User login
New long-term data for antipsychotic in pediatric bipolar depression
The antipsychotic lurasidone (Latuda, Sunovion Pharmaceuticals) has long-term efficacy in the treatment of bipolar depression (BD) in children and adolescents, new research suggests.
In an open-label extension study involving patients aged 10-17 years, up to 2 years of treatment with lurasidone was associated with continued improvement in depressive symptoms. There were progressively higher rates of remission, recovery, and sustained remission.
Coinvestigator Manpreet K. Singh, MD, director of the Stanford Pediatric Mood Disorders Program, Stanford (Calif.) University, noted that early onset of BD is common. Although in pediatric populations, prevalence has been fairly stable at around 1.8%, these patients have “a very limited number of treatment options available for the depressed phases of BD,” which is often predominant and can be difficult to identify.
“A lot of youths who are experiencing depressive symptoms in the context of having had a manic episode will often have a relapsing and remitting course, even after the acute phase of treatment, so because kids can be on medications for long periods of time, a better understanding of what works ... is very important,” Dr. Singh said in an interview.
The findings were presented at the virtual American Society of Clinical Psychopharmacology (ASCP) 2020 annual meeting.
Long-term Efficacy
The Food and Drug Administration approved lurasidone as monotherapy for BD in children and adolescents in 2018. The aim of the current study was to evaluate the drug’s long-term efficacy in achieving response or remission in this population.
A total of 305 children who completed an initial 6-week double-blind study of lurasidone versus placebo entered the 2-year, open-label extension study. In the extension, they either continued taking lurasidone or were switched from placebo to lurasidone 20-80 mg/day. Of this group, 195 children completed 52 weeks of treatment, and 93 completed 104 weeks of treatment.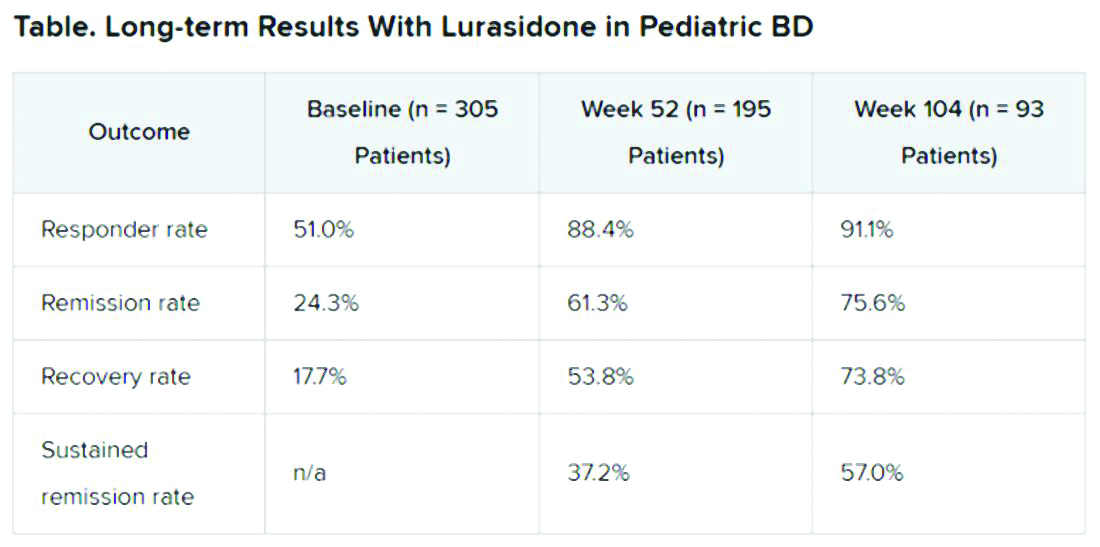
Efficacy was measured with the Children’s Depression Rating Scale, Revised (CDRS-R) and the Clinical Global Impression, Bipolar Depression Severity scale (CGI-BP-S). Functioning was evaluated with the clinician-rated Children’s Global Assessment Scale (CGAS); on that scale, a score of 70 or higher indicates no clinically meaningful functional impairment.
Remission criteria were met if a patient achieved a CDRS-R total score of 28 or less, a Young Mania Rating Scale (YMRS) total score of 8 or less, and a CGI-BP-S depression score of 3 or less.
Recovery criteria were met if a patient achieved remission and had a CGAS score of at least 70.
Sustained remission, a more stringent outcome, required that the patient meet remission criteria for at least 24 consecutive weeks.
In addition, there was a strong inverse correlation (r = –0.71) between depression severity, as measured by CDRS-R total score, and functioning, as measured by the CGAS.
“That’s the cool thing: As the depression symptoms and severity came down, the overall functioning in these kids improved,” Dr. Singh noted.
“This improvement in functioning ends up being much more clinically relevant and useful to clinicians than just showing an improvement in a set of symptoms because what brings a kid – or even an adult, for that matter – to see a clinician to get treatment is because something about their symptoms is causing significant functional impairment,” she said.
“So this is the take-home message: You can see that lurasidone ... demonstrates not just recovery from depressive symptoms but that this reduction in depressive symptoms corresponds to an improvement in functioning for these youths,” she added.
Potential Limitations
Commenting on the study, Christoph U. Correll, MD, professor of child and adolescent psychiatry, Charite Universitatsmedizin, Berlin, Germany, noted that BD is difficult to treat, especially for patients who are going through “a developmentally vulnerable phase of their lives.”
