User login
Study finds no standard for treatment discontinuation in myeloma
BOSTON — There is “no standard of care and no clear pattern” for discontinuing treatment in multiple myeloma, according to a speaker at the International Myeloma Workshop.
Data from a large, observational study revealed that a wide range of treatment regimens are used for first-, second-, and third-line therapy in multiple myeloma. The duration of therapy and time to next treatment were shorter in this real-world study than in prior clinical trials, and reasons for treatment discontinuation varied by regimen and line of therapy.
Katja Weisel, MD, of University Medical Center Hamburg-Eppendorf in Germany, presented these findings at the workshop, held by the International Myeloma Society.
The study, INSIGHT MM, is the largest global, prospective, observational study of multiple myeloma to date, according to Dr. Weisel. The study, which began July 1, 2016, has enrolled patients in the United States (n = 1,004), Europe (n = 1,612), Latin America (n = 367), and Asia (n = 218).
Dr. Weisel and her colleagues evaluated duration of therapy, reasons for treatment discontinuation, and subsequent therapies in a subset of patients on INSIGHT MM. The researchers’ analysis revealed “broad heterogeneity” across lines of therapy, Dr. Weisel said, adding that patients are receiving multiple regimens in addition to the most commonly prescribed regimens in myeloma.
First-line therapy
“In first-line treatment, we see predominantly bortezomib-based triplets ... regardless of transplant-eligible or transplant-ineligible patients,” Dr. Weisel said. “This is followed by doublets and other more rarely [applied] regimens.”
First-line therapies in 1,175 patients included:
- Bortezomib, cyclophosphamide, and dexamethasone (VCd) – 323 patients.
- Bortezomib, lenalidomide, and dexamethasone (VRd) – 321 patients.
- Bortezomib, thalidomide, and dexamethasone (VTd) – 200 patients.
- Bortezomib and dexamethasone (Vd) – 102 patients.
- Lenalidomide and dexamethasone (Rd) – 90 patients.
- Bortezomib, melphalan, and prednisone (VMP) – 53 patients.
- Carfilzomib, lenalidomide, and dexamethasone (KRd) – 47 patients.
- Daratumumab-based regimens (Dara) – 32 patients.
- Carfilzomib and dexamethasone (Kd) – 5 patients.
- Ixazomib, lenalidomide, and dexamethasone (IRd) – 2 patients.
Of the 1,175 newly diagnosed patients, 894 did not proceed to transplant after first-line therapy, but 281 did. Most of the patients who went on to transplant had received VRd (n = 82), VTd (n = 76), or VCd (n = 75).
Second- and third-line therapies
“In second-line treatment, we have still a dominance of the len-dex regimen all over the world,” Dr. Weisel said. “There is an emerging use of daratumumab in various combinations, and then you see the whole spectrum of approved triplet and doublet regimens.”
In the third line, the most commonly used regimens are daratumumab-based combinations and Rd.
There were 548 patients who received second-line treatment and 332 who received third-line therapy. The regimens used were:
- Rd – 130 patients second line, 71 third line.
- Dara – 121 patients second line, 105 third line.
- KRd – 61 patients second line, 17 third line.
- VCd – 57 patients second line, 19 third line.
- Vd – 48 patients second line, 29 third line.
- VRd – 36 patients second line, 8 third line.
- Kd – 33 patients for both second and third line.
- IRd – 29 patients second line, 43 third line.
- VTd – 25 patients second line, 4 third line.
- VMP – 8 patients second line, 3 third line.
Duration of therapy
Most transplant-eligible patients received any first-line therapy (VRd, VTd, or VCd) for longer than 12 months. Among transplant-ineligible patients, Rd was the first-line therapy most likely to be given for 12 months or more.
None of the second-line regimens lasted longer than 12 months in a majority of patients, but daratumumab-based regimens and IRd were the therapies most likely to exceed 12 months’ duration in both second- and third-line treatment.
Time to next treatment
“The vast majority of [transplant-eligible] patients, close to 90% ... do not need a second-line treatment during the first year of treatment,” Dr. Weisel said. “However, for transplant-ineligible patients, this accounts only for the most effective regimens, VMP and Rd.”
For second- and third-line therapies, a 12-month or longer time to next treatment was most likely among patients who received IRd or daratumumab-based regimens.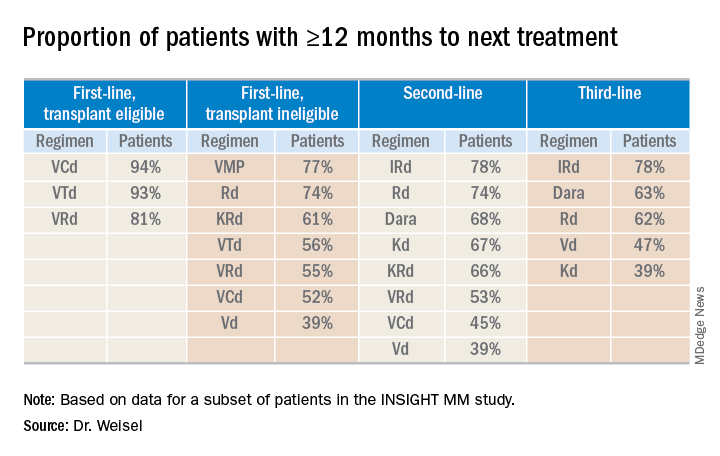
Reasons for discontinuation
“Planned end of therapy only accounts for a small proportion of treatment discontinuations, especially in the relapsed setting,” Dr. Weisel said. “Patients are discontinuing treatment due to reasons other than relapse, ultimately receiving fixed-duration therapy.”
The most common reasons for discontinuation of first-line therapy were:
- Relapse for VCd.
- Planned end of therapy for VRd.
- Adverse events (AEs) for VD and VTd.
- AEs and “other reasons” for Rd.
The most common reasons for discontinuation of second-line therapy were:
- Planned end of therapy for VCd.
- AEs, relapse, and other reasons for VRd.
- Relapse for VD, KRd, and Dara.
- AEs for Rd and IRd.
- AEs and other reasons for Kd.
The most common reasons for discontinuation of third-line therapy were:
- AEs for VCd, Vd, and KRd.
- Relapse for Kd, IRd, and Dara.
- Relapse and other reasons for VRd.
- AEs and other reasons for Rd.
The most common AE leading to discontinuation, across all treatment regimens, was peripheral neuropathy. This suggests peripheral neuropathy is still the “biggest impediment for continuous treatment,” Dr. Weisel said.
INSIGHT MM is sponsored by Takeda. Dr. Weisel reported relationships with Takeda and several other companies.
SOURCE: Weisel K et al. IMW 2019, Abstract OAB-005.
BOSTON — There is “no standard of care and no clear pattern” for discontinuing treatment in multiple myeloma, according to a speaker at the International Myeloma Workshop.
Data from a large, observational study revealed that a wide range of treatment regimens are used for first-, second-, and third-line therapy in multiple myeloma. The duration of therapy and time to next treatment were shorter in this real-world study than in prior clinical trials, and reasons for treatment discontinuation varied by regimen and line of therapy.
Katja Weisel, MD, of University Medical Center Hamburg-Eppendorf in Germany, presented these findings at the workshop, held by the International Myeloma Society.
The study, INSIGHT MM, is the largest global, prospective, observational study of multiple myeloma to date, according to Dr. Weisel. The study, which began July 1, 2016, has enrolled patients in the United States (n = 1,004), Europe (n = 1,612), Latin America (n = 367), and Asia (n = 218).
Dr. Weisel and her colleagues evaluated duration of therapy, reasons for treatment discontinuation, and subsequent therapies in a subset of patients on INSIGHT MM. The researchers’ analysis revealed “broad heterogeneity” across lines of therapy, Dr. Weisel said, adding that patients are receiving multiple regimens in addition to the most commonly prescribed regimens in myeloma.
First-line therapy
“In first-line treatment, we see predominantly bortezomib-based triplets ... regardless of transplant-eligible or transplant-ineligible patients,” Dr. Weisel said. “This is followed by doublets and other more rarely [applied] regimens.”
First-line therapies in 1,175 patients included:
- Bortezomib, cyclophosphamide, and dexamethasone (VCd) – 323 patients.
- Bortezomib, lenalidomide, and dexamethasone (VRd) – 321 patients.
- Bortezomib, thalidomide, and dexamethasone (VTd) – 200 patients.
- Bortezomib and dexamethasone (Vd) – 102 patients.
- Lenalidomide and dexamethasone (Rd) – 90 patients.
- Bortezomib, melphalan, and prednisone (VMP) – 53 patients.
- Carfilzomib, lenalidomide, and dexamethasone (KRd) – 47 patients.
- Daratumumab-based regimens (Dara) – 32 patients.
- Carfilzomib and dexamethasone (Kd) – 5 patients.
- Ixazomib, lenalidomide, and dexamethasone (IRd) – 2 patients.
Of the 1,175 newly diagnosed patients, 894 did not proceed to transplant after first-line therapy, but 281 did. Most of the patients who went on to transplant had received VRd (n = 82), VTd (n = 76), or VCd (n = 75).
Second- and third-line therapies
“In second-line treatment, we have still a dominance of the len-dex regimen all over the world,” Dr. Weisel said. “There is an emerging use of daratumumab in various combinations, and then you see the whole spectrum of approved triplet and doublet regimens.”
In the third line, the most commonly used regimens are daratumumab-based combinations and Rd.
There were 548 patients who received second-line treatment and 332 who received third-line therapy. The regimens used were:
- Rd – 130 patients second line, 71 third line.
- Dara – 121 patients second line, 105 third line.
- KRd – 61 patients second line, 17 third line.
- VCd – 57 patients second line, 19 third line.
- Vd – 48 patients second line, 29 third line.
- VRd – 36 patients second line, 8 third line.
- Kd – 33 patients for both second and third line.
- IRd – 29 patients second line, 43 third line.
- VTd – 25 patients second line, 4 third line.
- VMP – 8 patients second line, 3 third line.
Duration of therapy
Most transplant-eligible patients received any first-line therapy (VRd, VTd, or VCd) for longer than 12 months. Among transplant-ineligible patients, Rd was the first-line therapy most likely to be given for 12 months or more.
None of the second-line regimens lasted longer than 12 months in a majority of patients, but daratumumab-based regimens and IRd were the therapies most likely to exceed 12 months’ duration in both second- and third-line treatment.
Time to next treatment
“The vast majority of [transplant-eligible] patients, close to 90% ... do not need a second-line treatment during the first year of treatment,” Dr. Weisel said. “However, for transplant-ineligible patients, this accounts only for the most effective regimens, VMP and Rd.”
For second- and third-line therapies, a 12-month or longer time to next treatment was most likely among patients who received IRd or daratumumab-based regimens.
Reasons for discontinuation
“Planned end of therapy only accounts for a small proportion of treatment discontinuations, especially in the relapsed setting,” Dr. Weisel said. “Patients are discontinuing treatment due to reasons other than relapse, ultimately receiving fixed-duration therapy.”
The most common reasons for discontinuation of first-line therapy were:
- Relapse for VCd.
- Planned end of therapy for VRd.
- Adverse events (AEs) for VD and VTd.
- AEs and “other reasons” for Rd.
The most common reasons for discontinuation of second-line therapy were:
- Planned end of therapy for VCd.
- AEs, relapse, and other reasons for VRd.
- Relapse for VD, KRd, and Dara.
- AEs for Rd and IRd.
- AEs and other reasons for Kd.
The most common reasons for discontinuation of third-line therapy were:
- AEs for VCd, Vd, and KRd.
- Relapse for Kd, IRd, and Dara.
- Relapse and other reasons for VRd.
- AEs and other reasons for Rd.
The most common AE leading to discontinuation, across all treatment regimens, was peripheral neuropathy. This suggests peripheral neuropathy is still the “biggest impediment for continuous treatment,” Dr. Weisel said.
INSIGHT MM is sponsored by Takeda. Dr. Weisel reported relationships with Takeda and several other companies.
SOURCE: Weisel K et al. IMW 2019, Abstract OAB-005.
BOSTON — There is “no standard of care and no clear pattern” for discontinuing treatment in multiple myeloma, according to a speaker at the International Myeloma Workshop.
Data from a large, observational study revealed that a wide range of treatment regimens are used for first-, second-, and third-line therapy in multiple myeloma. The duration of therapy and time to next treatment were shorter in this real-world study than in prior clinical trials, and reasons for treatment discontinuation varied by regimen and line of therapy.
Katja Weisel, MD, of University Medical Center Hamburg-Eppendorf in Germany, presented these findings at the workshop, held by the International Myeloma Society.
The study, INSIGHT MM, is the largest global, prospective, observational study of multiple myeloma to date, according to Dr. Weisel. The study, which began July 1, 2016, has enrolled patients in the United States (n = 1,004), Europe (n = 1,612), Latin America (n = 367), and Asia (n = 218).
Dr. Weisel and her colleagues evaluated duration of therapy, reasons for treatment discontinuation, and subsequent therapies in a subset of patients on INSIGHT MM. The researchers’ analysis revealed “broad heterogeneity” across lines of therapy, Dr. Weisel said, adding that patients are receiving multiple regimens in addition to the most commonly prescribed regimens in myeloma.
First-line therapy
“In first-line treatment, we see predominantly bortezomib-based triplets ... regardless of transplant-eligible or transplant-ineligible patients,” Dr. Weisel said. “This is followed by doublets and other more rarely [applied] regimens.”
First-line therapies in 1,175 patients included:
- Bortezomib, cyclophosphamide, and dexamethasone (VCd) – 323 patients.
- Bortezomib, lenalidomide, and dexamethasone (VRd) – 321 patients.
- Bortezomib, thalidomide, and dexamethasone (VTd) – 200 patients.
