: Monitoring creatinine clearance every 6 months is optimal in patients taking tenofovir/emtricitabine for pre-exposure prophylaxis against HIV infection.
Major finding: Fewer than 1% of patients experienced a decline in creatinine clearance to below 60 mL/min during their first 12 months on oral daily tenofovir/emtricitabine for pre-exposure prophylaxis against HIV infection.
Data source: This was a secondary analysis of 5,359 adults whose creatinine clearance was measured every 3 or 6 months while on oral daily tenofovir/emtricitabine for pre-exposure prophylaxis against HIV infection in a randomized trial or open-label demonstration project.
Disclosures: The studies were funded by NIH, the Bill and Melinda Gates Foundation, and the U.S. Agency for International Development. The presenter reported having no financial conflicts of interest.
CMV viremia not culprit in high mortality of TB/HIV coinfection
DURBAN, SOUTH AFRICA – Cytomegalovirus viremia is common among patients hospitalized for HIV-associated tuberculosis, but it appears to be a bystander rather than a contributor to the high mortality seen in this population, Amy Ward, MD, said at the 21st International AIDS Conference.
“CMV [cytomegalovirus] viremia is likely a marker of more severe immunodeficiency rather than a direct contributor to mortality,” she concluded based upon the findings of her prospective cohort study. The finding means therapies for CMV viremia will not open up a new avenue of potentially life-saving treatments for these patients.
In other severe immunodeficiency conditions, such as after organ transplant, CMV viremia is directly related to increased mortality, and ganciclovir therapy can prevent progression to clinical disease and death, explained Dr. Ward of the University of Cape Town, South Africa.
She presented a prospective cohort study of 256 HIV-infected South African adults, median age 36 years, who were hospitalized with a new diagnosis of TB. At enrollment, their median CD4 count was 64 cells/mm3. Only 35% were on antiretroviral therapy (ART); 44% had previously been on ART, 21% were ART-naive, and 41% had a positive TB blood culture.
CMV viremia was present in 31%, and CMV viral load was 1,000 copies/mL or more in half of them. None had CMV retinitis, based on panoptic fundoscopy at enrollment. HIV-related retinal pathologies at enrollment included disseminated cryptococcal disease, ocular TB granules, and HIV retinitis.
The primary endpoint of the study was mortality at 12 weeks on anti-TB therapy. The mortality rate was 38% in the CMV viremic group, significantly higher than the 17.8% mortality rate seen in the CMV-negative patients.
In a univariate Cox proportional hazards regression analysis, CMV viremia was associated with a 2.1-fold increased risk for 12-week mortality. But advancing age, a low CD4 count, and decreasing serum albumin were also risk factors.
When these variables were incorporated in a multivariate regression analysis along with HIV viral load, tuberculosis blood culture results, and gender, CMV viremia was no longer a significant risk factor for 12-week mortality. Age was the sole significant predictor of death. Patients who were at least 36 years old had a 32.8% mortality rate, compared with a 14.1% rate in those who were younger. The CD4 count didn’t differ significantly by age; however, the prevalence of CMV viremia was 38% in the older group and 26.3% in patients under age 36.
“Those patients who were 36 years old and above had a higher mortality and were more likely to have CMV viremia, both findings perhaps reflecting premature aging of the immune system,” Dr. Ward said.
Also, no dose-response was seen between CMV viral load and mortality risk. The 12-week mortality rate was 33.3% in patients with a CMV viral load below 1,000 copies/mL and similar at 34.1% in those with a viral load above that cutpoint, she noted.
The study was funded by the Wellcome Trust and the South African Medical Research Council. Dr. Ward reported having no financial conflicts of interest.
DURBAN, SOUTH AFRICA – Cytomegalovirus viremia is common among patients hospitalized for HIV-associated tuberculosis, but it appears to be a bystander rather than a contributor to the high mortality seen in this population, Amy Ward, MD, said at the 21st International AIDS Conference.
“CMV [cytomegalovirus] viremia is likely a marker of more severe immunodeficiency rather than a direct contributor to mortality,” she concluded based upon the findings of her prospective cohort study. The finding means therapies for CMV viremia will not open up a new avenue of potentially life-saving treatments for these patients.
In other severe immunodeficiency conditions, such as after organ transplant, CMV viremia is directly related to increased mortality, and ganciclovir therapy can prevent progression to clinical disease and death, explained Dr. Ward of the University of Cape Town, South Africa.
She presented a prospective cohort study of 256 HIV-infected South African adults, median age 36 years, who were hospitalized with a new diagnosis of TB. At enrollment, their median CD4 count was 64 cells/mm3. Only 35% were on antiretroviral therapy (ART); 44% had previously been on ART, 21% were ART-naive, and 41% had a positive TB blood culture.
CMV viremia was present in 31%, and CMV viral load was 1,000 copies/mL or more in half of them. None had CMV retinitis, based on panoptic fundoscopy at enrollment. HIV-related retinal pathologies at enrollment included disseminated cryptococcal disease, ocular TB granules, and HIV retinitis.
The primary endpoint of the study was mortality at 12 weeks on anti-TB therapy. The mortality rate was 38% in the CMV viremic group, significantly higher than the 17.8% mortality rate seen in the CMV-negative patients.