“Lurasidone is the only monotherapy approved for bipolar depression in youth and is fairly well tolerated,” said Dr. Correll, who was not part of the research. He added that the long-term effectiveness data on response and remission “add relevant information” to the field.
However, he noted that it is not clear whether the high and increasing rates of response and remission were based on the reporting of observed cases or on last-observation-carried-forward analyses. “Given the naturally high dropout rate in such a long-term study and the potential for a survival bias, this is a relevant methodological question that affects the interpretation of the data,” he said.
“Nevertheless, the very favorable results for cumulative response, remission, and sustained remission add to the evidence that lurasidone is an effective treatment for youth with bipolar depression. Since efficacy cannot be interpreted in isolation, data describing the tolerability, including long-term cardiometabolic effects, will be important complementary data to consider,” Dr. Correll said.
The study was funded by Sunovion Pharmaceuticals. Dr. Singh is on the advisory board for Sunovion, is a consultant for Google X and Limbix, and receives royalties from American Psychiatric Association Publishing. She has also received research support from Stanford’s Maternal Child Health Research Institute and Department of Psychiatry, the National Institute of Mental Health, the National Institute on Aging, Johnson and Johnson, Allergan, PCORI, and the Brain and Behavior Research Foundation. Dr. Correll has been a consultant or adviser to and has received honoraria from Sunovion, as well as Acadia, Alkermes, Allergan, Angelini, Axsome, Gedeon Richter, Gerson Lehrman Group, Intra-Cellular Therapies, Janssen/J&J, LB Pharma, Lundbeck, MedAvante-ProPhase, Medscape, Neurocrine, Noven, Otsuka, Pfizer, Recordati, Rovi, Sumitomo Dainippon, Supernus, Takeda, and Teva.
A version of this article originally appeared on Medscape.com.
The antipsychotic lurasidone (Latuda, Sunovion Pharmaceuticals) has long-term efficacy in the treatment of bipolar depression (BD) in children and adolescents, new research suggests.
In an open-label extension study involving patients aged 10-17 years, up to 2 years of treatment with lurasidone was associated with continued improvement in depressive symptoms. There were progressively higher rates of remission, recovery, and sustained remission.
Coinvestigator Manpreet K. Singh, MD, director of the Stanford Pediatric Mood Disorders Program, Stanford (Calif.) University, noted that early onset of BD is common. Although in pediatric populations, prevalence has been fairly stable at around 1.8%, these patients have “a very limited number of treatment options available for the depressed phases of BD,” which is often predominant and can be difficult to identify.
“A lot of youths who are experiencing depressive symptoms in the context of having had a manic episode will often have a relapsing and remitting course, even after the acute phase of treatment, so because kids can be on medications for long periods of time, a better understanding of what works ... is very important,” Dr. Singh said in an interview.
The findings were presented at the virtual American Society of Clinical Psychopharmacology (ASCP) 2020 annual meeting.
Long-term Efficacy
The Food and Drug Administration approved lurasidone as monotherapy for BD in children and adolescents in 2018. The aim of the current study was to evaluate the drug’s long-term efficacy in achieving response or remission in this population.
A total of 305 children who completed an initial 6-week double-blind study of lurasidone versus placebo entered the 2-year, open-label extension study. In the extension, they either continued taking lurasidone or were switched from placebo to lurasidone 20-80 mg/day. Of this group, 195 children completed 52 weeks of treatment, and 93 completed 104 weeks of treatment.
Efficacy was measured with the Children’s Depression Rating Scale, Revised (CDRS-R) and the Clinical Global Impression, Bipolar Depression Severity scale (CGI-BP-S). Functioning was evaluated with the clinician-rated Children’s Global Assessment Scale (CGAS); on that scale, a score of 70 or higher indicates no clinically meaningful functional impairment.
Remission criteria were met if a patient achieved a CDRS-R total score of 28 or less, a Young Mania Rating Scale (YMRS) total score of 8 or less, and a CGI-BP-S depression score of 3 or less.
Recovery criteria were met if a patient achieved remission and had a CGAS score of at least 70.
Sustained remission, a more stringent outcome, required that the patient meet remission criteria for at least 24 consecutive weeks.
In addition, there was a strong inverse correlation (r = –0.71) between depression severity, as measured by CDRS-R total score, and functioning, as measured by the CGAS.
“That’s the cool thing: As the depression symptoms and severity came down, the overall functioning in these kids improved,” Dr. Singh noted.
“This improvement in functioning ends up being much more clinically relevant and useful to clinicians than just showing an improvement in a set of symptoms because what brings a kid – or even an adult, for that matter – to see a clinician to get treatment is because something about their symptoms is causing significant functional impairment,” she said.
“So this is the take-home message: You can see that lurasidone ... demonstrates not just recovery from depressive symptoms but that this reduction in depressive symptoms corresponds to an improvement in functioning for these youths,” she added.
Potential Limitations
Commenting on the study, Christoph U. Correll, MD, professor of child and adolescent psychiatry, Charite Universitatsmedizin, Berlin, Germany, noted that BD is difficult to treat, especially for patients who are going through “a developmentally vulnerable phase of their lives.”
“Lurasidone is the only monotherapy approved for bipolar depression in youth and is fairly well tolerated,” said Dr. Correll, who was not part of the research. He added that the long-term effectiveness data on response and remission “add relevant information” to the field.
However, he noted that it is not clear whether the high and increasing rates of response and remission were based on the reporting of observed cases or on last-observation-carried-forward analyses. “Given the naturally high dropout rate in such a long-term study and the potential for a survival bias, this is a relevant methodological question that affects the interpretation of the data,” he said.