- Bortezomib and dexamethasone (Vd) – 102 patients.
- Lenalidomide and dexamethasone (Rd) – 90 patients.
- Bortezomib, melphalan, and prednisone (VMP) – 53 patients.
- Carfilzomib, lenalidomide, and dexamethasone (KRd) – 47 patients.
- Daratumumab-based regimens (Dara) – 32 patients.
- Carfilzomib and dexamethasone (Kd) – 5 patients.
- Ixazomib, lenalidomide, and dexamethasone (IRd) – 2 patients.
Of the 1,175 newly diagnosed patients, 894 did not proceed to transplant after first-line therapy, but 281 did. Most of the patients who went on to transplant had received VRd (n = 82), VTd (n = 76), or VCd (n = 75).
Second- and third-line therapies
“In second-line treatment, we have still a dominance of the len-dex regimen all over the world,” Dr. Weisel said. “There is an emerging use of daratumumab in various combinations, and then you see the whole spectrum of approved triplet and doublet regimens.”
In the third line, the most commonly used regimens are daratumumab-based combinations and Rd.
There were 548 patients who received second-line treatment and 332 who received third-line therapy. The regimens used were:
- Rd – 130 patients second line, 71 third line.
- Dara – 121 patients second line, 105 third line.
- KRd – 61 patients second line, 17 third line.
- VCd – 57 patients second line, 19 third line.
- Vd – 48 patients second line, 29 third line.
- VRd – 36 patients second line, 8 third line.
- Kd – 33 patients for both second and third line.
- IRd – 29 patients second line, 43 third line.
- VTd – 25 patients second line, 4 third line.
- VMP – 8 patients second line, 3 third line.
Duration of therapy
Most transplant-eligible patients received any first-line therapy (VRd, VTd, or VCd) for longer than 12 months. Among transplant-ineligible patients, Rd was the first-line therapy most likely to be given for 12 months or more.
None of the second-line regimens lasted longer than 12 months in a majority of patients, but daratumumab-based regimens and IRd were the therapies most likely to exceed 12 months’ duration in both second- and third-line treatment.
Time to next treatment
“The vast majority of [transplant-eligible] patients, close to 90% ... do not need a second-line treatment during the first year of treatment,” Dr. Weisel said. “However, for transplant-ineligible patients, this accounts only for the most effective regimens, VMP and Rd.”
For second- and third-line therapies, a 12-month or longer time to next treatment was most likely among patients who received IRd or daratumumab-based regimens.
Reasons for discontinuation
“Planned end of therapy only accounts for a small proportion of treatment discontinuations, especially in the relapsed setting,” Dr. Weisel said. “Patients are discontinuing treatment due to reasons other than relapse, ultimately receiving fixed-duration therapy.”
The most common reasons for discontinuation of first-line therapy were:
- Relapse for VCd.
- Planned end of therapy for VRd.
- Adverse events (AEs) for VD and VTd.
- AEs and “other reasons” for Rd.
The most common reasons for discontinuation of second-line therapy were:
- Planned end of therapy for VCd.
- AEs, relapse, and other reasons for VRd.
- Relapse for VD, KRd, and Dara.
- AEs for Rd and IRd.
- AEs and other reasons for Kd.
The most common reasons for discontinuation of third-line therapy were:
- AEs for VCd, Vd, and KRd.
- Relapse for Kd, IRd, and Dara.
- Relapse and other reasons for VRd.
- AEs and other reasons for Rd.
The most common AE leading to discontinuation, across all treatment regimens, was peripheral neuropathy. This suggests peripheral neuropathy is still the “biggest impediment for continuous treatment,” Dr. Weisel said.
INSIGHT MM is sponsored by Takeda. Dr. Weisel reported relationships with Takeda and several other companies.
SOURCE: Weisel K et al. IMW 2019, Abstract OAB-005.
REPORTING FROM IMW 2019
Second-generation anti-BCMA CAR T-cell therapy shows promise in myeloma trial
BOSTON – CT053, a chimeric antigen receptor (CAR) T-cell therapy, has demonstrated efficacy and tolerability in a phase 1 trial of patients with relapsed/refractory multiple myeloma.
CT053 produced an objective response rate of 87.5% and a complete response rate of 79.2%. All patients experienced grade 3 or higher adverse events (AEs), but none developed grade 3 or higher cytokine-release syndrome (CRS).
Siguo Hao, MD, of Xinhua Hospital, Shanghai (China) Jiaotong University, presented these results at the International Myeloma Workshop held by the International Myeloma Society.
Dr. Hao explained that CT053 consists of autologous T cells modified with a second-generation CAR that incorporates a fully human anti–B-cell maturation antigen single-chain fragment variant, a 4-1BB costimulatory domain, and a CD3-zeta–signaling domain.
In preclinical studies, CT053 induced dose-dependent cytotoxic effects on multiple myeloma cell lines and completely eradicated myeloma in mice.
Dr. Hao and his colleagues conducted the phase 1 study of CT053 at three sites (NCT03716856, NCT03302403, and NCT03380039). The study enrolled 30 patients with relapsed/refractory multiple myeloma, and 24 ultimately received CT053.
In the 24 patients, the median age was 60.2 years (range, 38.5-70.0 years), and the median time since diagnosis was 3.5 years (range, 0.3-10.8 years). Nine patients had progressive disease at baseline.
The patients had received a median of 5 prior therapies (range, 2-12). All patients had received a proteasome inhibitor, 22 had received an immunomodulatory agent, 10 had undergone a transplant, and 5 had received an anti-CD38 monoclonal antibody.
For this study, patients received conditioning with fludarabine and cyclophosphamide, followed by a single infusion of CT053. Most patients (n = 21) received 1.5 x 108 cells, but three received 0.5 x 108, 1 x 108, and 1.8 x 108 cells, respectively.
The median follow-up was 333 days. CAR T cells were detectable 1-7 days after infusion and peaked at 7-21 days. The cells persisted for a median of 172 days (range, 21-341 days).
A total of 21 patients responded to treatment (87.5%). There were 19 patients with a complete response or stringent complete response, 1 patient with a very good partial response, and 1 with a partial response.
Dr. Hao noted that CT053 was effective even at the lowest dose. The patient who received 0.5 x 108 cells initially achieved a very good partial response that deepened to a stringent complete response on day 502 after infusion.
Ten patients still had a stringent complete response at last follow-up, and two had a complete response. Nine patients progressed, and one patient relapsed after achieving a complete response. Three patients died, two from disease progression and one from a serious AE (neutropenic infection).
All 24 patients experienced a treatment-related AE, and all had grade 3 or higher hematologic AEs. Six patients had grade 3 or higher fever, six had grade 3 or higher infections and infestations, and one had grade 3 or higher neurotoxicity.
Three patients had grade 1 CRS, and 12 had grade 2 CRS. None of the patients had grade 3 or higher CRS.
This trial was sponsored by Xinhua Hospital/Shanghai Jiao Tong University School of Medicine, First Affiliated Hospital of Zhejiang University, and First Affiliated Hospital of Wenzhou Medical University in collaboration with Carsgen Therapeutics. Dr. Hao did not disclose any conflicts of interest.
SOURCE: Hao S et al. IMW 2019, Abstract OAB-082.
BOSTON – CT053, a chimeric antigen receptor (CAR) T-cell therapy, has demonstrated efficacy and tolerability in a phase 1 trial of patients with relapsed/refractory multiple myeloma.
CT053 produced an objective response rate of 87.5% and a complete response rate of 79.2%. All patients experienced grade 3 or higher adverse events (AEs), but none developed grade 3 or higher cytokine-release syndrome (CRS).
Siguo Hao, MD, of Xinhua Hospital, Shanghai (China) Jiaotong University, presented these results at the International Myeloma Workshop held by the International Myeloma Society.
Dr. Hao explained that CT053 consists of autologous T cells modified with a second-generation CAR that incorporates a fully human anti–B-cell maturation antigen single-chain fragment variant, a 4-1BB costimulatory domain, and a CD3-zeta–signaling domain.
In preclinical studies, CT053 induced dose-dependent cytotoxic effects on multiple myeloma cell lines and completely eradicated myeloma in mice.
Dr. Hao and his colleagues conducted the phase 1 study of CT053 at three sites (NCT03716856, NCT03302403, and NCT03380039). The study enrolled 30 patients with relapsed/refractory multiple myeloma, and 24 ultimately received CT053.
In the 24 patients, the median age was 60.2 years (range, 38.5-70.0 years), and the median time since diagnosis was 3.5 years (range, 0.3-10.8 years). Nine patients had progressive disease at baseline.
The patients had received a median of 5 prior therapies (range, 2-12). All patients had received a proteasome inhibitor, 22 had received an immunomodulatory agent, 10 had undergone a transplant, and 5 had received an anti-CD38 monoclonal antibody.
For this study, patients received conditioning with fludarabine and cyclophosphamide, followed by a single infusion of CT053. Most patients (n = 21) received 1.5 x 108 cells, but three received 0.5 x 108, 1 x 108, and 1.8 x 108 cells, respectively.
The median follow-up was 333 days. CAR T cells were detectable 1-7 days after infusion and peaked at 7-21 days. The cells persisted for a median of 172 days (range, 21-341 days).
A total of 21 patients responded to treatment (87.5%). There were 19 patients with a complete response or stringent complete response, 1 patient with a very good partial response, and 1 with a partial response.
Dr. Hao noted that CT053 was effective even at the lowest dose. The patient who received 0.5 x 108 cells initially achieved a very good partial response that deepened to a stringent complete response on day 502 after infusion.
Ten patients still had a stringent complete response at last follow-up, and two had a complete response. Nine patients progressed, and one patient relapsed after achieving a complete response. Three patients died, two from disease progression and one from a serious AE (neutropenic infection).
All 24 patients experienced a treatment-related AE, and all had grade 3 or higher hematologic AEs. Six patients had grade 3 or higher fever, six had grade 3 or higher infections and infestations, and one had grade 3 or higher neurotoxicity.
Three patients had grade 1 CRS, and 12 had grade 2 CRS. None of the patients had grade 3 or higher CRS.
This trial was sponsored by Xinhua Hospital/Shanghai Jiao Tong University School of Medicine, First Affiliated Hospital of Zhejiang University, and First Affiliated Hospital of Wenzhou Medical University in collaboration with Carsgen Therapeutics. Dr. Hao did not disclose any conflicts of interest.
SOURCE: Hao S et al. IMW 2019, Abstract OAB-082.
BOSTON – CT053, a chimeric antigen receptor (CAR) T-cell therapy, has demonstrated efficacy and tolerability in a phase 1 trial of patients with relapsed/refractory multiple myeloma.
CT053 produced an objective response rate of 87.5% and a complete response rate of 79.2%. All patients experienced grade 3 or higher adverse events (AEs), but none developed grade 3 or higher cytokine-release syndrome (CRS).
Siguo Hao, MD, of Xinhua Hospital, Shanghai (China) Jiaotong University, presented these results at the International Myeloma Workshop held by the International Myeloma Society.
Dr. Hao explained that CT053 consists of autologous T cells modified with a second-generation CAR that incorporates a fully human anti–B-cell maturation antigen single-chain fragment variant, a 4-1BB costimulatory domain, and a CD3-zeta–signaling domain.
In preclinical studies, CT053 induced dose-dependent cytotoxic effects on multiple myeloma cell lines and completely eradicated myeloma in mice.
Dr. Hao and his colleagues conducted the phase 1 study of CT053 at three sites (NCT03716856, NCT03302403, and NCT03380039). The study enrolled 30 patients with relapsed/refractory multiple myeloma, and 24 ultimately received CT053.
In the 24 patients, the median age was 60.2 years (range, 38.5-70.0 years), and the median time since diagnosis was 3.5 years (range, 0.3-10.8 years). Nine patients had progressive disease at baseline.
The patients had received a median of 5 prior therapies (range, 2-12). All patients had received a proteasome inhibitor, 22 had received an immunomodulatory agent, 10 had undergone a transplant, and 5 had received an anti-CD38 monoclonal antibody.
For this study, patients received conditioning with fludarabine and cyclophosphamide, followed by a single infusion of CT053. Most patients (n = 21) received 1.5 x 108 cells, but three received 0.5 x 108, 1 x 108, and 1.8 x 108 cells, respectively.
The median follow-up was 333 days. CAR T cells were detectable 1-7 days after infusion and peaked at 7-21 days. The cells persisted for a median of 172 days (range, 21-341 days).
A total of 21 patients responded to treatment (87.5%). There were 19 patients with a complete response or stringent complete response, 1 patient with a very good partial response, and 1 with a partial response.
Dr. Hao noted that CT053 was effective even at the lowest dose. The patient who received 0.5 x 108 cells initially achieved a very good partial response that deepened to a stringent complete response on day 502 after infusion.
Ten patients still had a stringent complete response at last follow-up, and two had a complete response. Nine patients progressed, and one patient relapsed after achieving a complete response. Three patients died, two from disease progression and one from a serious AE (neutropenic infection).
All 24 patients experienced a treatment-related AE, and all had grade 3 or higher hematologic AEs. Six patients had grade 3 or higher fever, six had grade 3 or higher infections and infestations, and one had grade 3 or higher neurotoxicity.
Three patients had grade 1 CRS, and 12 had grade 2 CRS. None of the patients had grade 3 or higher CRS.
This trial was sponsored by Xinhua Hospital/Shanghai Jiao Tong University School of Medicine, First Affiliated Hospital of Zhejiang University, and First Affiliated Hospital of Wenzhou Medical University in collaboration with Carsgen Therapeutics. Dr. Hao did not disclose any conflicts of interest.
SOURCE: Hao S et al. IMW 2019, Abstract OAB-082.