In a univariate Cox proportional hazards regression analysis, CMV viremia was associated with a 2.1-fold increased risk for 12-week mortality. But advancing age, a low CD4 count, and decreasing serum albumin were also risk factors.
When these variables were incorporated in a multivariate regression analysis along with HIV viral load, tuberculosis blood culture results, and gender, CMV viremia was no longer a significant risk factor for 12-week mortality. Age was the sole significant predictor of death. Patients who were at least 36 years old had a 32.8% mortality rate, compared with a 14.1% rate in those who were younger. The CD4 count didn’t differ significantly by age; however, the prevalence of CMV viremia was 38% in the older group and 26.3% in patients under age 36.
“Those patients who were 36 years old and above had a higher mortality and were more likely to have CMV viremia, both findings perhaps reflecting premature aging of the immune system,” Dr. Ward said.
Also, no dose-response was seen between CMV viral load and mortality risk. The 12-week mortality rate was 33.3% in patients with a CMV viral load below 1,000 copies/mL and similar at 34.1% in those with a viral load above that cutpoint, she noted.
The study was funded by the Wellcome Trust and the South African Medical Research Council. Dr. Ward reported having no financial conflicts of interest.
DURBAN, SOUTH AFRICA – Cytomegalovirus viremia is common among patients hospitalized for HIV-associated tuberculosis, but it appears to be a bystander rather than a contributor to the high mortality seen in this population, Amy Ward, MD, said at the 21st International AIDS Conference.
“CMV [cytomegalovirus] viremia is likely a marker of more severe immunodeficiency rather than a direct contributor to mortality,” she concluded based upon the findings of her prospective cohort study. The finding means therapies for CMV viremia will not open up a new avenue of potentially life-saving treatments for these patients.
In other severe immunodeficiency conditions, such as after organ transplant, CMV viremia is directly related to increased mortality, and ganciclovir therapy can prevent progression to clinical disease and death, explained Dr. Ward of the University of Cape Town, South Africa.
She presented a prospective cohort study of 256 HIV-infected South African adults, median age 36 years, who were hospitalized with a new diagnosis of TB. At enrollment, their median CD4 count was 64 cells/mm3. Only 35% were on antiretroviral therapy (ART); 44% had previously been on ART, 21% were ART-naive, and 41% had a positive TB blood culture.
CMV viremia was present in 31%, and CMV viral load was 1,000 copies/mL or more in half of them. None had CMV retinitis, based on panoptic fundoscopy at enrollment. HIV-related retinal pathologies at enrollment included disseminated cryptococcal disease, ocular TB granules, and HIV retinitis.
The primary endpoint of the study was mortality at 12 weeks on anti-TB therapy. The mortality rate was 38% in the CMV viremic group, significantly higher than the 17.8% mortality rate seen in the CMV-negative patients.
In a univariate Cox proportional hazards regression analysis, CMV viremia was associated with a 2.1-fold increased risk for 12-week mortality. But advancing age, a low CD4 count, and decreasing serum albumin were also risk factors.
When these variables were incorporated in a multivariate regression analysis along with HIV viral load, tuberculosis blood culture results, and gender, CMV viremia was no longer a significant risk factor for 12-week mortality. Age was the sole significant predictor of death. Patients who were at least 36 years old had a 32.8% mortality rate, compared with a 14.1% rate in those who were younger. The CD4 count didn’t differ significantly by age; however, the prevalence of CMV viremia was 38% in the older group and 26.3% in patients under age 36.
“Those patients who were 36 years old and above had a higher mortality and were more likely to have CMV viremia, both findings perhaps reflecting premature aging of the immune system,” Dr. Ward said.
Also, no dose-response was seen between CMV viral load and mortality risk. The 12-week mortality rate was 33.3% in patients with a CMV viral load below 1,000 copies/mL and similar at 34.1% in those with a viral load above that cutpoint, she noted.
The study was funded by the Wellcome Trust and the South African Medical Research Council. Dr. Ward reported having no financial conflicts of interest.
AT AIDS 2016
Key clinical point: Cytomegalovirus viremia is common in patients hospitalized for HIV-associated tuberculosis, but treating the CMV infection is unlikely to reduce the coinfected group’s high mortality rate.
Major finding: Cytomegalovirus viremia was present in nearly one-third of a group of hospitalized patients with HIV infection and tuberculosis, but was not an independent risk factor for their 23% mortality rate at 12 weeks.
Data source: This was a prospective cohort study including 256 hospitalized patients coinfected with HIV and newly diagnosed tuberculosis.
Disclosures: The study was funded by the Wellcome Trust and the South African Medical Research Council. The presenter reported having no financial conflicts of interest.
Same-day PrEP protects MSM from HIV
DURBAN, SOUTH AFRICA – On-demand preexposure prophylaxis using oral antiretroviral therapy taken by men as little as 2 hours before engaging in high-risk sex with other men proved even more effective in preventing HIV transmission in the IPERGAY open-label extension study than in the original randomized, double-blind, placebo-controlled IPERGAY trial, Jean-Michel Molina, MD, reported at the 21st International AIDS Conference.