“Nevertheless, the very favorable results for cumulative response, remission, and sustained remission add to the evidence that lurasidone is an effective treatment for youth with bipolar depression. Since efficacy cannot be interpreted in isolation, data describing the tolerability, including long-term cardiometabolic effects, will be important complementary data to consider,” Dr. Correll said.
The study was funded by Sunovion Pharmaceuticals. Dr. Singh is on the advisory board for Sunovion, is a consultant for Google X and Limbix, and receives royalties from American Psychiatric Association Publishing. She has also received research support from Stanford’s Maternal Child Health Research Institute and Department of Psychiatry, the National Institute of Mental Health, the National Institute on Aging, Johnson and Johnson, Allergan, PCORI, and the Brain and Behavior Research Foundation. Dr. Correll has been a consultant or adviser to and has received honoraria from Sunovion, as well as Acadia, Alkermes, Allergan, Angelini, Axsome, Gedeon Richter, Gerson Lehrman Group, Intra-Cellular Therapies, Janssen/J&J, LB Pharma, Lundbeck, MedAvante-ProPhase, Medscape, Neurocrine, Noven, Otsuka, Pfizer, Recordati, Rovi, Sumitomo Dainippon, Supernus, Takeda, and Teva.
A version of this article originally appeared on Medscape.com.
The antipsychotic lurasidone (Latuda, Sunovion Pharmaceuticals) has long-term efficacy in the treatment of bipolar depression (BD) in children and adolescents, new research suggests.
In an open-label extension study involving patients aged 10-17 years, up to 2 years of treatment with lurasidone was associated with continued improvement in depressive symptoms. There were progressively higher rates of remission, recovery, and sustained remission.
Coinvestigator Manpreet K. Singh, MD, director of the Stanford Pediatric Mood Disorders Program, Stanford (Calif.) University, noted that early onset of BD is common. Although in pediatric populations, prevalence has been fairly stable at around 1.8%, these patients have “a very limited number of treatment options available for the depressed phases of BD,” which is often predominant and can be difficult to identify.
“A lot of youths who are experiencing depressive symptoms in the context of having had a manic episode will often have a relapsing and remitting course, even after the acute phase of treatment, so because kids can be on medications for long periods of time, a better understanding of what works ... is very important,” Dr. Singh said in an interview.
The findings were presented at the virtual American Society of Clinical Psychopharmacology (ASCP) 2020 annual meeting.
Long-term Efficacy
The Food and Drug Administration approved lurasidone as monotherapy for BD in children and adolescents in 2018. The aim of the current study was to evaluate the drug’s long-term efficacy in achieving response or remission in this population.
A total of 305 children who completed an initial 6-week double-blind study of lurasidone versus placebo entered the 2-year, open-label extension study. In the extension, they either continued taking lurasidone or were switched from placebo to lurasidone 20-80 mg/day. Of this group, 195 children completed 52 weeks of treatment, and 93 completed 104 weeks of treatment.
Efficacy was measured with the Children’s Depression Rating Scale, Revised (CDRS-R) and the Clinical Global Impression, Bipolar Depression Severity scale (CGI-BP-S). Functioning was evaluated with the clinician-rated Children’s Global Assessment Scale (CGAS); on that scale, a score of 70 or higher indicates no clinically meaningful functional impairment.
Remission criteria were met if a patient achieved a CDRS-R total score of 28 or less, a Young Mania Rating Scale (YMRS) total score of 8 or less, and a CGI-BP-S depression score of 3 or less.
Recovery criteria were met if a patient achieved remission and had a CGAS score of at least 70.
Sustained remission, a more stringent outcome, required that the patient meet remission criteria for at least 24 consecutive weeks.
In addition, there was a strong inverse correlation (r = –0.71) between depression severity, as measured by CDRS-R total score, and functioning, as measured by the CGAS.
“That’s the cool thing: As the depression symptoms and severity came down, the overall functioning in these kids improved,” Dr. Singh noted.
“This improvement in functioning ends up being much more clinically relevant and useful to clinicians than just showing an improvement in a set of symptoms because what brings a kid – or even an adult, for that matter – to see a clinician to get treatment is because something about their symptoms is causing significant functional impairment,” she said.
“So this is the take-home message: You can see that lurasidone ... demonstrates not just recovery from depressive symptoms but that this reduction in depressive symptoms corresponds to an improvement in functioning for these youths,” she added.
Potential Limitations
Commenting on the study, Christoph U. Correll, MD, professor of child and adolescent psychiatry, Charite Universitatsmedizin, Berlin, Germany, noted that BD is difficult to treat, especially for patients who are going through “a developmentally vulnerable phase of their lives.”
“Lurasidone is the only monotherapy approved for bipolar depression in youth and is fairly well tolerated,” said Dr. Correll, who was not part of the research. He added that the long-term effectiveness data on response and remission “add relevant information” to the field.
However, he noted that it is not clear whether the high and increasing rates of response and remission were based on the reporting of observed cases or on last-observation-carried-forward analyses. “Given the naturally high dropout rate in such a long-term study and the potential for a survival bias, this is a relevant methodological question that affects the interpretation of the data,” he said.
“Nevertheless, the very favorable results for cumulative response, remission, and sustained remission add to the evidence that lurasidone is an effective treatment for youth with bipolar depression. Since efficacy cannot be interpreted in isolation, data describing the tolerability, including long-term cardiometabolic effects, will be important complementary data to consider,” Dr. Correll said.