REPORTING FROM IMW 2019
Subcutaneous and IV daratumumab combos appear comparable in myeloma
BOSTON – Subcutaneous daratumumab in combination with standard care is comparable to intravenous daratumumab plus standard care in patients with newly diagnosed or relapsed/refractory multiple myeloma, according to a speaker at the International Myeloma Workshop.
Overall response rates (ORRs) observed with subcutaneous daratumumab–based combinations in the phase 2 PLEIADES trial were similar to ORRs observed with intravenous daratumumab–based combinations in three other trials – GRIFFIN, ALCYONE, and POLLUX.
Ajai Chari, MD, of the Icahn School of Medicine at Mount Sinai, New York, presented these findings at the workshop, which is held by the International Myeloma Society.
In the PLEIADES trial, researchers tested subcutaneous daratumumab (D) in combination with:
- Bortezomib, lenalidomide, and dexamethasone (VRd) in transplant-eligible patients with newly diagnosed multiple myeloma
- Bortezomib, melphalan, and prednisone (VMP) in transplant-ineligible patients with newly diagnosed multiple myeloma
- Lenalidomide and dexamethasone (Rd) in patients with relapsed/refractory multiple myeloma who had received at least one prior line of therapy.
There were 67 patients in the D-VRd arm, and they had a median age of 59 years (range, 33-76 years). There were 67 patients in the D-VMP arm, and they had a median age of 75 years (range, 66-86 years). There were 65 patients in the D-Rd arm, they had a median age of 69 years (range, 33-82 years), and they had received a median of one (range, one to five) prior therapies.
Dr. Chari noted that baseline characteristics in this study were “pretty comparable” to characteristics in the studies of intravenous daratumumab. He also pointed out that the median administration time for subcutaneous daratumumab was 5 minutes in this study, which is “substantially” shorter than the typical administration time for intravenous daratumumab.
The median number of treatment cycles was 4 (range, 1-4) in the D-VRd arm, 8 (range, 1-10) in the D-VMP arm, and 12 (range, 1-15) in the D-Rd arm. The median duration of treatment was 2.6 months, 10.6 months, and 11.1 months, respectively.
The proportion of patients who discontinued treatment was 3% in the D-VRd arm, 10.4% in the D-VMP arm, and 20% in the D-Rd arm.
Response
Dr. Chari said response rates in the three arms of PLEAIDES were similar to response rates in corresponding groups from the studies of intravenous daratumumab–based combinations.
After four induction cycles, subcutaneous D-VRd produced an ORR of 97% in PLEAIDES, and intravenous D-VRd produced an ORR of 98% in the GRIFFIN trial (IMW 2019. Abstract OAB-087).
Subcutaneous D-VMP produced an ORR of 89.6% at a median follow-up of 11 months. In the ALCYONE trial, intravenous D-VMP produced an ORR of 90.9% at a median follow-up of 16.5 months (N Engl J Med. 2018; 378:518-28).
Subcutaneous D-Rd produced an ORR of 93.8% at a median follow-up of 11.2 months. In the POLLUX trial, intravenous D-Rd produced an ORR of 92.9% at a median follow-up of 13.5 months (N Engl J Med. 2016; 375:1319-31).
Safety
All patients in PLEIADES had treatment-related adverse events (TEAEs). The rate of serious TEAEs was 28.4% in the D-VRd arm, 38.8% in the D-VMP arm, and 47.7% in the D-Rd arm. The rate of grade 3/4 TEAEs was 56.7%, 68.7%, and 83.1%, respectively. There was one fatal TEAE in the D-VRd arm, two fatal TEAEs in the D-VMP arm, and two in the D-Rd arm.
Infusion-related reactions occurred in 7.5% of all patients (15/199). Most infusion-related reactions were grade 1/2. One patient had a grade 3 reaction, and there were no grade 4 reactions. The median time to onset was 3.3 hours.
“Daratumumab in combination with standard of care, when given subcutaneously, demonstrated comparable clinical activity and safety and corresponded to daratumumab intravenous–containing regimens,” Dr. Chari said. “These results support the use of flat-dose 1,800 mg [subcutaneous daratumumab] in combination with standard treatment regimens.”
The PLEIADES trial was sponsored by Janssen Research & Development. Dr. Chari reported relationships with Janssen and several other companies.
SOURCE: Chari A et al. IMW 2019, Abstract OAB-022.
BOSTON – Subcutaneous daratumumab in combination with standard care is comparable to intravenous daratumumab plus standard care in patients with newly diagnosed or relapsed/refractory multiple myeloma, according to a speaker at the International Myeloma Workshop.
Overall response rates (ORRs) observed with subcutaneous daratumumab–based combinations in the phase 2 PLEIADES trial were similar to ORRs observed with intravenous daratumumab–based combinations in three other trials – GRIFFIN, ALCYONE, and POLLUX.
Ajai Chari, MD, of the Icahn School of Medicine at Mount Sinai, New York, presented these findings at the workshop, which is held by the International Myeloma Society.
In the PLEIADES trial, researchers tested subcutaneous daratumumab (D) in combination with:
- Bortezomib, lenalidomide, and dexamethasone (VRd) in transplant-eligible patients with newly diagnosed multiple myeloma
- Bortezomib, melphalan, and prednisone (VMP) in transplant-ineligible patients with newly diagnosed multiple myeloma
- Lenalidomide and dexamethasone (Rd) in patients with relapsed/refractory multiple myeloma who had received at least one prior line of therapy.
There were 67 patients in the D-VRd arm, and they had a median age of 59 years (range, 33-76 years). There were 67 patients in the D-VMP arm, and they had a median age of 75 years (range, 66-86 years). There were 65 patients in the D-Rd arm, they had a median age of 69 years (range, 33-82 years), and they had received a median of one (range, one to five) prior therapies.
Dr. Chari noted that baseline characteristics in this study were “pretty comparable” to characteristics in the studies of intravenous daratumumab. He also pointed out that the median administration time for subcutaneous daratumumab was 5 minutes in this study, which is “substantially” shorter than the typical administration time for intravenous daratumumab.
The median number of treatment cycles was 4 (range, 1-4) in the D-VRd arm, 8 (range, 1-10) in the D-VMP arm, and 12 (range, 1-15) in the D-Rd arm. The median duration of treatment was 2.6 months, 10.6 months, and 11.1 months, respectively.
The proportion of patients who discontinued treatment was 3% in the D-VRd arm, 10.4% in the D-VMP arm, and 20% in the D-Rd arm.
Response
Dr. Chari said response rates in the three arms of PLEAIDES were similar to response rates in corresponding groups from the studies of intravenous daratumumab–based combinations.
After four induction cycles, subcutaneous D-VRd produced an ORR of 97% in PLEAIDES, and intravenous D-VRd produced an ORR of 98% in the GRIFFIN trial (IMW 2019. Abstract OAB-087).
Subcutaneous D-VMP produced an ORR of 89.6% at a median follow-up of 11 months. In the ALCYONE trial, intravenous D-VMP produced an ORR of 90.9% at a median follow-up of 16.5 months (N Engl J Med. 2018; 378:518-28).
Subcutaneous D-Rd produced an ORR of 93.8% at a median follow-up of 11.2 months. In the POLLUX trial, intravenous D-Rd produced an ORR of 92.9% at a median follow-up of 13.5 months (N Engl J Med. 2016; 375:1319-31).
Safety
All patients in PLEIADES had treatment-related adverse events (TEAEs). The rate of serious TEAEs was 28.4% in the D-VRd arm, 38.8% in the D-VMP arm, and 47.7% in the D-Rd arm. The rate of grade 3/4 TEAEs was 56.7%, 68.7%, and 83.1%, respectively. There was one fatal TEAE in the D-VRd arm, two fatal TEAEs in the D-VMP arm, and two in the D-Rd arm.
Infusion-related reactions occurred in 7.5% of all patients (15/199). Most infusion-related reactions were grade 1/2. One patient had a grade 3 reaction, and there were no grade 4 reactions. The median time to onset was 3.3 hours.
“Daratumumab in combination with standard of care, when given subcutaneously, demonstrated comparable clinical activity and safety and corresponded to daratumumab intravenous–containing regimens,” Dr. Chari said. “These results support the use of flat-dose 1,800 mg [subcutaneous daratumumab] in combination with standard treatment regimens.”
The PLEIADES trial was sponsored by Janssen Research & Development. Dr. Chari reported relationships with Janssen and several other companies.
SOURCE: Chari A et al. IMW 2019, Abstract OAB-022.
BOSTON – Subcutaneous daratumumab in combination with standard care is comparable to intravenous daratumumab plus standard care in patients with newly diagnosed or relapsed/refractory multiple myeloma, according to a speaker at the International Myeloma Workshop.
Overall response rates (ORRs) observed with subcutaneous daratumumab–based combinations in the phase 2 PLEIADES trial were similar to ORRs observed with intravenous daratumumab–based combinations in three other trials – GRIFFIN, ALCYONE, and POLLUX.
Ajai Chari, MD, of the Icahn School of Medicine at Mount Sinai, New York, presented these findings at the workshop, which is held by the International Myeloma Society.
In the PLEIADES trial, researchers tested subcutaneous daratumumab (D) in combination with:
- Bortezomib, lenalidomide, and dexamethasone (VRd) in transplant-eligible patients with newly diagnosed multiple myeloma
- Bortezomib, melphalan, and prednisone (VMP) in transplant-ineligible patients with newly diagnosed multiple myeloma
- Lenalidomide and dexamethasone (Rd) in patients with relapsed/refractory multiple myeloma who had received at least one prior line of therapy.
There were 67 patients in the D-VRd arm, and they had a median age of 59 years (range, 33-76 years). There were 67 patients in the D-VMP arm, and they had a median age of 75 years (range, 66-86 years). There were 65 patients in the D-Rd arm, they had a median age of 69 years (range, 33-82 years), and they had received a median of one (range, one to five) prior therapies.
Dr. Chari noted that baseline characteristics in this study were “pretty comparable” to characteristics in the studies of intravenous daratumumab. He also pointed out that the median administration time for subcutaneous daratumumab was 5 minutes in this study, which is “substantially” shorter than the typical administration time for intravenous daratumumab.
The median number of treatment cycles was 4 (range, 1-4) in the D-VRd arm, 8 (range, 1-10) in the D-VMP arm, and 12 (range, 1-15) in the D-Rd arm. The median duration of treatment was 2.6 months, 10.6 months, and 11.1 months, respectively.
The proportion of patients who discontinued treatment was 3% in the D-VRd arm, 10.4% in the D-VMP arm, and 20% in the D-Rd arm.
Response
Dr. Chari said response rates in the three arms of PLEAIDES were similar to response rates in corresponding groups from the studies of intravenous daratumumab–based combinations.
After four induction cycles, subcutaneous D-VRd produced an ORR of 97% in PLEAIDES, and intravenous D-VRd produced an ORR of 98% in the GRIFFIN trial (IMW 2019. Abstract OAB-087).
Subcutaneous D-VMP produced an ORR of 89.6% at a median follow-up of 11 months. In the ALCYONE trial, intravenous D-VMP produced an ORR of 90.9% at a median follow-up of 16.5 months (N Engl J Med. 2018; 378:518-28).
Subcutaneous D-Rd produced an ORR of 93.8% at a median follow-up of 11.2 months. In the POLLUX trial, intravenous D-Rd produced an ORR of 92.9% at a median follow-up of 13.5 months (N Engl J Med. 2016; 375:1319-31).
Safety
All patients in PLEIADES had treatment-related adverse events (TEAEs). The rate of serious TEAEs was 28.4% in the D-VRd arm, 38.8% in the D-VMP arm, and 47.7% in the D-Rd arm. The rate of grade 3/4 TEAEs was 56.7%, 68.7%, and 83.1%, respectively. There was one fatal TEAE in the D-VRd arm, two fatal TEAEs in the D-VMP arm, and two in the D-Rd arm.
Infusion-related reactions occurred in 7.5% of all patients (15/199). Most infusion-related reactions were grade 1/2. One patient had a grade 3 reaction, and there were no grade 4 reactions. The median time to onset was 3.3 hours.
“Daratumumab in combination with standard of care, when given subcutaneously, demonstrated comparable clinical activity and safety and corresponded to daratumumab intravenous–containing regimens,” Dr. Chari said. “These results support the use of flat-dose 1,800 mg [subcutaneous daratumumab] in combination with standard treatment regimens.”
The PLEIADES trial was sponsored by Janssen Research & Development. Dr. Chari reported relationships with Janssen and several other companies.
SOURCE: Chari A et al. IMW 2019, Abstract OAB-022.
REPORTING FROM IMW 2019
Quadruplet prolongs progression-free survival in newly diagnosed myeloma
BOSTON – A carfilzomib-based quadruplet can improve outcomes in transplant-eligible patients with newly diagnosed multiple myeloma, a phase 3 trial suggests.
In the Myeloma XI trial, carfilzomib plus cyclophosphamide, lenalidomide, and dexamethasone (KCRD) significantly prolonged progression-free survival (PFS), compared with cyclophosphamide-lenalidomide-dexamethasone (CRD) or cyclophosphamide-thalidomide-dexamethasone (CTD).
“KCRD was associated with a very high response rate and a high MRD [minimal residual disease]-negative rate at the end of induction, and it significantly improved progression-free survival compared to the triplet combinations,” said Charlotte Pawlyn, PhD, of The Institute of Cancer Research in London.
Dr. Pawlyn reported these findings at the International Myeloma Workshop held by the International Myeloma Society.
The phase 3 Myeloma XI trial enrolled 1,056 patients with newly diagnosed myeloma who were eligible for transplant. The patients were randomized to receive KCRD (n = 526), CRD (n = 265), or CTD (n = 265) as induction.