In the previously reported landmark double-blind IPERGAY trial, the incidence of HIV infection was 0.91%/year with tenofovir/emtricitabine (Truvada) for preexposure prophylaxis (PrEP) during 219 person-years of follow-up and 6.6%/year with placebo during 212 person-years of follow-up, for an 86% relative risk reduction (N Engl J Med. 2015 Dec 3;373[23]:2237-46). In the 299 participants in the open-label extension of IPERGAY, HIV infection occurred at a rate of just 0.19%/year during 515 person-years of prospective follow-up, translating to a 97% reduction in risk, compared with the placebo-treated controls in the parent study.
Moreover, the one patient who became HIV positive in the open-label extension study hadn’t used PrEP for months beforehand because he’d been in a stable relationship with a single partner, who turned out to be HIV infected, according to Dr. Molina, professor of infectious diseases at the University of Paris-Diderot and head of the infectious diseases department at Saint-Louis Hospital.
The rationale for on-demand, as-needed PrEP is that patients find it less burdensome than chronic daily therapy. The IPERGAY regimen entails taking a loading dose of two tablets of Truvada, each containing a fixed dose of 300 mg of tenofovir and 200 mg of emtricitabine, with food 2-24 hours before sex, followed by a third tablet 24 hours after the loading dose and a fourth tablet 24 hours after that. If a patient has multiple consecutive sexual episodes, he is to take one tablet per day until the last episode, then take the two postexposure tablets at 24-hour intervals.
Participants in the open-label extension study engaged in high-risk sex. At baseline they had averaged seven different sexual partners in the previous 2 months and engaged in condomless anal sex with at least two different partners in the prior 6 months.
At baseline 77% of subjects indicated their last experience of receptive anal intercourse was condomless. And even though all study participants received risk reduction counseling and free condoms, at the study’s end 86% of subjects said they were condomless for their most recent experience of receptive anal intercourse, a significant increase, compared with baseline.
The rate of non-HIV sexually transmitted infections was disturbingly high in both the open-label extension study and parent randomized trial. This will need to be addressed in future prevention studies, Dr. Molina said. During the open-label study, for example, 34% of men developed one or more chlamydia infections, 32% had gonorrhea, 19% syphilis, and 1% became infected with hepatitis C virus.
Patient questionnaire responses indicated 50% of subjects in the open-label study used PrEP correctly, a significantly better adherence rate than the 42% seen in the earlier double-blind study. Adherence is often better in open-label studies because participants are counseled that the regimen has been shown to be effective and there’s no chance they’ll receive a placebo. But the 24% rate of suboptimal PrEP use and 26% rate of no PrEP at last sexual intercourse in the extension study leave ample room for improvement, the researcher noted.
Adverse events in the open-label extension study were similar to those seen in the double-blind trial. Nausea/vomiting and other gastrointestinal symptoms were the most common adverse events, affecting 13% of subjects in the open-label study. All three study dropouts because of adverse events resulted from a drop in plasma creatinine clearance.
The IPERGAY studies were funded by the French National Agency for Research on AIDS and Viral Hepatitis. Dr. Molina reported serving as an advisor to Gilead Sciences, which markets Truvada, as well as a handful of other pharmaceutical companies.
DURBAN, SOUTH AFRICA – On-demand preexposure prophylaxis using oral antiretroviral therapy taken by men as little as 2 hours before engaging in high-risk sex with other men proved even more effective in preventing HIV transmission in the IPERGAY open-label extension study than in the original randomized, double-blind, placebo-controlled IPERGAY trial, Jean-Michel Molina, MD, reported at the 21st International AIDS Conference.
In the previously reported landmark double-blind IPERGAY trial, the incidence of HIV infection was 0.91%/year with tenofovir/emtricitabine (Truvada) for preexposure prophylaxis (PrEP) during 219 person-years of follow-up and 6.6%/year with placebo during 212 person-years of follow-up, for an 86% relative risk reduction (N Engl J Med. 2015 Dec 3;373[23]:2237-46). In the 299 participants in the open-label extension of IPERGAY, HIV infection occurred at a rate of just 0.19%/year during 515 person-years of prospective follow-up, translating to a 97% reduction in risk, compared with the placebo-treated controls in the parent study.
Moreover, the one patient who became HIV positive in the open-label extension study hadn’t used PrEP for months beforehand because he’d been in a stable relationship with a single partner, who turned out to be HIV infected, according to Dr. Molina, professor of infectious diseases at the University of Paris-Diderot and head of the infectious diseases department at Saint-Louis Hospital.
The rationale for on-demand, as-needed PrEP is that patients find it less burdensome than chronic daily therapy. The IPERGAY regimen entails taking a loading dose of two tablets of Truvada, each containing a fixed dose of 300 mg of tenofovir and 200 mg of emtricitabine, with food 2-24 hours before sex, followed by a third tablet 24 hours after the loading dose and a fourth tablet 24 hours after that. If a patient has multiple consecutive sexual episodes, he is to take one tablet per day until the last episode, then take the two postexposure tablets at 24-hour intervals.
Participants in the open-label extension study engaged in high-risk sex. At baseline they had averaged seven different sexual partners in the previous 2 months and engaged in condomless anal sex with at least two different partners in the prior 6 months.