The study was funded by Sunovion Pharmaceuticals. Dr. Singh is on the advisory board for Sunovion, is a consultant for Google X and Limbix, and receives royalties from American Psychiatric Association Publishing. She has also received research support from Stanford’s Maternal Child Health Research Institute and Department of Psychiatry, the National Institute of Mental Health, the National Institute on Aging, Johnson and Johnson, Allergan, PCORI, and the Brain and Behavior Research Foundation. Dr. Correll has been a consultant or adviser to and has received honoraria from Sunovion, as well as Acadia, Alkermes, Allergan, Angelini, Axsome, Gedeon Richter, Gerson Lehrman Group, Intra-Cellular Therapies, Janssen/J&J, LB Pharma, Lundbeck, MedAvante-ProPhase, Medscape, Neurocrine, Noven, Otsuka, Pfizer, Recordati, Rovi, Sumitomo Dainippon, Supernus, Takeda, and Teva.
A version of this article originally appeared on Medscape.com.
FROM ASCP 2020
Irritability strongly linked to suicidal behavior in major depression
Irritability in adults with major depressive disorder (MDD) and stimulant use disorder (SUD) is strongly linked to suicidality and should be assessed by clinicians.
Three clinical trials of adults with MDD and one trial of adults with SUD showed that the link between irritability and suicidality was stronger than the association between depression severity and suicidal behaviors.
“Irritability is an important construct that is not often studied in adults with major depressive disorder,” Manish K. Jha, MD, of Icahn School of Medicine at Mount Sinai, New York, said in an interview.
“If you look at current diagnostic convention, irritability is not considered a symptom of major depressive episodes in adults, but below age 18, it is considered one of the two main symptoms,” Dr. Jha said.
The findings were presented at the virtual American Society of Clinical Psychopharmacology 2020 annual Meeting.
Clinically useful
Irritability is assessed using age-related norms of behavior, Dr. Jha said.
“The best way to conceptualize it is that it is the propensity to get angry easily or more frequently as compared to peers in response to frustration. I have a 2½-year old, and if he throws a tantrum, that is perfectly age appropriate. But if I do the same thing, it would be extreme irritability. The pediatric literature uses the word ‘grouchiness,’ but it is a little bit difficult to define, in part because it hasn’t been studied extensively,” he said.
To better understand the potential association between irritability and suicidality, the investigators reviewed results of three trials involving adults with MDD. These trials were CO-MED (Combining Medications to Enhance Depression Outcomes), which included 665 patients; EMBARC (Establishing Moderators and Biosignatures of Antidepressant Response in Clinical Care), which included 296 patients; and SAMS (Suicide Assessment Methodology Study), which included 266 patients.
They also examined the STRIDE (Stimulant Reduction Intervention Using Dosed Exercise) study, which was conducted in 302 adults with SUD.
All studies assessed irritability using the Concise Associated Symptom Tracking scale, a 5-point Likert scale. The trials also assessed suicidality with the Concise Health Risk Tracking Suicidal Thoughts.
The investigators found that irritability and suicidality were positively correlated. The association between irritability and suicidality was 2-11 times stronger than the link to overall depression.
Higher irritability at baseline predicted higher levels of suicidality at week 9 in CO-MED (P = .011), EMBARC (P < .0001), and STRIDE (P = .007), but not in SAMS (P = .21).
Greater reduction in irritability from baseline to week 4 predicted lower levels of suicidality at week 8 in CO-MED (P = .007), EMBARC (P < .0001), and STRIDE (P < .0001), but not in SAMS (P = .065).
Similarly, lower baseline levels and greater reductions in irritability were associated with lower levels of suicidality at week 28 of CO-MED, week 16 of EMBARC, and week 36 of STRIDE.
, and he believes that measuring irritability in MDD “has clinical utility.”
A common and disabling symptom
Commenting on the study, Sanjay J. Mathew, MD, professor of psychiatry and behavioral sciences at Baylor College of Medicine, Houston, said the findings provide further support that irritability is a relatively common and disabling symptom associated with major depression.
“The presence of significant irritability was associated with higher levels of suicidal ideation and is therefore highly relevant for clinicians to assess,” said Dr. Mathew, who was not part of the study.
“Early improvements in irritability are associated with better longer-term outcomes with antidepressant treatments, and this highlights the need for careful clinical evaluation early on in the course of antidepressant therapy, ideally within the first 2 weeks,” he said.
Dr. Jha reports financial relationships with Acadia Pharmaceuticals and Janssen Research & Development. Dr. Mathew reports financial relationships with Allergan, Vistagen, Janssen, Clexio, and Biohaven.
A version of this article originally appeared on Medscape.com.
Irritability in adults with major depressive disorder (MDD) and stimulant use disorder (SUD) is strongly linked to suicidality and should be assessed by clinicians.
Three clinical trials of adults with MDD and one trial of adults with SUD showed that the link between irritability and suicidality was stronger than the association between depression severity and suicidal behaviors.
“Irritability is an important construct that is not often studied in adults with major depressive disorder,” Manish K. Jha, MD, of Icahn School of Medicine at Mount Sinai, New York, said in an interview.
“If you look at current diagnostic convention, irritability is not considered a symptom of major depressive episodes in adults, but below age 18, it is considered one of the two main symptoms,” Dr. Jha said.
The findings were presented at the virtual American Society of Clinical Psychopharmacology 2020 annual Meeting.
Clinically useful
Irritability is assessed using age-related norms of behavior, Dr. Jha said.