Baseline characteristics were well balanced between the treatment arms. The median age was 61 years in the KCRD and CTD arms and 62 years in the CRD arm (overall range, 33-75 years). Roughly 60% of patients in each arm were men.
About 50%-60% of patients in each arm had standard-risk cytogenetics, which was defined as the absence of any cytogenetic lesions. About 30%-40% of patients in each arm had high-risk cytogenetics, meaning they had one of the following lesions: t(4;14), t(14;16), t(14;20), del (17p), or gain(1q). About 10% of patients in each arm had ultra-high-risk cytogenetics, which was defined as having more than one lesion.
Treatment
For induction, patients were randomized to KCRD, CRD, or CTD. All patients in the KCRD arm and patients in the CRD/CTD arms who achieved a partial response or better went straight to autologous transplant after induction. Nonresponders in the CTD and CRD arms received intensification with cyclophosphamide, bortezomib, and dexamethasone before transplant.
After transplant, all eligible patients were randomized to lenalidomide maintenance or observation. Patients were eligible for this randomization if they didn’t respond to induction, had progressive disease, or had previous or concurrent active malignancies.
The median follow-up was 34.5 months. The median number of induction cycles completed was 4 (range, 1-12) in the KCRD arm, 5 (range, 1-15) in the CRD arm, and 6 (range, 1-13) in the CTD arm.
Response
At the end of induction, the rate of very good partial response or better was 82.3% in the KCRD arm, 64.9% in the CRD arm, 52.8% in the CTD arm, and 58.9% in the CTD-CRD arms combined. The odds ratio for the KCRD group compared to the triplets combined was 4.35 (P less than .0001).
At 100 days after transplant, the rate of very good partial response or better was 91.9% in the KCRD arm, 82.1% in the CRD arm, 76.1% in the CTD arm, and 79.3% in the CTD-CRD arms combined. The odds ratio for the KCRD group compared to the triplets combined was 3.01 (P less than .0001).
KCRD produced a higher proportion of MRD-negative responses both before and after transplant. After induction, the rate of MRD-negative response was 11% in the CTD arm, 21% in the CRD arm, and 55% in the KCRD arm. After transplant, the rates were 51%, 49%, and 77%, respectively.
Survival
KCRD improved PFS. The 3-year PFS rate was 64.5% in the KCRD arm and 50.3% in the CTD-CRD arms combined. The hazard ratio (HR) was 0.63 (P less than .0001).
The PFS benefit with KCRD was present in all patient subgroups. For example, KCRD improved PFS, compared with CTD-CRD, in patients with standard-risk (HR = 0.62), high-risk (HR = 0.68), and ultra-high-risk (HR = 0.50) cytogenetics.
Patients who achieved an MRD-negative response had better PFS, and early achievement of MRD negativity was associated with improved PFS, Dr. Pawlyn noted.
“But what’s also notable ... is that those patients who received KCRD and achieved MRD negativity ... had better outcomes than patients who achieved MRD negativity whilst receiving a triplet combination,” Dr. Pawlyn said. “So this suggests that the induction regimen delivered is important, not just the achievement of MRD negativity at a defined cutoff.”
Dr. Pawlyn added that overall survival data from this study are not yet mature, but the researchers did assess PFS2. PFS2 was defined as the time from randomization to second disease progression. The 3-year PFS2 was 81.8% in the KCRD arm and 75.1% in the CTD-CRD arms combined. The HR was 0.75 (P = .0451).
Myeloma XI is sponsored by University of Leeds in collaboration with Celgene, Merck Sharp & Dohme, and Amgen. Dr. Pawlyn reported relationships with Amgen, Celgene, and other companies.
SOURCE: Pawlyn C et al. IMW 2019, Abstract OAB-002.
BOSTON – A carfilzomib-based quadruplet can improve outcomes in transplant-eligible patients with newly diagnosed multiple myeloma, a phase 3 trial suggests.
In the Myeloma XI trial, carfilzomib plus cyclophosphamide, lenalidomide, and dexamethasone (KCRD) significantly prolonged progression-free survival (PFS), compared with cyclophosphamide-lenalidomide-dexamethasone (CRD) or cyclophosphamide-thalidomide-dexamethasone (CTD).
“KCRD was associated with a very high response rate and a high MRD [minimal residual disease]-negative rate at the end of induction, and it significantly improved progression-free survival compared to the triplet combinations,” said Charlotte Pawlyn, PhD, of The Institute of Cancer Research in London.
Dr. Pawlyn reported these findings at the International Myeloma Workshop held by the International Myeloma Society.
The phase 3 Myeloma XI trial enrolled 1,056 patients with newly diagnosed myeloma who were eligible for transplant. The patients were randomized to receive KCRD (n = 526), CRD (n = 265), or CTD (n = 265) as induction.
Baseline characteristics were well balanced between the treatment arms. The median age was 61 years in the KCRD and CTD arms and 62 years in the CRD arm (overall range, 33-75 years). Roughly 60% of patients in each arm were men.
About 50%-60% of patients in each arm had standard-risk cytogenetics, which was defined as the absence of any cytogenetic lesions. About 30%-40% of patients in each arm had high-risk cytogenetics, meaning they had one of the following lesions: t(4;14), t(14;16), t(14;20), del (17p), or gain(1q). About 10% of patients in each arm had ultra-high-risk cytogenetics, which was defined as having more than one lesion.
Treatment
For induction, patients were randomized to KCRD, CRD, or CTD. All patients in the KCRD arm and patients in the CRD/CTD arms who achieved a partial response or better went straight to autologous transplant after induction. Nonresponders in the CTD and CRD arms received intensification with cyclophosphamide, bortezomib, and dexamethasone before transplant.
After transplant, all eligible patients were randomized to lenalidomide maintenance or observation. Patients were eligible for this randomization if they didn’t respond to induction, had progressive disease, or had previous or concurrent active malignancies.
The median follow-up was 34.5 months. The median number of induction cycles completed was 4 (range, 1-12) in the KCRD arm, 5 (range, 1-15) in the CRD arm, and 6 (range, 1-13) in the CTD arm.
Response
At the end of induction, the rate of very good partial response or better was 82.3% in the KCRD arm, 64.9% in the CRD arm, 52.8% in the CTD arm, and 58.9% in the CTD-CRD arms combined. The odds ratio for the KCRD group compared to the triplets combined was 4.35 (P less than .0001).
At 100 days after transplant, the rate of very good partial response or better was 91.9% in the KCRD arm, 82.1% in the CRD arm, 76.1% in the CTD arm, and 79.3% in the CTD-CRD arms combined. The odds ratio for the KCRD group compared to the triplets combined was 3.01 (P less than .0001).
KCRD produced a higher proportion of MRD-negative responses both before and after transplant. After induction, the rate of MRD-negative response was 11% in the CTD arm, 21% in the CRD arm, and 55% in the KCRD arm. After transplant, the rates were 51%, 49%, and 77%, respectively.
Survival
KCRD improved PFS. The 3-year PFS rate was 64.5% in the KCRD arm and 50.3% in the CTD-CRD arms combined. The hazard ratio (HR) was 0.63 (P less than .0001).
The PFS benefit with KCRD was present in all patient subgroups. For example, KCRD improved PFS, compared with CTD-CRD, in patients with standard-risk (HR = 0.62), high-risk (HR = 0.68), and ultra-high-risk (HR = 0.50) cytogenetics.
Patients who achieved an MRD-negative response had better PFS, and early achievement of MRD negativity was associated with improved PFS, Dr. Pawlyn noted.
“But what’s also notable ... is that those patients who received KCRD and achieved MRD negativity ... had better outcomes than patients who achieved MRD negativity whilst receiving a triplet combination,” Dr. Pawlyn said. “So this suggests that the induction regimen delivered is important, not just the achievement of MRD negativity at a defined cutoff.”
Dr. Pawlyn added that overall survival data from this study are not yet mature, but the researchers did assess PFS2. PFS2 was defined as the time from randomization to second disease progression. The 3-year PFS2 was 81.8% in the KCRD arm and 75.1% in the CTD-CRD arms combined. The HR was 0.75 (P = .0451).
Myeloma XI is sponsored by University of Leeds in collaboration with Celgene, Merck Sharp & Dohme, and Amgen. Dr. Pawlyn reported relationships with Amgen, Celgene, and other companies.
SOURCE: Pawlyn C et al. IMW 2019, Abstract OAB-002.
BOSTON – A carfilzomib-based quadruplet can improve outcomes in transplant-eligible patients with newly diagnosed multiple myeloma, a phase 3 trial suggests.
In the Myeloma XI trial, carfilzomib plus cyclophosphamide, lenalidomide, and dexamethasone (KCRD) significantly prolonged progression-free survival (PFS), compared with cyclophosphamide-lenalidomide-dexamethasone (CRD) or cyclophosphamide-thalidomide-dexamethasone (CTD).
“KCRD was associated with a very high response rate and a high MRD [minimal residual disease]-negative rate at the end of induction, and it significantly improved progression-free survival compared to the triplet combinations,” said Charlotte Pawlyn, PhD, of The Institute of Cancer Research in London.
Dr. Pawlyn reported these findings at the International Myeloma Workshop held by the International Myeloma Society.
The phase 3 Myeloma XI trial enrolled 1,056 patients with newly diagnosed myeloma who were eligible for transplant. The patients were randomized to receive KCRD (n = 526), CRD (n = 265), or CTD (n = 265) as induction.
Baseline characteristics were well balanced between the treatment arms. The median age was 61 years in the KCRD and CTD arms and 62 years in the CRD arm (overall range, 33-75 years). Roughly 60% of patients in each arm were men.
About 50%-60% of patients in each arm had standard-risk cytogenetics, which was defined as the absence of any cytogenetic lesions. About 30%-40% of patients in each arm had high-risk cytogenetics, meaning they had one of the following lesions: t(4;14), t(14;16), t(14;20), del (17p), or gain(1q). About 10% of patients in each arm had ultra-high-risk cytogenetics, which was defined as having more than one lesion.
Treatment
For induction, patients were randomized to KCRD, CRD, or CTD. All patients in the KCRD arm and patients in the CRD/CTD arms who achieved a partial response or better went straight to autologous transplant after induction. Nonresponders in the CTD and CRD arms received intensification with cyclophosphamide, bortezomib, and dexamethasone before transplant.
After transplant, all eligible patients were randomized to lenalidomide maintenance or observation. Patients were eligible for this randomization if they didn’t respond to induction, had progressive disease, or had previous or concurrent active malignancies.
The median follow-up was 34.5 months. The median number of induction cycles completed was 4 (range, 1-12) in the KCRD arm, 5 (range, 1-15) in the CRD arm, and 6 (range, 1-13) in the CTD arm.
Response
At the end of induction, the rate of very good partial response or better was 82.3% in the KCRD arm, 64.9% in the CRD arm, 52.8% in the CTD arm, and 58.9% in the CTD-CRD arms combined. The odds ratio for the KCRD group compared to the triplets combined was 4.35 (P less than .0001).
At 100 days after transplant, the rate of very good partial response or better was 91.9% in the KCRD arm, 82.1% in the CRD arm, 76.1% in the CTD arm, and 79.3% in the CTD-CRD arms combined. The odds ratio for the KCRD group compared to the triplets combined was 3.01 (P less than .0001).
KCRD produced a higher proportion of MRD-negative responses both before and after transplant. After induction, the rate of MRD-negative response was 11% in the CTD arm, 21% in the CRD arm, and 55% in the KCRD arm. After transplant, the rates were 51%, 49%, and 77%, respectively.
Survival
KCRD improved PFS. The 3-year PFS rate was 64.5% in the KCRD arm and 50.3% in the CTD-CRD arms combined. The hazard ratio (HR) was 0.63 (P less than .0001).
The PFS benefit with KCRD was present in all patient subgroups. For example, KCRD improved PFS, compared with CTD-CRD, in patients with standard-risk (HR = 0.62), high-risk (HR = 0.68), and ultra-high-risk (HR = 0.50) cytogenetics.
Patients who achieved an MRD-negative response had better PFS, and early achievement of MRD negativity was associated with improved PFS, Dr. Pawlyn noted.
“But what’s also notable ... is that those patients who received KCRD and achieved MRD negativity ... had better outcomes than patients who achieved MRD negativity whilst receiving a triplet combination,” Dr. Pawlyn said. “So this suggests that the induction regimen delivered is important, not just the achievement of MRD negativity at a defined cutoff.”
Dr. Pawlyn added that overall survival data from this study are not yet mature, but the researchers did assess PFS2. PFS2 was defined as the time from randomization to second disease progression. The 3-year PFS2 was 81.8% in the KCRD arm and 75.1% in the CTD-CRD arms combined. The HR was 0.75 (P = .0451).
Myeloma XI is sponsored by University of Leeds in collaboration with Celgene, Merck Sharp & Dohme, and Amgen. Dr. Pawlyn reported relationships with Amgen, Celgene, and other companies.
SOURCE: Pawlyn C et al. IMW 2019, Abstract OAB-002.
REPORTING FROM IMW 2019
Oral triplet shows promise for relapsed/refractory myeloma
BOSTON – The all-oral combination of selinexor, lenalidomide, and dexamethasone has demonstrated efficacy in patients with relapsed/refractory multiple myeloma, particularly those who are lenalidomide naive.
In the phase 1/2 STOMP trial (NCT02343042), selinexor plus lenalidomide and dexamethasone produced an overall response rate (ORR) of 60% in all evaluable patients and an ORR of 92% in lenalidomide-naive patients.
Darrell White, MD, of Dalhousie University in Halifax, N.S., presented these results at the International Myeloma Workshop held by the International Myeloma Society.
Dr. White discussed results in myeloma patients who had received at least one prior line of therapy. This arm of the STOMP trial has enrolled 24 patients. Their median age at baseline was 67 years (range, 49-84 years), and their median time from diagnosis was 4.5 years (range, less than 1-22 years).