At baseline 77% of subjects indicated their last experience of receptive anal intercourse was condomless. And even though all study participants received risk reduction counseling and free condoms, at the study’s end 86% of subjects said they were condomless for their most recent experience of receptive anal intercourse, a significant increase, compared with baseline.
The rate of non-HIV sexually transmitted infections was disturbingly high in both the open-label extension study and parent randomized trial. This will need to be addressed in future prevention studies, Dr. Molina said. During the open-label study, for example, 34% of men developed one or more chlamydia infections, 32% had gonorrhea, 19% syphilis, and 1% became infected with hepatitis C virus.
Patient questionnaire responses indicated 50% of subjects in the open-label study used PrEP correctly, a significantly better adherence rate than the 42% seen in the earlier double-blind study. Adherence is often better in open-label studies because participants are counseled that the regimen has been shown to be effective and there’s no chance they’ll receive a placebo. But the 24% rate of suboptimal PrEP use and 26% rate of no PrEP at last sexual intercourse in the extension study leave ample room for improvement, the researcher noted.
Adverse events in the open-label extension study were similar to those seen in the double-blind trial. Nausea/vomiting and other gastrointestinal symptoms were the most common adverse events, affecting 13% of subjects in the open-label study. All three study dropouts because of adverse events resulted from a drop in plasma creatinine clearance.
The IPERGAY studies were funded by the French National Agency for Research on AIDS and Viral Hepatitis. Dr. Molina reported serving as an advisor to Gilead Sciences, which markets Truvada, as well as a handful of other pharmaceutical companies.
DURBAN, SOUTH AFRICA – On-demand preexposure prophylaxis using oral antiretroviral therapy taken by men as little as 2 hours before engaging in high-risk sex with other men proved even more effective in preventing HIV transmission in the IPERGAY open-label extension study than in the original randomized, double-blind, placebo-controlled IPERGAY trial, Jean-Michel Molina, MD, reported at the 21st International AIDS Conference.
In the previously reported landmark double-blind IPERGAY trial, the incidence of HIV infection was 0.91%/year with tenofovir/emtricitabine (Truvada) for preexposure prophylaxis (PrEP) during 219 person-years of follow-up and 6.6%/year with placebo during 212 person-years of follow-up, for an 86% relative risk reduction (N Engl J Med. 2015 Dec 3;373[23]:2237-46). In the 299 participants in the open-label extension of IPERGAY, HIV infection occurred at a rate of just 0.19%/year during 515 person-years of prospective follow-up, translating to a 97% reduction in risk, compared with the placebo-treated controls in the parent study.
Moreover, the one patient who became HIV positive in the open-label extension study hadn’t used PrEP for months beforehand because he’d been in a stable relationship with a single partner, who turned out to be HIV infected, according to Dr. Molina, professor of infectious diseases at the University of Paris-Diderot and head of the infectious diseases department at Saint-Louis Hospital.
The rationale for on-demand, as-needed PrEP is that patients find it less burdensome than chronic daily therapy. The IPERGAY regimen entails taking a loading dose of two tablets of Truvada, each containing a fixed dose of 300 mg of tenofovir and 200 mg of emtricitabine, with food 2-24 hours before sex, followed by a third tablet 24 hours after the loading dose and a fourth tablet 24 hours after that. If a patient has multiple consecutive sexual episodes, he is to take one tablet per day until the last episode, then take the two postexposure tablets at 24-hour intervals.
Participants in the open-label extension study engaged in high-risk sex. At baseline they had averaged seven different sexual partners in the previous 2 months and engaged in condomless anal sex with at least two different partners in the prior 6 months.
At baseline 77% of subjects indicated their last experience of receptive anal intercourse was condomless. And even though all study participants received risk reduction counseling and free condoms, at the study’s end 86% of subjects said they were condomless for their most recent experience of receptive anal intercourse, a significant increase, compared with baseline.
The rate of non-HIV sexually transmitted infections was disturbingly high in both the open-label extension study and parent randomized trial. This will need to be addressed in future prevention studies, Dr. Molina said. During the open-label study, for example, 34% of men developed one or more chlamydia infections, 32% had gonorrhea, 19% syphilis, and 1% became infected with hepatitis C virus.
Patient questionnaire responses indicated 50% of subjects in the open-label study used PrEP correctly, a significantly better adherence rate than the 42% seen in the earlier double-blind study. Adherence is often better in open-label studies because participants are counseled that the regimen has been shown to be effective and there’s no chance they’ll receive a placebo. But the 24% rate of suboptimal PrEP use and 26% rate of no PrEP at last sexual intercourse in the extension study leave ample room for improvement, the researcher noted.
Adverse events in the open-label extension study were similar to those seen in the double-blind trial. Nausea/vomiting and other gastrointestinal symptoms were the most common adverse events, affecting 13% of subjects in the open-label study. All three study dropouts because of adverse events resulted from a drop in plasma creatinine clearance.
The IPERGAY studies were funded by the French National Agency for Research on AIDS and Viral Hepatitis. Dr. Molina reported serving as an advisor to Gilead Sciences, which markets Truvada, as well as a handful of other pharmaceutical companies.