“The best way to conceptualize it is that it is the propensity to get angry easily or more frequently as compared to peers in response to frustration. I have a 2½-year old, and if he throws a tantrum, that is perfectly age appropriate. But if I do the same thing, it would be extreme irritability. The pediatric literature uses the word ‘grouchiness,’ but it is a little bit difficult to define, in part because it hasn’t been studied extensively,” he said.
To better understand the potential association between irritability and suicidality, the investigators reviewed results of three trials involving adults with MDD. These trials were CO-MED (Combining Medications to Enhance Depression Outcomes), which included 665 patients; EMBARC (Establishing Moderators and Biosignatures of Antidepressant Response in Clinical Care), which included 296 patients; and SAMS (Suicide Assessment Methodology Study), which included 266 patients.
They also examined the STRIDE (Stimulant Reduction Intervention Using Dosed Exercise) study, which was conducted in 302 adults with SUD.
All studies assessed irritability using the Concise Associated Symptom Tracking scale, a 5-point Likert scale. The trials also assessed suicidality with the Concise Health Risk Tracking Suicidal Thoughts.
The investigators found that irritability and suicidality were positively correlated. The association between irritability and suicidality was 2-11 times stronger than the link to overall depression.
Higher irritability at baseline predicted higher levels of suicidality at week 9 in CO-MED (P = .011), EMBARC (P < .0001), and STRIDE (P = .007), but not in SAMS (P = .21).
Greater reduction in irritability from baseline to week 4 predicted lower levels of suicidality at week 8 in CO-MED (P = .007), EMBARC (P < .0001), and STRIDE (P < .0001), but not in SAMS (P = .065).
Similarly, lower baseline levels and greater reductions in irritability were associated with lower levels of suicidality at week 28 of CO-MED, week 16 of EMBARC, and week 36 of STRIDE.
, and he believes that measuring irritability in MDD “has clinical utility.”
A common and disabling symptom
Commenting on the study, Sanjay J. Mathew, MD, professor of psychiatry and behavioral sciences at Baylor College of Medicine, Houston, said the findings provide further support that irritability is a relatively common and disabling symptom associated with major depression.
“The presence of significant irritability was associated with higher levels of suicidal ideation and is therefore highly relevant for clinicians to assess,” said Dr. Mathew, who was not part of the study.
“Early improvements in irritability are associated with better longer-term outcomes with antidepressant treatments, and this highlights the need for careful clinical evaluation early on in the course of antidepressant therapy, ideally within the first 2 weeks,” he said.
Dr. Jha reports financial relationships with Acadia Pharmaceuticals and Janssen Research & Development. Dr. Mathew reports financial relationships with Allergan, Vistagen, Janssen, Clexio, and Biohaven.
A version of this article originally appeared on Medscape.com.
Irritability in adults with major depressive disorder (MDD) and stimulant use disorder (SUD) is strongly linked to suicidality and should be assessed by clinicians.
Three clinical trials of adults with MDD and one trial of adults with SUD showed that the link between irritability and suicidality was stronger than the association between depression severity and suicidal behaviors.
“Irritability is an important construct that is not often studied in adults with major depressive disorder,” Manish K. Jha, MD, of Icahn School of Medicine at Mount Sinai, New York, said in an interview.
“If you look at current diagnostic convention, irritability is not considered a symptom of major depressive episodes in adults, but below age 18, it is considered one of the two main symptoms,” Dr. Jha said.
The findings were presented at the virtual American Society of Clinical Psychopharmacology 2020 annual Meeting.
Clinically useful
Irritability is assessed using age-related norms of behavior, Dr. Jha said.
“The best way to conceptualize it is that it is the propensity to get angry easily or more frequently as compared to peers in response to frustration. I have a 2½-year old, and if he throws a tantrum, that is perfectly age appropriate. But if I do the same thing, it would be extreme irritability. The pediatric literature uses the word ‘grouchiness,’ but it is a little bit difficult to define, in part because it hasn’t been studied extensively,” he said.
To better understand the potential association between irritability and suicidality, the investigators reviewed results of three trials involving adults with MDD. These trials were CO-MED (Combining Medications to Enhance Depression Outcomes), which included 665 patients; EMBARC (Establishing Moderators and Biosignatures of Antidepressant Response in Clinical Care), which included 296 patients; and SAMS (Suicide Assessment Methodology Study), which included 266 patients.
They also examined the STRIDE (Stimulant Reduction Intervention Using Dosed Exercise) study, which was conducted in 302 adults with SUD.
All studies assessed irritability using the Concise Associated Symptom Tracking scale, a 5-point Likert scale. The trials also assessed suicidality with the Concise Health Risk Tracking Suicidal Thoughts.
The investigators found that irritability and suicidality were positively correlated. The association between irritability and suicidality was 2-11 times stronger than the link to overall depression.
Higher irritability at baseline predicted higher levels of suicidality at week 9 in CO-MED (P = .011), EMBARC (P < .0001), and STRIDE (P = .007), but not in SAMS (P = .21).
Greater reduction in irritability from baseline to week 4 predicted lower levels of suicidality at week 8 in CO-MED (P = .007), EMBARC (P < .0001), and STRIDE (P < .0001), but not in SAMS (P = .065).
Similarly, lower baseline levels and greater reductions in irritability were associated with lower levels of suicidality at week 28 of CO-MED, week 16 of EMBARC, and week 36 of STRIDE.
, and he believes that measuring irritability in MDD “has clinical utility.”
A common and disabling symptom
Commenting on the study, Sanjay J. Mathew, MD, professor of psychiatry and behavioral sciences at Baylor College of Medicine, Houston, said the findings provide further support that irritability is a relatively common and disabling symptom associated with major depression.