The patients had received a median of 1 (range, 1-8) prior therapies. Half of patients had received a transplant. All patients had received a proteasome inhibitor (65% refractory), and 38% had received prior lenalidomide (21% refractory).
Dosing
Patients received selinexor at 60 mg once or twice weekly or at 80 mg once weekly on a 28-day cycle. They received dexamethasone at 20 mg twice weekly or 40 mg once weekly and lenalidomide at 25 mg once daily every 21 days.
The recommended phase 2 dosing schedule was selinexor at 60 mg once weekly plus lenalidomide at 25 mg daily and dexamethasone at 40 mg once weekly. Half of patients (n = 12) received this dosing schedule.
There were no dose-limiting toxicities (DLTs) at the recommended phase 2 dose. When selinexor was given at 60 mg twice weekly, DLTs included grade 3 fatigue, grade 3 anorexia and weight loss, and grade 4 thrombocytopenia (n = 2). When selinexor was given at 80 mg once weekly, the DLTs were grade 4 thrombocytopenia (n = 2).
Safety
“The side-effect profile was as expected, with mainly grade 3/4 toxicity being hematologic and primarily thrombocytopenia and neutropenia,” Dr. White said. “Frequent gastrointestinal side effects [were] expected. Investigators were able to manage that with appropriate supportive care and antiemetics in particular.”
Among patients who received the recommended phase 2 dose, the grade 4 treatment-related adverse events (AEs) were thrombocytopenia (n = 4) and neutropenia (n = 4). Grade 3 treatment-related AEs were thrombocytopenia (n = 3), neutropenia (n = 4), anemia (n = 1), nausea (n = 1), asthenia (n = 1), fatigue (n = 2), and dehydration (n = 1). Common grade 1/2 treatment-related AEs in patients who received the recommended phase 2 dose were nausea (n = 6), anorexia (n = 5), weight loss (n = 5), vomiting (n = 4), diarrhea (n = 4), and fatigue (n = 4).
Efficacy
In the 20 patients evaluable for response, the ORR was 60% (n = 12). One patient achieved a stringent complete response, three had a very good partial response, and eight had a partial response. The median time to response was 1 month.
The ORR was 92% (11/12) in lenalidomide-naive patients and 13% (1/8) in lenalidomide-exposed patients. The single responder in the lenalidomide-exposed group achieved a partial response.
Among lenalidomide-naive patients who received the recommended phase 2 dose, the median time on treatment was 12 months. The patient who achieved a stringent complete response remained on treatment at last follow-up, as did one partial responder and one patient with a very good partial response.
These results suggest the combination of selinexor, lenalidomide, and dexamethasone “is active, relatively well tolerated, and could warrant further investigation,” Dr. White said.
The STOMP trial is sponsored by Karyopharm Therapeutics. Dr. White disclosed relationships with Karyopharm, Amgen, Celgene, Janssen, Sanofi, and Takeda.
SOURCE: White D et al. IMW 2019, Abstract OAB-083.
BOSTON – The all-oral combination of selinexor, lenalidomide, and dexamethasone has demonstrated efficacy in patients with relapsed/refractory multiple myeloma, particularly those who are lenalidomide naive.
In the phase 1/2 STOMP trial (NCT02343042), selinexor plus lenalidomide and dexamethasone produced an overall response rate (ORR) of 60% in all evaluable patients and an ORR of 92% in lenalidomide-naive patients.
Darrell White, MD, of Dalhousie University in Halifax, N.S., presented these results at the International Myeloma Workshop held by the International Myeloma Society.
Dr. White discussed results in myeloma patients who had received at least one prior line of therapy. This arm of the STOMP trial has enrolled 24 patients. Their median age at baseline was 67 years (range, 49-84 years), and their median time from diagnosis was 4.5 years (range, less than 1-22 years).
The patients had received a median of 1 (range, 1-8) prior therapies. Half of patients had received a transplant. All patients had received a proteasome inhibitor (65% refractory), and 38% had received prior lenalidomide (21% refractory).
Dosing
Patients received selinexor at 60 mg once or twice weekly or at 80 mg once weekly on a 28-day cycle. They received dexamethasone at 20 mg twice weekly or 40 mg once weekly and lenalidomide at 25 mg once daily every 21 days.
The recommended phase 2 dosing schedule was selinexor at 60 mg once weekly plus lenalidomide at 25 mg daily and dexamethasone at 40 mg once weekly. Half of patients (n = 12) received this dosing schedule.
There were no dose-limiting toxicities (DLTs) at the recommended phase 2 dose. When selinexor was given at 60 mg twice weekly, DLTs included grade 3 fatigue, grade 3 anorexia and weight loss, and grade 4 thrombocytopenia (n = 2). When selinexor was given at 80 mg once weekly, the DLTs were grade 4 thrombocytopenia (n = 2).
Safety
“The side-effect profile was as expected, with mainly grade 3/4 toxicity being hematologic and primarily thrombocytopenia and neutropenia,” Dr. White said. “Frequent gastrointestinal side effects [were] expected. Investigators were able to manage that with appropriate supportive care and antiemetics in particular.”
Among patients who received the recommended phase 2 dose, the grade 4 treatment-related adverse events (AEs) were thrombocytopenia (n = 4) and neutropenia (n = 4). Grade 3 treatment-related AEs were thrombocytopenia (n = 3), neutropenia (n = 4), anemia (n = 1), nausea (n = 1), asthenia (n = 1), fatigue (n = 2), and dehydration (n = 1). Common grade 1/2 treatment-related AEs in patients who received the recommended phase 2 dose were nausea (n = 6), anorexia (n = 5), weight loss (n = 5), vomiting (n = 4), diarrhea (n = 4), and fatigue (n = 4).
Efficacy
In the 20 patients evaluable for response, the ORR was 60% (n = 12). One patient achieved a stringent complete response, three had a very good partial response, and eight had a partial response. The median time to response was 1 month.
The ORR was 92% (11/12) in lenalidomide-naive patients and 13% (1/8) in lenalidomide-exposed patients. The single responder in the lenalidomide-exposed group achieved a partial response.
Among lenalidomide-naive patients who received the recommended phase 2 dose, the median time on treatment was 12 months. The patient who achieved a stringent complete response remained on treatment at last follow-up, as did one partial responder and one patient with a very good partial response.
These results suggest the combination of selinexor, lenalidomide, and dexamethasone “is active, relatively well tolerated, and could warrant further investigation,” Dr. White said.
The STOMP trial is sponsored by Karyopharm Therapeutics. Dr. White disclosed relationships with Karyopharm, Amgen, Celgene, Janssen, Sanofi, and Takeda.
SOURCE: White D et al. IMW 2019, Abstract OAB-083.
BOSTON – The all-oral combination of selinexor, lenalidomide, and dexamethasone has demonstrated efficacy in patients with relapsed/refractory multiple myeloma, particularly those who are lenalidomide naive.
In the phase 1/2 STOMP trial (NCT02343042), selinexor plus lenalidomide and dexamethasone produced an overall response rate (ORR) of 60% in all evaluable patients and an ORR of 92% in lenalidomide-naive patients.
Darrell White, MD, of Dalhousie University in Halifax, N.S., presented these results at the International Myeloma Workshop held by the International Myeloma Society.
Dr. White discussed results in myeloma patients who had received at least one prior line of therapy. This arm of the STOMP trial has enrolled 24 patients. Their median age at baseline was 67 years (range, 49-84 years), and their median time from diagnosis was 4.5 years (range, less than 1-22 years).
The patients had received a median of 1 (range, 1-8) prior therapies. Half of patients had received a transplant. All patients had received a proteasome inhibitor (65% refractory), and 38% had received prior lenalidomide (21% refractory).
Dosing
Patients received selinexor at 60 mg once or twice weekly or at 80 mg once weekly on a 28-day cycle. They received dexamethasone at 20 mg twice weekly or 40 mg once weekly and lenalidomide at 25 mg once daily every 21 days.
The recommended phase 2 dosing schedule was selinexor at 60 mg once weekly plus lenalidomide at 25 mg daily and dexamethasone at 40 mg once weekly. Half of patients (n = 12) received this dosing schedule.
There were no dose-limiting toxicities (DLTs) at the recommended phase 2 dose. When selinexor was given at 60 mg twice weekly, DLTs included grade 3 fatigue, grade 3 anorexia and weight loss, and grade 4 thrombocytopenia (n = 2). When selinexor was given at 80 mg once weekly, the DLTs were grade 4 thrombocytopenia (n = 2).
Safety
“The side-effect profile was as expected, with mainly grade 3/4 toxicity being hematologic and primarily thrombocytopenia and neutropenia,” Dr. White said. “Frequent gastrointestinal side effects [were] expected. Investigators were able to manage that with appropriate supportive care and antiemetics in particular.”
Among patients who received the recommended phase 2 dose, the grade 4 treatment-related adverse events (AEs) were thrombocytopenia (n = 4) and neutropenia (n = 4). Grade 3 treatment-related AEs were thrombocytopenia (n = 3), neutropenia (n = 4), anemia (n = 1), nausea (n = 1), asthenia (n = 1), fatigue (n = 2), and dehydration (n = 1). Common grade 1/2 treatment-related AEs in patients who received the recommended phase 2 dose were nausea (n = 6), anorexia (n = 5), weight loss (n = 5), vomiting (n = 4), diarrhea (n = 4), and fatigue (n = 4).
Efficacy
In the 20 patients evaluable for response, the ORR was 60% (n = 12). One patient achieved a stringent complete response, three had a very good partial response, and eight had a partial response. The median time to response was 1 month.
The ORR was 92% (11/12) in lenalidomide-naive patients and 13% (1/8) in lenalidomide-exposed patients. The single responder in the lenalidomide-exposed group achieved a partial response.
Among lenalidomide-naive patients who received the recommended phase 2 dose, the median time on treatment was 12 months. The patient who achieved a stringent complete response remained on treatment at last follow-up, as did one partial responder and one patient with a very good partial response.
These results suggest the combination of selinexor, lenalidomide, and dexamethasone “is active, relatively well tolerated, and could warrant further investigation,” Dr. White said.
The STOMP trial is sponsored by Karyopharm Therapeutics. Dr. White disclosed relationships with Karyopharm, Amgen, Celgene, Janssen, Sanofi, and Takeda.
SOURCE: White D et al. IMW 2019, Abstract OAB-083.
REPORTING FROM IMW 2019
Combo could be new standard for transplant-eligible, newly diagnosed myeloma patients
BOSTON — Daratumumab plus bortezomib, lenalidomide, and dexamethasone (D-RVd) may be a new standard of care for transplant-eligible patients with newly diagnosed multiple myeloma, according to a speaker at the International Myeloma Workshop.
In the phase 2 GRIFFIN trial, adding daratumumab to RVd deepened responses at all time points and improved rates of stringent complete response and minimal residual disease (MRD) negativity post consolidation.
These results might convince “early adopters of therapy” to change their practice, said Peter M. Voorhees, MD, of Levine Cancer Institute at Atrium Health in Charlotte, N.C., who presented results from GRIFFIN as a late-breaking abstract at the workshop, which is held by the International Myeloma Society.
“But I think you do have to be careful,” Dr. Voorhees added. “We’ll have to see how these patients do over time. Is the MRD sustained, and does that MRD negativity improve progression-free survival?”
Dr. Voorhees presented data on 207 adults with transplant-eligible, newly diagnosed multiple myeloma who were enrolled in the GRIFFIN trial. The patients received RVd, with or without daratumumab, for induction (cycles 1-4). They received granulocyte colony stimulating factor, with or without plerixafor, for stem cell mobilization, and melphalan for conditioning prior to transplant.
Patients received consolidation with D-RVd or RVd (cycles 5-6) and maintenance with lenalidomide alone or in combination with daratumumab (cycles 7-32). Patients could continue maintenance with lenalidomide alone beyond cycle 32.
The D-RVd arm comprised 104 patients, and the RVd arm comprised 103 patients. Baseline characteristics were well balanced between the treatment arms. The median age was 59 years (range, 29-70 years) in the D-RVd arm and 61 years (range, 40-70 years) in the RVd arm.
Most patients had stage I (47% in the D-RVd arm and 49% in the RVd arm) or stage II disease (39% and 36%, respectively) according to the International Staging System. And most patients had standard risk cytogenetics (84% and 86%, respectively).
Response, MRD, and engraftment
The study’s primary endpoint was stringent complete response by the end of consolidation, which was achieved by 42.4% of patients in the D-RVd arm and 32.0% in the RVd arm (odds ratio [OR] = 1.57; P = .068). The overall response rate at that time point was 99.0% and 91.8%, respectively (P = .0160).
Responses deepened over time, and response rates were greater for D-RVd than for RVd at all time points. The complete response rate was 19.2% in the D-RVd arm and 13.4% in the RVd arm at the end of induction; 27.3% and 19.6%, respectively, at the end of transplant; 51.5% and 42.3%, respectively, at the end of consolidation; and 62.6% and 47.4%, respectively, at the clinical cutoff.
D-RVd also improved MRD negativity (10-5) rates at the end of consolidation. MRD negativity was 44.2% in the D-RVd arm and 14.6% in the RVd arm (OR = 4.70; P less than .0001). The rate of MRD negativity in patients with a complete response or better was 28.8% and 9.7%, respectively (OR = 3.73; P = .0007).
Dr. Voorhees noted that D-RVd was favored across all subgroups for MRD negativity and stringent compete response, except among patients with high-risk cytogenetics and stage III disease.
He also pointed out that stem cell mobilization was “feasible” in the D-RVd arm, and daratumumab did not impact engraftment. The median time to neutrophil engraftment was 12 days in both treatment arms. The median time to platelet engraftment was 13 days in the D-RVd arm and 12 days in the RVd arm.