AT AIDS 2016
Key clinical point: The safety and efficacy of same-day preexposure prophylaxis (PrEP) antiretroviral therapy in preventing HIV infection in high-risk men who have sex with men, as earlier demonstrated in a French double-blind randomized trial, has now been confirmed in an open-label extension study.
Major finding: The incidence of HIV infection among men who have high-risk sex with men was 0.19%/year in an open-label study of same-day antiretroviral prophylaxis.
Data source: This open-label extension study of on-demand preexposure prophylaxis against HIV infection included 299 men who have sex with men followed prospectively for 515 person-years.
Disclosures: The study was funded by the French National Agency for Research on AIDS and Viral Hepatitis. The presenter reported serving as an advisor to Gilead Sciences, which markets Truvada, as well as a handful of other pharmaceutical companies.
HIV chemoprophylaxis shown effective in 15-year-olds
DURBAN, SOUTH AFRICA – Oral emtricitabine/tenofovir for pre-exposure prophylaxis against HIV acquisition in high-risk 15- to 17-year-old males proved safe and effective in the first clinical trial looking at the drug’s effects in a population so young, Sybil Hosek, PhD, reported at the 21st International AIDS Conference.
Based upon these encouraging findings, the drug’s manufacturer, Gilead Sciences, plans to file a request for the Food and Drug Administration to grant an expanded indication for emtricitabine/tenofovir (Truvada) for pre-exposure prophylaxis (PrEP) against HIV infection in teens as young as 15 years. The drug is currently approved for use only in patients aged 18 and up because there were no data in younger patients, said Dr. Hosek of John H. Stroger, Jr. Hospital, Chicago.
The prospect of an expanded indication in younger adolescents is most welcome news, she added.
“I really want to strongly, strongly, strongly say that adolescents need access to PrEP,” Dr. Hosek declared. “This is one of the best prevention options we’ve had in a long time.”
Co-investigator Craig M. Wilson, MD, concurred. “The epicenter of the HIV/AIDS epidemic in the U.S. is in 13- to 24-year-old males who have sex with males, particular MSM of color,” noted Dr. Wilson, professor of epidemiology, pediatrics, and director of the Sparkman Center for Global Health at the University of Alabama, Birmingham.
Dr. Hosek reported on 77 male teens ages 15-17 at high self-reported risk for HIV infection because of behaviors such as condomless anal intercourse with an HIV-positive or unknown-status partner. All 77 were negative for HIV at enrollment, which didn’t require parental permission. Prior to embarking on 48 months of once-daily, open-label emtricitabine/tenofovir for PrEP in this multicenter U.S. trial, they received personalized risk reduction, adherence, and behavior counseling. As part of the study protocol they had clinic visits monthly for the first 12 weeks. At that point the visits, which included testing for HIV and other STIs as well as measurement of blood drug levels as an indicator of adherence, were scaled back to once every 3 months.
The PrEP was safe and well tolerated. No one discontinued treatment because of side effects. The only adverse event of note was weight loss of 10%-19% in two patients. New STIs were diagnosed and treated in 12.3% of participants in the first 24 weeks of the study and in 10.6% in the next 24 weeks.
Three patients seroconverted during the 48-week study, for a hefty HIV infection rate of 6.41% per year. One of these patients never took the PrEP medication, the other two did so on and off but had no or very low blood levels of the drug at the time of seroconversion.
Adherence was a major issue, according to Dr. Hosek. She deemed adherence to be “really good” during the first 12 weeks of the study. During that period, the majority of participants had blood levels indicating they were taking their medication at least 4 days per week, providing high-level protection. More than 95% of subjects had detectable levels of drug, indicating they were at least trying to keep up with their medication schedule. However, once the clinic visits were scaled back from monthly to quarterly, adherence fell off drastically.
Audience member Carlos del Rio, MD, commented that he found the poor adherence over time to be really discouraging.
“The adolescent challenge is tremendous. All the studies show us that this group isn’t getting any protection. Are we trying to fit a square peg in a round hole? Is this something that’s just not going to happen, so we should look at alternatives such as long-acting injectables? It looks to me like we’re not going to get the adherence we need in adolescents with any of the things that are out there at this moment,” said Dr. del Rio, professor and chair of the department of global health and codirector of the center for AIDS research at Emory University in Atlanta.
Dr. Hosek replied that she found heartening the “outstanding” treatment adherence rate when patients were being seen monthly.
“Young people need more time,” Dr. Hosek observed. “And if they need that time from us, we have to give it to them. If they need to see us more frequently, if they need to text with us, if they need interim phone calls, a peer support group, an adherence club – whatever they need, if they want PrEP and they want to make it work, then we need to help them make it work. That’s our responsibility, to give them the time and attention they need.”
Loss of bone mineral density with PrEP
Dr. Wilson said an issue that bears watching, assuming a large increase in the use of emtricitabine/tenofovir for HIV PrEP in adolescents is in store in the near future, is drug-related loss in bone mineral density.
He presented data on changes in bone mineral density (BMD) as measured by dual-energy x-ray absorptiometry with results assessed at a core laboratory every 6 months in a companion study to the one presented by Dr. Hosek, this one involving 72 high-HIV-risk patients aged 18-22 years on 48 weeks of open-label emtricitabine/tenofovir followed by 48 weeks off PrEP.