“The presence of significant irritability was associated with higher levels of suicidal ideation and is therefore highly relevant for clinicians to assess,” said Dr. Mathew, who was not part of the study.
“Early improvements in irritability are associated with better longer-term outcomes with antidepressant treatments, and this highlights the need for careful clinical evaluation early on in the course of antidepressant therapy, ideally within the first 2 weeks,” he said.
Dr. Jha reports financial relationships with Acadia Pharmaceuticals and Janssen Research & Development. Dr. Mathew reports financial relationships with Allergan, Vistagen, Janssen, Clexio, and Biohaven.
A version of this article originally appeared on Medscape.com.
First-in-class antipsychotic linked to lower cardiometabolic risk
A recently approved first-in-class antipsychotic appears to have fewer adverse cardiometabolic effects than standard care with risperidone, new research suggests.
In post hoc analyses of two short-term randomized controlled trials plus an open-label long-term study, patients with schizophrenia on lumateperone (Caplyta, Intra-Cellular Therapies) had reduced rates of metabolic syndrome, compared with their counterparts taking placebo or the antipsychotic risperidone.
In the short-term studies, rates of metabolic syndrome were similar between groups at baseline, but by the end of 4 and 6 weeks of treatment, 25% of patients taking lumateperone no longer met criteria for metabolic syndrome. A similar finding occurred in 36% of patients in the 1-year open label study.
“One of the major advantages that we found during the drug’s development was that it has a very favorable profile with regard to changes in weight, and other [parameters] associated with cardiovascular disease risk, such as elevated glucose and lipids,” study investigator Andrew Satlin, MD, chief medical officer at Intra-Cellular Therapies, New York, told this news organization.
“So we went back to our data and looked to see whether the changes that we saw had an impact on either the development or the resolution of metabolic syndrome in the patients who came into our studies,” he said.
The findings were presented at the American Society of Clinical Psychopharmacology 2020 Virtual Conference.
Reduced cholesterol
Lumateperone was approved in December by the Food and Drug Administration. The drug acts synergistically through the serotonergic, dopaminergic, and glutamatergic systems.
The short-term studies included 511 patients randomly assigned to receive lumateperone 42 mg (n = 256 patients) or risperidone 4 mg (n = 255 patients).
At baseline, rates of metabolic syndrome were 16% in the lumateperone group and 19% in the risperidone group. At the end of treatment, metabolic syndrome was less common in the lumateperone group (13%) vs. those receiving risperidone (25%).
In addition, 46% of lumateperone patients with metabolic syndrome at baseline no longer had it at the end of the study period. This compared with 25% of patients on risperidone.
The differences in metabolic syndrome conversion rates appeared to be driven by greater reductions in total cholesterol with lumateperone, compared with risperidone (–2.8 mg/dL with lumateperone vs. 4.8 mg/dL with risperidone) and triglycerides (–0.7 mg/dL with lumateperone vs. 20.4 mg/dL with risperidone).
Greater increases in blood glucose were also seen with risperidone (7.7 mg/dL) than with lumateperone (0.9 mg/dL).
The long-term study included 602 patients with stable schizophrenia. All received lumateperone 42 mg, and 197 patients (33%) had metabolic syndrome at baseline.
At the end of the 1-year study, 72 of these patients (36%) no longer met criteria for metabolic syndrome.
“Safest antipsychotic so far”
“Lumateperone seems to be the safest antipsychotic we have seen so far,” Christoph U. Correll, MD, professor of child and adolescent psychiatry, Charité Universitatsmedizin, Berlin, who was also involved in clinical trials of lumateperone, told this news organization.
“It seems to be very safe when it comes to cardiometabolic parameters, and it shows similar reduction in symptoms as risperidone. It is certainly an agent one should consider, particularly when a patient cannot tolerate other medications or may not be in full adherence,” said Dr. Correll, who has a joint appointment as professor of psychiatry and molecular medicine at the Zucker School of Medicine at Hofstra University in Hempstead, New York.
The drug’s safety and efficacy profile would make it a good candidate in patients initiating antipsychotic treatment, but reimbursement issues may be a barrier, at least for now, he added.
He said that the drug may prevent the onset of metabolic side effects and added that once payers are willing to reimburse the drug it should become the “first-line standard of care.”
It is well known that atypical antipsychotics are associated with adverse and rapid metabolic changes. Dr. Correll noted that particularly early-phase and first-episode patients can be “very sensitive” to the side effects of these drugs and often experience rapid weight gain and other adverse metabolic changes. Lumateperone, he added, may help avoid some of this cardiometabolic risk.
Time will tell
Jessica M. Gannon, MD, a psychiatrist at the University of Pittsburgh said in commenting on the findings that the drug’s favorable metabolic profile has previously been reported.
She also noted that there has been some interest in lumateperone because of possible “downstream effects on NMDA-type glutamate receptor activity, a larger binding ratio at dopamine-2:5HT1A receptors than other atypical antipsychotics, and presynaptic D2 partial agonism and a postsynaptic D2 antagonism.”
“This latter feature may explain the reported low extrapyramidal symptom incidence in the clinical trials,” she said .
“While I think future studies and clinical use can help determine how clinically efficacious this medication will be for our patients when compared to others on the market, its favorable metabolic and EPS profile do make it of interest,” added Gannon, who was not involved in researching the drug.