Safety
“The adverse events are what you would expect,” Dr. Voorhees said. “Grade 3 and 4 neutropenia and thrombocytopenia were seen more often in the dara arm of the trial compared to the RVd arm.”
The most common grade 3/4 treatment-emergent adverse events (in the D-RVd and RVd arms, respectively) were neutropenia (32% and 15%), lymphopenia (23% in both), thrombocytopenia (16% and 8%), and leukopenia (15% and 7%).
“Nonhematologic toxicities were generally equal between the two groups, but I do want to stress that there was a higher rate of infection in the dara arm,” Dr. Voorhees noted.
The incidence of infection was 82% in the D-RVd arm and 55% in the RVd arm, but the rate of grade 3/4 infection was 17% in both arms. The rate of pneumonia was 10% in the D-RVd arm and 9% in the RVd arm.
All-grade infusion-related reactions occurred in 41% of patients in the D-RVd arm, and grade 3/4 infusion-related reactions occurred in 5%.
The trial was sponsored by Janssen. Dr. Voorhees reported relationships with Janssen and several other companies.
SOURCE: Voorhees PM et al. IMW 2019. Abstract OAB-087.
BOSTON — Daratumumab plus bortezomib, lenalidomide, and dexamethasone (D-RVd) may be a new standard of care for transplant-eligible patients with newly diagnosed multiple myeloma, according to a speaker at the International Myeloma Workshop.
In the phase 2 GRIFFIN trial, adding daratumumab to RVd deepened responses at all time points and improved rates of stringent complete response and minimal residual disease (MRD) negativity post consolidation.
These results might convince “early adopters of therapy” to change their practice, said Peter M. Voorhees, MD, of Levine Cancer Institute at Atrium Health in Charlotte, N.C., who presented results from GRIFFIN as a late-breaking abstract at the workshop, which is held by the International Myeloma Society.
“But I think you do have to be careful,” Dr. Voorhees added. “We’ll have to see how these patients do over time. Is the MRD sustained, and does that MRD negativity improve progression-free survival?”
Dr. Voorhees presented data on 207 adults with transplant-eligible, newly diagnosed multiple myeloma who were enrolled in the GRIFFIN trial. The patients received RVd, with or without daratumumab, for induction (cycles 1-4). They received granulocyte colony stimulating factor, with or without plerixafor, for stem cell mobilization, and melphalan for conditioning prior to transplant.
Patients received consolidation with D-RVd or RVd (cycles 5-6) and maintenance with lenalidomide alone or in combination with daratumumab (cycles 7-32). Patients could continue maintenance with lenalidomide alone beyond cycle 32.
The D-RVd arm comprised 104 patients, and the RVd arm comprised 103 patients. Baseline characteristics were well balanced between the treatment arms. The median age was 59 years (range, 29-70 years) in the D-RVd arm and 61 years (range, 40-70 years) in the RVd arm.
Most patients had stage I (47% in the D-RVd arm and 49% in the RVd arm) or stage II disease (39% and 36%, respectively) according to the International Staging System. And most patients had standard risk cytogenetics (84% and 86%, respectively).
Response, MRD, and engraftment
The study’s primary endpoint was stringent complete response by the end of consolidation, which was achieved by 42.4% of patients in the D-RVd arm and 32.0% in the RVd arm (odds ratio [OR] = 1.57; P = .068). The overall response rate at that time point was 99.0% and 91.8%, respectively (P = .0160).
Responses deepened over time, and response rates were greater for D-RVd than for RVd at all time points. The complete response rate was 19.2% in the D-RVd arm and 13.4% in the RVd arm at the end of induction; 27.3% and 19.6%, respectively, at the end of transplant; 51.5% and 42.3%, respectively, at the end of consolidation; and 62.6% and 47.4%, respectively, at the clinical cutoff.
D-RVd also improved MRD negativity (10-5) rates at the end of consolidation. MRD negativity was 44.2% in the D-RVd arm and 14.6% in the RVd arm (OR = 4.70; P less than .0001). The rate of MRD negativity in patients with a complete response or better was 28.8% and 9.7%, respectively (OR = 3.73; P = .0007).
Dr. Voorhees noted that D-RVd was favored across all subgroups for MRD negativity and stringent compete response, except among patients with high-risk cytogenetics and stage III disease.
He also pointed out that stem cell mobilization was “feasible” in the D-RVd arm, and daratumumab did not impact engraftment. The median time to neutrophil engraftment was 12 days in both treatment arms. The median time to platelet engraftment was 13 days in the D-RVd arm and 12 days in the RVd arm.
Safety
“The adverse events are what you would expect,” Dr. Voorhees said. “Grade 3 and 4 neutropenia and thrombocytopenia were seen more often in the dara arm of the trial compared to the RVd arm.”
The most common grade 3/4 treatment-emergent adverse events (in the D-RVd and RVd arms, respectively) were neutropenia (32% and 15%), lymphopenia (23% in both), thrombocytopenia (16% and 8%), and leukopenia (15% and 7%).
“Nonhematologic toxicities were generally equal between the two groups, but I do want to stress that there was a higher rate of infection in the dara arm,” Dr. Voorhees noted.
The incidence of infection was 82% in the D-RVd arm and 55% in the RVd arm, but the rate of grade 3/4 infection was 17% in both arms. The rate of pneumonia was 10% in the D-RVd arm and 9% in the RVd arm.
All-grade infusion-related reactions occurred in 41% of patients in the D-RVd arm, and grade 3/4 infusion-related reactions occurred in 5%.
The trial was sponsored by Janssen. Dr. Voorhees reported relationships with Janssen and several other companies.
SOURCE: Voorhees PM et al. IMW 2019. Abstract OAB-087.
BOSTON — Daratumumab plus bortezomib, lenalidomide, and dexamethasone (D-RVd) may be a new standard of care for transplant-eligible patients with newly diagnosed multiple myeloma, according to a speaker at the International Myeloma Workshop.
In the phase 2 GRIFFIN trial, adding daratumumab to RVd deepened responses at all time points and improved rates of stringent complete response and minimal residual disease (MRD) negativity post consolidation.
These results might convince “early adopters of therapy” to change their practice, said Peter M. Voorhees, MD, of Levine Cancer Institute at Atrium Health in Charlotte, N.C., who presented results from GRIFFIN as a late-breaking abstract at the workshop, which is held by the International Myeloma Society.
“But I think you do have to be careful,” Dr. Voorhees added. “We’ll have to see how these patients do over time. Is the MRD sustained, and does that MRD negativity improve progression-free survival?”
Dr. Voorhees presented data on 207 adults with transplant-eligible, newly diagnosed multiple myeloma who were enrolled in the GRIFFIN trial. The patients received RVd, with or without daratumumab, for induction (cycles 1-4). They received granulocyte colony stimulating factor, with or without plerixafor, for stem cell mobilization, and melphalan for conditioning prior to transplant.
Patients received consolidation with D-RVd or RVd (cycles 5-6) and maintenance with lenalidomide alone or in combination with daratumumab (cycles 7-32). Patients could continue maintenance with lenalidomide alone beyond cycle 32.
The D-RVd arm comprised 104 patients, and the RVd arm comprised 103 patients. Baseline characteristics were well balanced between the treatment arms. The median age was 59 years (range, 29-70 years) in the D-RVd arm and 61 years (range, 40-70 years) in the RVd arm.
Most patients had stage I (47% in the D-RVd arm and 49% in the RVd arm) or stage II disease (39% and 36%, respectively) according to the International Staging System. And most patients had standard risk cytogenetics (84% and 86%, respectively).
Response, MRD, and engraftment
The study’s primary endpoint was stringent complete response by the end of consolidation, which was achieved by 42.4% of patients in the D-RVd arm and 32.0% in the RVd arm (odds ratio [OR] = 1.57; P = .068). The overall response rate at that time point was 99.0% and 91.8%, respectively (P = .0160).
Responses deepened over time, and response rates were greater for D-RVd than for RVd at all time points. The complete response rate was 19.2% in the D-RVd arm and 13.4% in the RVd arm at the end of induction; 27.3% and 19.6%, respectively, at the end of transplant; 51.5% and 42.3%, respectively, at the end of consolidation; and 62.6% and 47.4%, respectively, at the clinical cutoff.
D-RVd also improved MRD negativity (10-5) rates at the end of consolidation. MRD negativity was 44.2% in the D-RVd arm and 14.6% in the RVd arm (OR = 4.70; P less than .0001). The rate of MRD negativity in patients with a complete response or better was 28.8% and 9.7%, respectively (OR = 3.73; P = .0007).
Dr. Voorhees noted that D-RVd was favored across all subgroups for MRD negativity and stringent compete response, except among patients with high-risk cytogenetics and stage III disease.
He also pointed out that stem cell mobilization was “feasible” in the D-RVd arm, and daratumumab did not impact engraftment. The median time to neutrophil engraftment was 12 days in both treatment arms. The median time to platelet engraftment was 13 days in the D-RVd arm and 12 days in the RVd arm.
Safety
“The adverse events are what you would expect,” Dr. Voorhees said. “Grade 3 and 4 neutropenia and thrombocytopenia were seen more often in the dara arm of the trial compared to the RVd arm.”
The most common grade 3/4 treatment-emergent adverse events (in the D-RVd and RVd arms, respectively) were neutropenia (32% and 15%), lymphopenia (23% in both), thrombocytopenia (16% and 8%), and leukopenia (15% and 7%).
“Nonhematologic toxicities were generally equal between the two groups, but I do want to stress that there was a higher rate of infection in the dara arm,” Dr. Voorhees noted.
The incidence of infection was 82% in the D-RVd arm and 55% in the RVd arm, but the rate of grade 3/4 infection was 17% in both arms. The rate of pneumonia was 10% in the D-RVd arm and 9% in the RVd arm.
All-grade infusion-related reactions occurred in 41% of patients in the D-RVd arm, and grade 3/4 infusion-related reactions occurred in 5%.
The trial was sponsored by Janssen. Dr. Voorhees reported relationships with Janssen and several other companies.
SOURCE: Voorhees PM et al. IMW 2019. Abstract OAB-087.
FROM IMW 2019
Adding elotuzumab to lenalidomide/dexamethasone can prolong survival in relapsed/refractory myeloma
BOSTON – Adding elotuzumab to lenalidomide and dexamethasone can prolong overall survival in patients with relapsed/refractory multiple myeloma, according to final results from the ELOQUENT-2 trial.
At a minimum follow-up of 6 years, elotuzumab plus lenalidomide/dexamethasone (ELd) reduced the risk of death by 18% and prolonged the median overall survival by 8.7 months when compared to treatment with lenalidomide/dexamethasone (Ld).
“The combination of elotuzumab with lenalidomide and dexamethasone demonstrated a statistically significant and clinically meaningful 18% reduction in the risk of death,” said Meletios A. Dimopoulos, MD, PhD, of the National and Kapodistrian University of Athens. “This treatment combination is the only approved antibody-based regimen shown to prolong overall survival significantly in patients with relapsed or refractory myeloma in the context of a large, prospective, randomized trial.”
Dr. Dimopoulos presented results from this phase 3 trial at the International Myeloma Workshop, held by the International Myeloma Society.
The final analysis of ELOQUENT-2 included 646 patients with relapsed/refractory multiple myeloma who had received one to three prior lines of therapy at baseline. There were 321 patients randomized to ELd and 325 randomized to Ld. Baseline characteristics were well balanced between the treatment arms.
At the data cutoff of Oct. 3, 2018, 319 patients in the ELd arm and 316 in the Ld arm had received their assigned treatment. Ten percent (n = 33) of patients in the ELd arm and 4% (n = 14) in the Ld arm were still receiving their assigned treatment at the cutoff date.
The median number of treatment cycles was 19 (range, 9-42) in the ELd arm and 14 (range, 6-25) in the Ld arm. Most patients discontinued treatment due to disease progression (56% in the ELd arm and 57% in the Ld arm) or treatment-related toxicity (12% and 14%, respectively).
Survival
At a minimum follow-up of 71 months, the median overall survival was 48.3 months in the ELd arm and 39.6 months in the Ld arm. The hazard ratio was 0.82 (P = .0408).
The overall survival advantage with ELd was observed in all prespecified patient subgroups, Dr. Dimopoulos said. For example, overall survival favored ELd in patients aged 75 years and older (HR, 0.69), patients with International Staging System stage III disease at enrollment (HR, 0.74), those who had received two to three prior lines of therapy (HR, 0.71), and patients with del(17p) (HR, 0.71).
There were 212 deaths in the ELd arm and 225 in the Ld arm (67% and 71%, respectively). The most common causes of death were disease progression (41% in the ELd arm and 45% in the Ld arm), infection (9% and 6%, respectively), and “other” or unknown causes (7% and 9%, respectively). Two percent of patients in each arm (n = 7 in both) died from study treatment–related toxicity.
Dr. Dimopoulos pointed out that there were no imbalances in subsequent therapies between the ELd and Ld arms. The most common subsequent therapies (in the ELd and Ld arms, respectively) were bortezomib (38% and 42%), cyclophosphamide (30% in both arms), pomalidomide (26% and 29%), and lenalidomide (18% and 22%).
Safety
“Most of the adverse events which occurred throughout the study were due to the known side effects of lenalidomide and dexamethasone,” Dr. Dimopoulos said. “The contribution of elotuzumab to any kind of toxicity was minimal.”
Nearly all patients in both arms (99%) experienced adverse events. Serious adverse events were observed in 75% of patients in the ELd arm and 61% in the Ld arm. Adverse events leading to treatment discontinuation occurred in 36% and 33%, respectively, and grade 3-4 events leading to discontinuation occurred in 21% and 20%, respectively.