Consistent with what’s been seen in studies of adults on emtricitabine/tenofovir, statistically significant decreases in mean Z-scores adjusted for age, sex, and race were seen at the hip and lumbar spine in this younger population between baseline and week 48 of PrEP. The reductions in BMD were in the range of 0.1-0.2 standard deviation. That’s noteworthy because up until age 20, people are supposed to be accruing bone mineralization, he observed.
During the subsequent 48 weeks off-PrEP patients showed evidence of partial but not full remineralization.
“There’s nothing here to indicate we should stop using PrEP in this age group, but given that we’d like to see high-risk young patients remain on therapy for longer than in this 48-week study, I think it would be smart to get longer-term exposure data to ensure that we still believe it’s safe,” the pediatrician commented.
Reassuringly, there is no evidence of an increase in fractures or complaints of bone pain in any studies of HIV-positive patients on tenofovir, he observed.
Because it’s unrealistic to expect to be able to routinely do serial DEXA scans in young patients on emtricitabine/tenofovir once PrEP is ramped up to the scale HIV specialists are hoping for, Dr. Wilson said he and his coinvestigators are now looking at potential biomarkers of clinically significant bone loss in young patients on chemoprophylaxis.
Dr. Wilson drew attention to the disturbingly high HIV seroconversion rate of 7.2% per year following discontinuation of PrEP after 48 weeks.
“Remember, this is a population that had already gone through extensive counseling, behavioral interventions, and personalized prevention and adherence support during the 48 weeks they were on the study drug, so they had been informed as to what the risks were. Yet we still end up with one of the highest seroconversion rates observed in any PrEP study. That tells us we still have a lot of work to do in these particular young populations,” according to Dr. Wilson.
These clinical trials of PrEP in 15- to 17- and 18- to 22-year-olds were carried out by the Adolescent Medicine Trials Network for HIV/AIDS Interventions with funding from the National Institutes of Health. Dr. Husek and Dr. Wilson reported having no financial conflicts.
DURBAN, SOUTH AFRICA – Oral emtricitabine/tenofovir for pre-exposure prophylaxis against HIV acquisition in high-risk 15- to 17-year-old males proved safe and effective in the first clinical trial looking at the drug’s effects in a population so young, Sybil Hosek, PhD, reported at the 21st International AIDS Conference.
Based upon these encouraging findings, the drug’s manufacturer, Gilead Sciences, plans to file a request for the Food and Drug Administration to grant an expanded indication for emtricitabine/tenofovir (Truvada) for pre-exposure prophylaxis (PrEP) against HIV infection in teens as young as 15 years. The drug is currently approved for use only in patients aged 18 and up because there were no data in younger patients, said Dr. Hosek of John H. Stroger, Jr. Hospital, Chicago.
The prospect of an expanded indication in younger adolescents is most welcome news, she added.
“I really want to strongly, strongly, strongly say that adolescents need access to PrEP,” Dr. Hosek declared. “This is one of the best prevention options we’ve had in a long time.”
Co-investigator Craig M. Wilson, MD, concurred. “The epicenter of the HIV/AIDS epidemic in the U.S. is in 13- to 24-year-old males who have sex with males, particular MSM of color,” noted Dr. Wilson, professor of epidemiology, pediatrics, and director of the Sparkman Center for Global Health at the University of Alabama, Birmingham.
Dr. Hosek reported on 77 male teens ages 15-17 at high self-reported risk for HIV infection because of behaviors such as condomless anal intercourse with an HIV-positive or unknown-status partner. All 77 were negative for HIV at enrollment, which didn’t require parental permission. Prior to embarking on 48 months of once-daily, open-label emtricitabine/tenofovir for PrEP in this multicenter U.S. trial, they received personalized risk reduction, adherence, and behavior counseling. As part of the study protocol they had clinic visits monthly for the first 12 weeks. At that point the visits, which included testing for HIV and other STIs as well as measurement of blood drug levels as an indicator of adherence, were scaled back to once every 3 months.
The PrEP was safe and well tolerated. No one discontinued treatment because of side effects. The only adverse event of note was weight loss of 10%-19% in two patients. New STIs were diagnosed and treated in 12.3% of participants in the first 24 weeks of the study and in 10.6% in the next 24 weeks.
Three patients seroconverted during the 48-week study, for a hefty HIV infection rate of 6.41% per year. One of these patients never took the PrEP medication, the other two did so on and off but had no or very low blood levels of the drug at the time of seroconversion.
Adherence was a major issue, according to Dr. Hosek. She deemed adherence to be “really good” during the first 12 weeks of the study. During that period, the majority of participants had blood levels indicating they were taking their medication at least 4 days per week, providing high-level protection. More than 95% of subjects had detectable levels of drug, indicating they were at least trying to keep up with their medication schedule. However, once the clinic visits were scaled back from monthly to quarterly, adherence fell off drastically.
Audience member Carlos del Rio, MD, commented that he found the poor adherence over time to be really discouraging.