The study was funded by Intra-Cellular Therapies. Dr. Satlin is chief medical officer of Intracellular Therapies. Dr. Correll has been a consultant or advisor to and has received honoraria from Acadia, Alkermes, Allergan, Angelini, Axsome, Gedeon Richter, Gerson Lehrman Group, Intra-Cellular Therapies, Janssen/J&J, LB Pharma, Lundbeck, MedAvante-ProPhase, Medscape, Neurocrine, Noven, Otsuka, Pfizer, Recordati, Rovi, Sumitomo Dainippon, Sunovion, Supernus, Takeda, and Teva.
A version of this article originally appeared on Medscape.com.
A recently approved first-in-class antipsychotic appears to have fewer adverse cardiometabolic effects than standard care with risperidone, new research suggests.
In post hoc analyses of two short-term randomized controlled trials plus an open-label long-term study, patients with schizophrenia on lumateperone (Caplyta, Intra-Cellular Therapies) had reduced rates of metabolic syndrome, compared with their counterparts taking placebo or the antipsychotic risperidone.
In the short-term studies, rates of metabolic syndrome were similar between groups at baseline, but by the end of 4 and 6 weeks of treatment, 25% of patients taking lumateperone no longer met criteria for metabolic syndrome. A similar finding occurred in 36% of patients in the 1-year open label study.
“One of the major advantages that we found during the drug’s development was that it has a very favorable profile with regard to changes in weight, and other [parameters] associated with cardiovascular disease risk, such as elevated glucose and lipids,” study investigator Andrew Satlin, MD, chief medical officer at Intra-Cellular Therapies, New York, told this news organization.
“So we went back to our data and looked to see whether the changes that we saw had an impact on either the development or the resolution of metabolic syndrome in the patients who came into our studies,” he said.
The findings were presented at the American Society of Clinical Psychopharmacology 2020 Virtual Conference.
Reduced cholesterol
Lumateperone was approved in December by the Food and Drug Administration. The drug acts synergistically through the serotonergic, dopaminergic, and glutamatergic systems.
The short-term studies included 511 patients randomly assigned to receive lumateperone 42 mg (n = 256 patients) or risperidone 4 mg (n = 255 patients).
At baseline, rates of metabolic syndrome were 16% in the lumateperone group and 19% in the risperidone group. At the end of treatment, metabolic syndrome was less common in the lumateperone group (13%) vs. those receiving risperidone (25%).
In addition, 46% of lumateperone patients with metabolic syndrome at baseline no longer had it at the end of the study period. This compared with 25% of patients on risperidone.
The differences in metabolic syndrome conversion rates appeared to be driven by greater reductions in total cholesterol with lumateperone, compared with risperidone (–2.8 mg/dL with lumateperone vs. 4.8 mg/dL with risperidone) and triglycerides (–0.7 mg/dL with lumateperone vs. 20.4 mg/dL with risperidone).
Greater increases in blood glucose were also seen with risperidone (7.7 mg/dL) than with lumateperone (0.9 mg/dL).
The long-term study included 602 patients with stable schizophrenia. All received lumateperone 42 mg, and 197 patients (33%) had metabolic syndrome at baseline.
At the end of the 1-year study, 72 of these patients (36%) no longer met criteria for metabolic syndrome.
“Safest antipsychotic so far”
“Lumateperone seems to be the safest antipsychotic we have seen so far,” Christoph U. Correll, MD, professor of child and adolescent psychiatry, Charité Universitatsmedizin, Berlin, who was also involved in clinical trials of lumateperone, told this news organization.
“It seems to be very safe when it comes to cardiometabolic parameters, and it shows similar reduction in symptoms as risperidone. It is certainly an agent one should consider, particularly when a patient cannot tolerate other medications or may not be in full adherence,” said Dr. Correll, who has a joint appointment as professor of psychiatry and molecular medicine at the Zucker School of Medicine at Hofstra University in Hempstead, New York.
The drug’s safety and efficacy profile would make it a good candidate in patients initiating antipsychotic treatment, but reimbursement issues may be a barrier, at least for now, he added.
He said that the drug may prevent the onset of metabolic side effects and added that once payers are willing to reimburse the drug it should become the “first-line standard of care.”
It is well known that atypical antipsychotics are associated with adverse and rapid metabolic changes. Dr. Correll noted that particularly early-phase and first-episode patients can be “very sensitive” to the side effects of these drugs and often experience rapid weight gain and other adverse metabolic changes. Lumateperone, he added, may help avoid some of this cardiometabolic risk.
Time will tell
Jessica M. Gannon, MD, a psychiatrist at the University of Pittsburgh said in commenting on the findings that the drug’s favorable metabolic profile has previously been reported.
She also noted that there has been some interest in lumateperone because of possible “downstream effects on NMDA-type glutamate receptor activity, a larger binding ratio at dopamine-2:5HT1A receptors than other atypical antipsychotics, and presynaptic D2 partial agonism and a postsynaptic D2 antagonism.”
“This latter feature may explain the reported low extrapyramidal symptom incidence in the clinical trials,” she said .
“While I think future studies and clinical use can help determine how clinically efficacious this medication will be for our patients when compared to others on the market, its favorable metabolic and EPS profile do make it of interest,” added Gannon, who was not involved in researching the drug.
The study was funded by Intra-Cellular Therapies. Dr. Satlin is chief medical officer of Intracellular Therapies. Dr. Correll has been a consultant or advisor to and has received honoraria from Acadia, Alkermes, Allergan, Angelini, Axsome, Gedeon Richter, Gerson Lehrman Group, Intra-Cellular Therapies, Janssen/J&J, LB Pharma, Lundbeck, MedAvante-ProPhase, Medscape, Neurocrine, Noven, Otsuka, Pfizer, Recordati, Rovi, Sumitomo Dainippon, Sunovion, Supernus, Takeda, and Teva.