Grade 3-4 adverse events of special interest (in the ELd and Ld arms, respectively) were infections (35% and 27%), renal and urinary disorders (5% in both), cardiac disorders (6% and 8%), and lymphopenia (8% and 4%). Second primary malignancies occurred in 12% of patients in the ELd arm and 9% in the Ld arm.
This trial was sponsored by Bristol-Myers Squibb in collaboration with AbbVie. Dr. Dimopoulos reported relationships with Bristol-Myers Squibb, Amgen, Celgene, Janssen, and Takeda.
SOURCE: Dimopoulos MA et al. IMW 2019, Abstract OAB-021.
BOSTON – Adding elotuzumab to lenalidomide and dexamethasone can prolong overall survival in patients with relapsed/refractory multiple myeloma, according to final results from the ELOQUENT-2 trial.
At a minimum follow-up of 6 years, elotuzumab plus lenalidomide/dexamethasone (ELd) reduced the risk of death by 18% and prolonged the median overall survival by 8.7 months when compared to treatment with lenalidomide/dexamethasone (Ld).
“The combination of elotuzumab with lenalidomide and dexamethasone demonstrated a statistically significant and clinically meaningful 18% reduction in the risk of death,” said Meletios A. Dimopoulos, MD, PhD, of the National and Kapodistrian University of Athens. “This treatment combination is the only approved antibody-based regimen shown to prolong overall survival significantly in patients with relapsed or refractory myeloma in the context of a large, prospective, randomized trial.”
Dr. Dimopoulos presented results from this phase 3 trial at the International Myeloma Workshop, held by the International Myeloma Society.
The final analysis of ELOQUENT-2 included 646 patients with relapsed/refractory multiple myeloma who had received one to three prior lines of therapy at baseline. There were 321 patients randomized to ELd and 325 randomized to Ld. Baseline characteristics were well balanced between the treatment arms.
At the data cutoff of Oct. 3, 2018, 319 patients in the ELd arm and 316 in the Ld arm had received their assigned treatment. Ten percent (n = 33) of patients in the ELd arm and 4% (n = 14) in the Ld arm were still receiving their assigned treatment at the cutoff date.
The median number of treatment cycles was 19 (range, 9-42) in the ELd arm and 14 (range, 6-25) in the Ld arm. Most patients discontinued treatment due to disease progression (56% in the ELd arm and 57% in the Ld arm) or treatment-related toxicity (12% and 14%, respectively).
Survival
At a minimum follow-up of 71 months, the median overall survival was 48.3 months in the ELd arm and 39.6 months in the Ld arm. The hazard ratio was 0.82 (P = .0408).
The overall survival advantage with ELd was observed in all prespecified patient subgroups, Dr. Dimopoulos said. For example, overall survival favored ELd in patients aged 75 years and older (HR, 0.69), patients with International Staging System stage III disease at enrollment (HR, 0.74), those who had received two to three prior lines of therapy (HR, 0.71), and patients with del(17p) (HR, 0.71).
There were 212 deaths in the ELd arm and 225 in the Ld arm (67% and 71%, respectively). The most common causes of death were disease progression (41% in the ELd arm and 45% in the Ld arm), infection (9% and 6%, respectively), and “other” or unknown causes (7% and 9%, respectively). Two percent of patients in each arm (n = 7 in both) died from study treatment–related toxicity.
Dr. Dimopoulos pointed out that there were no imbalances in subsequent therapies between the ELd and Ld arms. The most common subsequent therapies (in the ELd and Ld arms, respectively) were bortezomib (38% and 42%), cyclophosphamide (30% in both arms), pomalidomide (26% and 29%), and lenalidomide (18% and 22%).
Safety
“Most of the adverse events which occurred throughout the study were due to the known side effects of lenalidomide and dexamethasone,” Dr. Dimopoulos said. “The contribution of elotuzumab to any kind of toxicity was minimal.”
Nearly all patients in both arms (99%) experienced adverse events. Serious adverse events were observed in 75% of patients in the ELd arm and 61% in the Ld arm. Adverse events leading to treatment discontinuation occurred in 36% and 33%, respectively, and grade 3-4 events leading to discontinuation occurred in 21% and 20%, respectively.
Grade 3-4 adverse events of special interest (in the ELd and Ld arms, respectively) were infections (35% and 27%), renal and urinary disorders (5% in both), cardiac disorders (6% and 8%), and lymphopenia (8% and 4%). Second primary malignancies occurred in 12% of patients in the ELd arm and 9% in the Ld arm.
This trial was sponsored by Bristol-Myers Squibb in collaboration with AbbVie. Dr. Dimopoulos reported relationships with Bristol-Myers Squibb, Amgen, Celgene, Janssen, and Takeda.
SOURCE: Dimopoulos MA et al. IMW 2019, Abstract OAB-021.
BOSTON – Adding elotuzumab to lenalidomide and dexamethasone can prolong overall survival in patients with relapsed/refractory multiple myeloma, according to final results from the ELOQUENT-2 trial.
At a minimum follow-up of 6 years, elotuzumab plus lenalidomide/dexamethasone (ELd) reduced the risk of death by 18% and prolonged the median overall survival by 8.7 months when compared to treatment with lenalidomide/dexamethasone (Ld).
“The combination of elotuzumab with lenalidomide and dexamethasone demonstrated a statistically significant and clinically meaningful 18% reduction in the risk of death,” said Meletios A. Dimopoulos, MD, PhD, of the National and Kapodistrian University of Athens. “This treatment combination is the only approved antibody-based regimen shown to prolong overall survival significantly in patients with relapsed or refractory myeloma in the context of a large, prospective, randomized trial.”
Dr. Dimopoulos presented results from this phase 3 trial at the International Myeloma Workshop, held by the International Myeloma Society.
The final analysis of ELOQUENT-2 included 646 patients with relapsed/refractory multiple myeloma who had received one to three prior lines of therapy at baseline. There were 321 patients randomized to ELd and 325 randomized to Ld. Baseline characteristics were well balanced between the treatment arms.
At the data cutoff of Oct. 3, 2018, 319 patients in the ELd arm and 316 in the Ld arm had received their assigned treatment. Ten percent (n = 33) of patients in the ELd arm and 4% (n = 14) in the Ld arm were still receiving their assigned treatment at the cutoff date.
The median number of treatment cycles was 19 (range, 9-42) in the ELd arm and 14 (range, 6-25) in the Ld arm. Most patients discontinued treatment due to disease progression (56% in the ELd arm and 57% in the Ld arm) or treatment-related toxicity (12% and 14%, respectively).
Survival
At a minimum follow-up of 71 months, the median overall survival was 48.3 months in the ELd arm and 39.6 months in the Ld arm. The hazard ratio was 0.82 (P = .0408).
The overall survival advantage with ELd was observed in all prespecified patient subgroups, Dr. Dimopoulos said. For example, overall survival favored ELd in patients aged 75 years and older (HR, 0.69), patients with International Staging System stage III disease at enrollment (HR, 0.74), those who had received two to three prior lines of therapy (HR, 0.71), and patients with del(17p) (HR, 0.71).
There were 212 deaths in the ELd arm and 225 in the Ld arm (67% and 71%, respectively). The most common causes of death were disease progression (41% in the ELd arm and 45% in the Ld arm), infection (9% and 6%, respectively), and “other” or unknown causes (7% and 9%, respectively). Two percent of patients in each arm (n = 7 in both) died from study treatment–related toxicity.
Dr. Dimopoulos pointed out that there were no imbalances in subsequent therapies between the ELd and Ld arms. The most common subsequent therapies (in the ELd and Ld arms, respectively) were bortezomib (38% and 42%), cyclophosphamide (30% in both arms), pomalidomide (26% and 29%), and lenalidomide (18% and 22%).
Safety
“Most of the adverse events which occurred throughout the study were due to the known side effects of lenalidomide and dexamethasone,” Dr. Dimopoulos said. “The contribution of elotuzumab to any kind of toxicity was minimal.”
Nearly all patients in both arms (99%) experienced adverse events. Serious adverse events were observed in 75% of patients in the ELd arm and 61% in the Ld arm. Adverse events leading to treatment discontinuation occurred in 36% and 33%, respectively, and grade 3-4 events leading to discontinuation occurred in 21% and 20%, respectively.
Grade 3-4 adverse events of special interest (in the ELd and Ld arms, respectively) were infections (35% and 27%), renal and urinary disorders (5% in both), cardiac disorders (6% and 8%), and lymphopenia (8% and 4%). Second primary malignancies occurred in 12% of patients in the ELd arm and 9% in the Ld arm.
This trial was sponsored by Bristol-Myers Squibb in collaboration with AbbVie. Dr. Dimopoulos reported relationships with Bristol-Myers Squibb, Amgen, Celgene, Janssen, and Takeda.
SOURCE: Dimopoulos MA et al. IMW 2019, Abstract OAB-021.
FROM IMW 2019
CT103A elicits responses after prior CAR T-cell relapse
BOSTON – CT103A, a chimeric antigen receptor (CAR) T-cell therapy, is “active and effective” in patients with relapsed/refractory multiple myeloma, according to a speaker at the International Myeloma Workshop, held by the International Myeloma Society.
The anti–B-cell maturation antigen (BCMA) CAR T-cell therapy produced a 100% response rate in patients with heavily pretreated multiple myeloma, and three of four patients who had failed a prior CAR T-cell therapy achieved a stringent complete response after CT103A.
Chunrui Li, MD, PhD, of Tongji Hospital and Tongji Medical College, Huazhong University of Science, Wuhan, China, presented these results at the workshop.
Dr. Li noted that anti-BCMA CAR T-cell therapy has produced responses in myeloma patients, but approximately half of patients typically relapse in about a year. CAR T-cell infusions after relapse have not been effective in these patients.
In an effort to change that, Dr. Li and his colleagues developed CT103A, a lentiviral vector containing a CAR structure with a fully human single-chain fragment variant; CD8a hinger; and transmembrane, 4-1BB co-stimulatory, and CD3z activation domains.
Dr. Li and his colleagues evaluated CT103A in a phase 0 trial (ChiCTR1800018137) of 18 patients who had received at least three prior lines of therapy and had disease refractory to a proteasome inhibitor and an immunomodulatory agent.
The patients’ median age was 53.3 years (range, 38-66 years), and their median time since diagnosis was 32 months (range, 8-92 months). They had received a median of 4 (range, 3-6) prior therapies. All had received prior bortezomib and lenalidomide, seven had undergone a transplant, and four had been treated on a trial of murine anti-BCMA CAR T-cell therapy.
For the current trial, patients received lymphodepletion with cyclophosphamide and fludarabine, followed by CT103A at 1x106, 3x106, or 6x106 CAR T cells/kg.
There was one dose-limiting toxicity at the highest dose level – grade 4 cytokine release syndrome (CRS) in a patient who died at day 19 after CT103A infusion.
In all, 17 patients developed CRS, four with grade 1, eight with grade 2, four with grade 3, and one with grade 4 CRS. None of the patients developed neurologic toxicity.
Serious adverse events related to lymphodepletion and/or CT103A included prolonged cytopenia (n = 3), pulmonary infection (n = 2), herpes zoster (n = 1), pleuritis (n = 1), and hypoxemia (n = 1).
There were 17 patients evaluable for efficacy, and all of them achieved a response at some point. In eight patients, responses have lasted more than 200 days.
At the data cutoff, there were 10 stringent complete responses, two complete responses, and three very good partial responses. One patient progressed after achieving a very good partial response, and one patient achieved a partial response but ultimately died (likely of respiratory failure attributable to a lung infection).
Of the four patients who had previously received murine CAR T-cell therapy, one progressed, and three achieved a stringent complete response.
This study was funded by Nanjing Iaso Biotherapeutics. Dr. Li did not disclose any conflicts of interest.
SOURCE: Li C et al. IMW 2019, Abstract OAB-033.
BOSTON – CT103A, a chimeric antigen receptor (CAR) T-cell therapy, is “active and effective” in patients with relapsed/refractory multiple myeloma, according to a speaker at the International Myeloma Workshop, held by the International Myeloma Society.
The anti–B-cell maturation antigen (BCMA) CAR T-cell therapy produced a 100% response rate in patients with heavily pretreated multiple myeloma, and three of four patients who had failed a prior CAR T-cell therapy achieved a stringent complete response after CT103A.
Chunrui Li, MD, PhD, of Tongji Hospital and Tongji Medical College, Huazhong University of Science, Wuhan, China, presented these results at the workshop.
Dr. Li noted that anti-BCMA CAR T-cell therapy has produced responses in myeloma patients, but approximately half of patients typically relapse in about a year. CAR T-cell infusions after relapse have not been effective in these patients.
In an effort to change that, Dr. Li and his colleagues developed CT103A, a lentiviral vector containing a CAR structure with a fully human single-chain fragment variant; CD8a hinger; and transmembrane, 4-1BB co-stimulatory, and CD3z activation domains.
Dr. Li and his colleagues evaluated CT103A in a phase 0 trial (ChiCTR1800018137) of 18 patients who had received at least three prior lines of therapy and had disease refractory to a proteasome inhibitor and an immunomodulatory agent.
The patients’ median age was 53.3 years (range, 38-66 years), and their median time since diagnosis was 32 months (range, 8-92 months). They had received a median of 4 (range, 3-6) prior therapies. All had received prior bortezomib and lenalidomide, seven had undergone a transplant, and four had been treated on a trial of murine anti-BCMA CAR T-cell therapy.
For the current trial, patients received lymphodepletion with cyclophosphamide and fludarabine, followed by CT103A at 1x106, 3x106, or 6x106 CAR T cells/kg.
There was one dose-limiting toxicity at the highest dose level – grade 4 cytokine release syndrome (CRS) in a patient who died at day 19 after CT103A infusion.