“The adolescent challenge is tremendous. All the studies show us that this group isn’t getting any protection. Are we trying to fit a square peg in a round hole? Is this something that’s just not going to happen, so we should look at alternatives such as long-acting injectables? It looks to me like we’re not going to get the adherence we need in adolescents with any of the things that are out there at this moment,” said Dr. del Rio, professor and chair of the department of global health and codirector of the center for AIDS research at Emory University in Atlanta.
Dr. Hosek replied that she found heartening the “outstanding” treatment adherence rate when patients were being seen monthly.
“Young people need more time,” Dr. Hosek observed. “And if they need that time from us, we have to give it to them. If they need to see us more frequently, if they need to text with us, if they need interim phone calls, a peer support group, an adherence club – whatever they need, if they want PrEP and they want to make it work, then we need to help them make it work. That’s our responsibility, to give them the time and attention they need.”
Loss of bone mineral density with PrEP
Dr. Wilson said an issue that bears watching, assuming a large increase in the use of emtricitabine/tenofovir for HIV PrEP in adolescents is in store in the near future, is drug-related loss in bone mineral density.
He presented data on changes in bone mineral density (BMD) as measured by dual-energy x-ray absorptiometry with results assessed at a core laboratory every 6 months in a companion study to the one presented by Dr. Hosek, this one involving 72 high-HIV-risk patients aged 18-22 years on 48 weeks of open-label emtricitabine/tenofovir followed by 48 weeks off PrEP.
Consistent with what’s been seen in studies of adults on emtricitabine/tenofovir, statistically significant decreases in mean Z-scores adjusted for age, sex, and race were seen at the hip and lumbar spine in this younger population between baseline and week 48 of PrEP. The reductions in BMD were in the range of 0.1-0.2 standard deviation. That’s noteworthy because up until age 20, people are supposed to be accruing bone mineralization, he observed.
During the subsequent 48 weeks off-PrEP patients showed evidence of partial but not full remineralization.
“There’s nothing here to indicate we should stop using PrEP in this age group, but given that we’d like to see high-risk young patients remain on therapy for longer than in this 48-week study, I think it would be smart to get longer-term exposure data to ensure that we still believe it’s safe,” the pediatrician commented.
Reassuringly, there is no evidence of an increase in fractures or complaints of bone pain in any studies of HIV-positive patients on tenofovir, he observed.
Because it’s unrealistic to expect to be able to routinely do serial DEXA scans in young patients on emtricitabine/tenofovir once PrEP is ramped up to the scale HIV specialists are hoping for, Dr. Wilson said he and his coinvestigators are now looking at potential biomarkers of clinically significant bone loss in young patients on chemoprophylaxis.
Dr. Wilson drew attention to the disturbingly high HIV seroconversion rate of 7.2% per year following discontinuation of PrEP after 48 weeks.
“Remember, this is a population that had already gone through extensive counseling, behavioral interventions, and personalized prevention and adherence support during the 48 weeks they were on the study drug, so they had been informed as to what the risks were. Yet we still end up with one of the highest seroconversion rates observed in any PrEP study. That tells us we still have a lot of work to do in these particular young populations,” according to Dr. Wilson.
These clinical trials of PrEP in 15- to 17- and 18- to 22-year-olds were carried out by the Adolescent Medicine Trials Network for HIV/AIDS Interventions with funding from the National Institutes of Health. Dr. Husek and Dr. Wilson reported having no financial conflicts.
DURBAN, SOUTH AFRICA – Oral emtricitabine/tenofovir for pre-exposure prophylaxis against HIV acquisition in high-risk 15- to 17-year-old males proved safe and effective in the first clinical trial looking at the drug’s effects in a population so young, Sybil Hosek, PhD, reported at the 21st International AIDS Conference.
Based upon these encouraging findings, the drug’s manufacturer, Gilead Sciences, plans to file a request for the Food and Drug Administration to grant an expanded indication for emtricitabine/tenofovir (Truvada) for pre-exposure prophylaxis (PrEP) against HIV infection in teens as young as 15 years. The drug is currently approved for use only in patients aged 18 and up because there were no data in younger patients, said Dr. Hosek of John H. Stroger, Jr. Hospital, Chicago.
The prospect of an expanded indication in younger adolescents is most welcome news, she added.
“I really want to strongly, strongly, strongly say that adolescents need access to PrEP,” Dr. Hosek declared. “This is one of the best prevention options we’ve had in a long time.”
Co-investigator Craig M. Wilson, MD, concurred. “The epicenter of the HIV/AIDS epidemic in the U.S. is in 13- to 24-year-old males who have sex with males, particular MSM of color,” noted Dr. Wilson, professor of epidemiology, pediatrics, and director of the Sparkman Center for Global Health at the University of Alabama, Birmingham.
Dr. Hosek reported on 77 male teens ages 15-17 at high self-reported risk for HIV infection because of behaviors such as condomless anal intercourse with an HIV-positive or unknown-status partner. All 77 were negative for HIV at enrollment, which didn’t require parental permission. Prior to embarking on 48 months of once-daily, open-label emtricitabine/tenofovir for PrEP in this multicenter U.S. trial, they received personalized risk reduction, adherence, and behavior counseling. As part of the study protocol they had clinic visits monthly for the first 12 weeks. At that point the visits, which included testing for HIV and other STIs as well as measurement of blood drug levels as an indicator of adherence, were scaled back to once every 3 months.