A version of this article originally appeared on Medscape.com.
A recently approved first-in-class antipsychotic appears to have fewer adverse cardiometabolic effects than standard care with risperidone, new research suggests.
In post hoc analyses of two short-term randomized controlled trials plus an open-label long-term study, patients with schizophrenia on lumateperone (Caplyta, Intra-Cellular Therapies) had reduced rates of metabolic syndrome, compared with their counterparts taking placebo or the antipsychotic risperidone.
In the short-term studies, rates of metabolic syndrome were similar between groups at baseline, but by the end of 4 and 6 weeks of treatment, 25% of patients taking lumateperone no longer met criteria for metabolic syndrome. A similar finding occurred in 36% of patients in the 1-year open label study.
“One of the major advantages that we found during the drug’s development was that it has a very favorable profile with regard to changes in weight, and other [parameters] associated with cardiovascular disease risk, such as elevated glucose and lipids,” study investigator Andrew Satlin, MD, chief medical officer at Intra-Cellular Therapies, New York, told this news organization.
“So we went back to our data and looked to see whether the changes that we saw had an impact on either the development or the resolution of metabolic syndrome in the patients who came into our studies,” he said.
The findings were presented at the American Society of Clinical Psychopharmacology 2020 Virtual Conference.
Reduced cholesterol
Lumateperone was approved in December by the Food and Drug Administration. The drug acts synergistically through the serotonergic, dopaminergic, and glutamatergic systems.
The short-term studies included 511 patients randomly assigned to receive lumateperone 42 mg (n = 256 patients) or risperidone 4 mg (n = 255 patients).
At baseline, rates of metabolic syndrome were 16% in the lumateperone group and 19% in the risperidone group. At the end of treatment, metabolic syndrome was less common in the lumateperone group (13%) vs. those receiving risperidone (25%).
In addition, 46% of lumateperone patients with metabolic syndrome at baseline no longer had it at the end of the study period. This compared with 25% of patients on risperidone.
The differences in metabolic syndrome conversion rates appeared to be driven by greater reductions in total cholesterol with lumateperone, compared with risperidone (–2.8 mg/dL with lumateperone vs. 4.8 mg/dL with risperidone) and triglycerides (–0.7 mg/dL with lumateperone vs. 20.4 mg/dL with risperidone).
Greater increases in blood glucose were also seen with risperidone (7.7 mg/dL) than with lumateperone (0.9 mg/dL).
The long-term study included 602 patients with stable schizophrenia. All received lumateperone 42 mg, and 197 patients (33%) had metabolic syndrome at baseline.
At the end of the 1-year study, 72 of these patients (36%) no longer met criteria for metabolic syndrome.
“Safest antipsychotic so far”
“Lumateperone seems to be the safest antipsychotic we have seen so far,” Christoph U. Correll, MD, professor of child and adolescent psychiatry, Charité Universitatsmedizin, Berlin, who was also involved in clinical trials of lumateperone, told this news organization.
“It seems to be very safe when it comes to cardiometabolic parameters, and it shows similar reduction in symptoms as risperidone. It is certainly an agent one should consider, particularly when a patient cannot tolerate other medications or may not be in full adherence,” said Dr. Correll, who has a joint appointment as professor of psychiatry and molecular medicine at the Zucker School of Medicine at Hofstra University in Hempstead, New York.
The drug’s safety and efficacy profile would make it a good candidate in patients initiating antipsychotic treatment, but reimbursement issues may be a barrier, at least for now, he added.
He said that the drug may prevent the onset of metabolic side effects and added that once payers are willing to reimburse the drug it should become the “first-line standard of care.”
It is well known that atypical antipsychotics are associated with adverse and rapid metabolic changes. Dr. Correll noted that particularly early-phase and first-episode patients can be “very sensitive” to the side effects of these drugs and often experience rapid weight gain and other adverse metabolic changes. Lumateperone, he added, may help avoid some of this cardiometabolic risk.
Time will tell
Jessica M. Gannon, MD, a psychiatrist at the University of Pittsburgh said in commenting on the findings that the drug’s favorable metabolic profile has previously been reported.
She also noted that there has been some interest in lumateperone because of possible “downstream effects on NMDA-type glutamate receptor activity, a larger binding ratio at dopamine-2:5HT1A receptors than other atypical antipsychotics, and presynaptic D2 partial agonism and a postsynaptic D2 antagonism.”
“This latter feature may explain the reported low extrapyramidal symptom incidence in the clinical trials,” she said .
“While I think future studies and clinical use can help determine how clinically efficacious this medication will be for our patients when compared to others on the market, its favorable metabolic and EPS profile do make it of interest,” added Gannon, who was not involved in researching the drug.
The study was funded by Intra-Cellular Therapies. Dr. Satlin is chief medical officer of Intracellular Therapies. Dr. Correll has been a consultant or advisor to and has received honoraria from Acadia, Alkermes, Allergan, Angelini, Axsome, Gedeon Richter, Gerson Lehrman Group, Intra-Cellular Therapies, Janssen/J&J, LB Pharma, Lundbeck, MedAvante-ProPhase, Medscape, Neurocrine, Noven, Otsuka, Pfizer, Recordati, Rovi, Sumitomo Dainippon, Sunovion, Supernus, Takeda, and Teva.
A version of this article originally appeared on Medscape.com.