The PrEP was safe and well tolerated. No one discontinued treatment because of side effects. The only adverse event of note was weight loss of 10%-19% in two patients. New STIs were diagnosed and treated in 12.3% of participants in the first 24 weeks of the study and in 10.6% in the next 24 weeks.
Three patients seroconverted during the 48-week study, for a hefty HIV infection rate of 6.41% per year. One of these patients never took the PrEP medication, the other two did so on and off but had no or very low blood levels of the drug at the time of seroconversion.
Adherence was a major issue, according to Dr. Hosek. She deemed adherence to be “really good” during the first 12 weeks of the study. During that period, the majority of participants had blood levels indicating they were taking their medication at least 4 days per week, providing high-level protection. More than 95% of subjects had detectable levels of drug, indicating they were at least trying to keep up with their medication schedule. However, once the clinic visits were scaled back from monthly to quarterly, adherence fell off drastically.
Audience member Carlos del Rio, MD, commented that he found the poor adherence over time to be really discouraging.
“The adolescent challenge is tremendous. All the studies show us that this group isn’t getting any protection. Are we trying to fit a square peg in a round hole? Is this something that’s just not going to happen, so we should look at alternatives such as long-acting injectables? It looks to me like we’re not going to get the adherence we need in adolescents with any of the things that are out there at this moment,” said Dr. del Rio, professor and chair of the department of global health and codirector of the center for AIDS research at Emory University in Atlanta.
Dr. Hosek replied that she found heartening the “outstanding” treatment adherence rate when patients were being seen monthly.
“Young people need more time,” Dr. Hosek observed. “And if they need that time from us, we have to give it to them. If they need to see us more frequently, if they need to text with us, if they need interim phone calls, a peer support group, an adherence club – whatever they need, if they want PrEP and they want to make it work, then we need to help them make it work. That’s our responsibility, to give them the time and attention they need.”
Loss of bone mineral density with PrEP
Dr. Wilson said an issue that bears watching, assuming a large increase in the use of emtricitabine/tenofovir for HIV PrEP in adolescents is in store in the near future, is drug-related loss in bone mineral density.
He presented data on changes in bone mineral density (BMD) as measured by dual-energy x-ray absorptiometry with results assessed at a core laboratory every 6 months in a companion study to the one presented by Dr. Hosek, this one involving 72 high-HIV-risk patients aged 18-22 years on 48 weeks of open-label emtricitabine/tenofovir followed by 48 weeks off PrEP.
Consistent with what’s been seen in studies of adults on emtricitabine/tenofovir, statistically significant decreases in mean Z-scores adjusted for age, sex, and race were seen at the hip and lumbar spine in this younger population between baseline and week 48 of PrEP. The reductions in BMD were in the range of 0.1-0.2 standard deviation. That’s noteworthy because up until age 20, people are supposed to be accruing bone mineralization, he observed.
During the subsequent 48 weeks off-PrEP patients showed evidence of partial but not full remineralization.
“There’s nothing here to indicate we should stop using PrEP in this age group, but given that we’d like to see high-risk young patients remain on therapy for longer than in this 48-week study, I think it would be smart to get longer-term exposure data to ensure that we still believe it’s safe,” the pediatrician commented.
Reassuringly, there is no evidence of an increase in fractures or complaints of bone pain in any studies of HIV-positive patients on tenofovir, he observed.
Because it’s unrealistic to expect to be able to routinely do serial DEXA scans in young patients on emtricitabine/tenofovir once PrEP is ramped up to the scale HIV specialists are hoping for, Dr. Wilson said he and his coinvestigators are now looking at potential biomarkers of clinically significant bone loss in young patients on chemoprophylaxis.
Dr. Wilson drew attention to the disturbingly high HIV seroconversion rate of 7.2% per year following discontinuation of PrEP after 48 weeks.
“Remember, this is a population that had already gone through extensive counseling, behavioral interventions, and personalized prevention and adherence support during the 48 weeks they were on the study drug, so they had been informed as to what the risks were. Yet we still end up with one of the highest seroconversion rates observed in any PrEP study. That tells us we still have a lot of work to do in these particular young populations,” according to Dr. Wilson.
These clinical trials of PrEP in 15- to 17- and 18- to 22-year-olds were carried out by the Adolescent Medicine Trials Network for HIV/AIDS Interventions with funding from the National Institutes of Health. Dr. Husek and Dr. Wilson reported having no financial conflicts.
AT AIDS 2016
Key clinical point: The licensed indication for daily emtricitabine/tenofovir for prevention of HIV infection might be expanded to include high-risk patients ages 15 and older based upon new study results.
Major finding: Emtricitabine/tenofovir was safe and well-tolerated for pre-exposure prophylaxis against HIV acquisition in teen males ages 15-17; however, adherence was a problem.
Data source: This prospective, open-label study included 77 male 15- to 17-year-olds at high risk for HIV infection who were placed on daily oral emtricitabine/tenofovir for chemoprophylaxis for 48 weeks.
Disclosures: The study was carried out by the Adolescent Medicine Trials Network for HIV/AIDS Interventions with funding from the National Institutes of Health. The presenter reported having no financial conflicts.