In all, 17 patients developed CRS, four with grade 1, eight with grade 2, four with grade 3, and one with grade 4 CRS. None of the patients developed neurologic toxicity.
Serious adverse events related to lymphodepletion and/or CT103A included prolonged cytopenia (n = 3), pulmonary infection (n = 2), herpes zoster (n = 1), pleuritis (n = 1), and hypoxemia (n = 1).
There were 17 patients evaluable for efficacy, and all of them achieved a response at some point. In eight patients, responses have lasted more than 200 days.
At the data cutoff, there were 10 stringent complete responses, two complete responses, and three very good partial responses. One patient progressed after achieving a very good partial response, and one patient achieved a partial response but ultimately died (likely of respiratory failure attributable to a lung infection).
Of the four patients who had previously received murine CAR T-cell therapy, one progressed, and three achieved a stringent complete response.
This study was funded by Nanjing Iaso Biotherapeutics. Dr. Li did not disclose any conflicts of interest.
SOURCE: Li C et al. IMW 2019, Abstract OAB-033.
BOSTON – CT103A, a chimeric antigen receptor (CAR) T-cell therapy, is “active and effective” in patients with relapsed/refractory multiple myeloma, according to a speaker at the International Myeloma Workshop, held by the International Myeloma Society.
The anti–B-cell maturation antigen (BCMA) CAR T-cell therapy produced a 100% response rate in patients with heavily pretreated multiple myeloma, and three of four patients who had failed a prior CAR T-cell therapy achieved a stringent complete response after CT103A.
Chunrui Li, MD, PhD, of Tongji Hospital and Tongji Medical College, Huazhong University of Science, Wuhan, China, presented these results at the workshop.
Dr. Li noted that anti-BCMA CAR T-cell therapy has produced responses in myeloma patients, but approximately half of patients typically relapse in about a year. CAR T-cell infusions after relapse have not been effective in these patients.
In an effort to change that, Dr. Li and his colleagues developed CT103A, a lentiviral vector containing a CAR structure with a fully human single-chain fragment variant; CD8a hinger; and transmembrane, 4-1BB co-stimulatory, and CD3z activation domains.
Dr. Li and his colleagues evaluated CT103A in a phase 0 trial (ChiCTR1800018137) of 18 patients who had received at least three prior lines of therapy and had disease refractory to a proteasome inhibitor and an immunomodulatory agent.
The patients’ median age was 53.3 years (range, 38-66 years), and their median time since diagnosis was 32 months (range, 8-92 months). They had received a median of 4 (range, 3-6) prior therapies. All had received prior bortezomib and lenalidomide, seven had undergone a transplant, and four had been treated on a trial of murine anti-BCMA CAR T-cell therapy.
For the current trial, patients received lymphodepletion with cyclophosphamide and fludarabine, followed by CT103A at 1x106, 3x106, or 6x106 CAR T cells/kg.
There was one dose-limiting toxicity at the highest dose level – grade 4 cytokine release syndrome (CRS) in a patient who died at day 19 after CT103A infusion.
In all, 17 patients developed CRS, four with grade 1, eight with grade 2, four with grade 3, and one with grade 4 CRS. None of the patients developed neurologic toxicity.
Serious adverse events related to lymphodepletion and/or CT103A included prolonged cytopenia (n = 3), pulmonary infection (n = 2), herpes zoster (n = 1), pleuritis (n = 1), and hypoxemia (n = 1).
There were 17 patients evaluable for efficacy, and all of them achieved a response at some point. In eight patients, responses have lasted more than 200 days.
At the data cutoff, there were 10 stringent complete responses, two complete responses, and three very good partial responses. One patient progressed after achieving a very good partial response, and one patient achieved a partial response but ultimately died (likely of respiratory failure attributable to a lung infection).
Of the four patients who had previously received murine CAR T-cell therapy, one progressed, and three achieved a stringent complete response.
This study was funded by Nanjing Iaso Biotherapeutics. Dr. Li did not disclose any conflicts of interest.
SOURCE: Li C et al. IMW 2019, Abstract OAB-033.
REPORTING FROM IMW 2019
Melflufen-dexamethasone active in patients with relapsed/refractory myeloma and EMD
BOSTON – Melflufen plus dexamethasone is active in patients with relapsed/refractory multiple myeloma, whether or not they have extramedullary disease (EMD), a phase 2 trial suggests.
In the HORIZON trial, melflufen-dexamethasone produced an overall response rate of 23% in patients with EMD and 27% in those without EMD.
Paul G. Richardson, MD, of Harvard Medical School and the Dana-Farber Cancer Institute, both in Boston, presented these results as a late-breaking abstract at the International Myeloma Workshop, held by the International Myeloma Society.
As of July 30, 2019, 136 patients had been treated on the HORIZON trial. The trial is enrolling patients with relapsed/refractory multiple myeloma refractory to pomalidomide, an anti-CD38 monoclonal antibody (mAb), or both. The patients must have received at least two prior lines of therapy, including a proteasome inhibitor (PI) and an immunomodulatory agent (IMiD).
Dr. Richardson presented results for 130 patients, 44 with EMD and 86 without it. The median age at baseline was 64 years in the EMD and non-EMD groups (overall range, 35-86 years). More than half of patients had high-risk cytogenetics (52% in the EMD group and 57% in the non-EMD group).
The median number of prior therapies was five in both the EMD and non-EMD groups. Most patients had received at least one prior transplant (73% in the EMD group and 69% in the non-EMD group). Most patients in both groups were refractory to an anti-CD38 mAb (93% EMD and 72% non-EMD); an IMiD and a PI (93% EMD and 90% non-EMD); an IMiD, a PI, and an anti-CD38 mAb (91% EMD and 63% non-EMD); and their last therapy (100% EMD and 95% non-EMD).
Dr. Richardson pointed out that the incidence of EMD in this trial is higher than has been reported previously. Of the 44 EMD patients, 26 had soft-tissue EMD, and 18 had bone-related EMD. Five patients had CNS involvement.
Another key finding, according to Dr. Richardson, was that EMD appeared to be associated with prior anti-CD38 therapy. Specifically, 40% of patients exposed to an anti-CD38 mAb had EMD, compared with 11% of patients who had not received an anti-CD38 mAb (P = .01).
“I don’t for a minute want to say that CD38-targeted therapy engenders extramedullary disease,” Dr. Richardson said. “I think what we can say, though, is that, once CD38 treatment fails a patient, extramedullary disease … is a very real challenge. Therefore, we need rationally targeted approaches, ideally in combination, to meet that challenge.”
The overall response rate was similar for EMD and non-EMD patients – 23% and 27%, respectively. The median duration of response was 3.4 months in the EMD patients and 4.4 months in the non-EMD group. The clinical benefit rate was 30% and 45%, respectively.
In the EMD group, the overall response rate was 19% in patients with soft-tissue EMD and 28% in bone-related EMD. None of the patients with CNS disease responded.
The median progression-free survival was 2.9 months for patients with EMD and 4.6 months for those without EMD. The median overall survival was 5.8 month and 11.6 months, respectively.
The median overall survival was 18.5 months in EMD responders and 17.2 months in non-EMD responders. The median overall survival was 5.1 months in EMD nonresponders and 8.5 months in non-EMD nonresponders.
In all, 54% of patients received subsequent therapy. There were no significant differences in outcomes between EMD and non-EMD patients.
The safety profiles were similar for EMD and non-EMD patients, Dr. Richardson said. Melflufen-dexamethasone was considered well tolerated overall, and there were no treatment-related deaths.
The most common treatment-emergent adverse events (grade 3 and 4, respectively) were thrombocytopenia (22% and 46%), neutropenia (32% and 35%), anemia (35% and 1%), white blood cell count decrease (10% and 7%), and pneumonia (7% and 1%).
This trial is sponsored by Oncopeptides. Dr. Richardson reported an advisory role and research funding from Oncopeptides.
SOURCE: Richardson PG et al. IMW 2019, Abstract OAB-086.
BOSTON – Melflufen plus dexamethasone is active in patients with relapsed/refractory multiple myeloma, whether or not they have extramedullary disease (EMD), a phase 2 trial suggests.
In the HORIZON trial, melflufen-dexamethasone produced an overall response rate of 23% in patients with EMD and 27% in those without EMD.
Paul G. Richardson, MD, of Harvard Medical School and the Dana-Farber Cancer Institute, both in Boston, presented these results as a late-breaking abstract at the International Myeloma Workshop, held by the International Myeloma Society.
As of July 30, 2019, 136 patients had been treated on the HORIZON trial. The trial is enrolling patients with relapsed/refractory multiple myeloma refractory to pomalidomide, an anti-CD38 monoclonal antibody (mAb), or both. The patients must have received at least two prior lines of therapy, including a proteasome inhibitor (PI) and an immunomodulatory agent (IMiD).
Dr. Richardson presented results for 130 patients, 44 with EMD and 86 without it. The median age at baseline was 64 years in the EMD and non-EMD groups (overall range, 35-86 years). More than half of patients had high-risk cytogenetics (52% in the EMD group and 57% in the non-EMD group).
The median number of prior therapies was five in both the EMD and non-EMD groups. Most patients had received at least one prior transplant (73% in the EMD group and 69% in the non-EMD group). Most patients in both groups were refractory to an anti-CD38 mAb (93% EMD and 72% non-EMD); an IMiD and a PI (93% EMD and 90% non-EMD); an IMiD, a PI, and an anti-CD38 mAb (91% EMD and 63% non-EMD); and their last therapy (100% EMD and 95% non-EMD).
Dr. Richardson pointed out that the incidence of EMD in this trial is higher than has been reported previously. Of the 44 EMD patients, 26 had soft-tissue EMD, and 18 had bone-related EMD. Five patients had CNS involvement.
Another key finding, according to Dr. Richardson, was that EMD appeared to be associated with prior anti-CD38 therapy. Specifically, 40% of patients exposed to an anti-CD38 mAb had EMD, compared with 11% of patients who had not received an anti-CD38 mAb (P = .01).
“I don’t for a minute want to say that CD38-targeted therapy engenders extramedullary disease,” Dr. Richardson said. “I think what we can say, though, is that, once CD38 treatment fails a patient, extramedullary disease … is a very real challenge. Therefore, we need rationally targeted approaches, ideally in combination, to meet that challenge.”
The overall response rate was similar for EMD and non-EMD patients – 23% and 27%, respectively. The median duration of response was 3.4 months in the EMD patients and 4.4 months in the non-EMD group. The clinical benefit rate was 30% and 45%, respectively.
In the EMD group, the overall response rate was 19% in patients with soft-tissue EMD and 28% in bone-related EMD. None of the patients with CNS disease responded.
The median progression-free survival was 2.9 months for patients with EMD and 4.6 months for those without EMD. The median overall survival was 5.8 month and 11.6 months, respectively.
The median overall survival was 18.5 months in EMD responders and 17.2 months in non-EMD responders. The median overall survival was 5.1 months in EMD nonresponders and 8.5 months in non-EMD nonresponders.
In all, 54% of patients received subsequent therapy. There were no significant differences in outcomes between EMD and non-EMD patients.
The safety profiles were similar for EMD and non-EMD patients, Dr. Richardson said. Melflufen-dexamethasone was considered well tolerated overall, and there were no treatment-related deaths.
The most common treatment-emergent adverse events (grade 3 and 4, respectively) were thrombocytopenia (22% and 46%), neutropenia (32% and 35%), anemia (35% and 1%), white blood cell count decrease (10% and 7%), and pneumonia (7% and 1%).
This trial is sponsored by Oncopeptides. Dr. Richardson reported an advisory role and research funding from Oncopeptides.
SOURCE: Richardson PG et al. IMW 2019, Abstract OAB-086.
BOSTON – Melflufen plus dexamethasone is active in patients with relapsed/refractory multiple myeloma, whether or not they have extramedullary disease (EMD), a phase 2 trial suggests.
In the HORIZON trial, melflufen-dexamethasone produced an overall response rate of 23% in patients with EMD and 27% in those without EMD.
Paul G. Richardson, MD, of Harvard Medical School and the Dana-Farber Cancer Institute, both in Boston, presented these results as a late-breaking abstract at the International Myeloma Workshop, held by the International Myeloma Society.
As of July 30, 2019, 136 patients had been treated on the HORIZON trial. The trial is enrolling patients with relapsed/refractory multiple myeloma refractory to pomalidomide, an anti-CD38 monoclonal antibody (mAb), or both. The patients must have received at least two prior lines of therapy, including a proteasome inhibitor (PI) and an immunomodulatory agent (IMiD).
Dr. Richardson presented results for 130 patients, 44 with EMD and 86 without it. The median age at baseline was 64 years in the EMD and non-EMD groups (overall range, 35-86 years). More than half of patients had high-risk cytogenetics (52% in the EMD group and 57% in the non-EMD group).
The median number of prior therapies was five in both the EMD and non-EMD groups. Most patients had received at least one prior transplant (73% in the EMD group and 69% in the non-EMD group). Most patients in both groups were refractory to an anti-CD38 mAb (93% EMD and 72% non-EMD); an IMiD and a PI (93% EMD and 90% non-EMD); an IMiD, a PI, and an anti-CD38 mAb (91% EMD and 63% non-EMD); and their last therapy (100% EMD and 95% non-EMD).
Dr. Richardson pointed out that the incidence of EMD in this trial is higher than has been reported previously. Of the 44 EMD patients, 26 had soft-tissue EMD, and 18 had bone-related EMD. Five patients had CNS involvement.
Another key finding, according to Dr. Richardson, was that EMD appeared to be associated with prior anti-CD38 therapy. Specifically, 40% of patients exposed to an anti-CD38 mAb had EMD, compared with 11% of patients who had not received an anti-CD38 mAb (P = .01).
“I don’t for a minute want to say that CD38-targeted therapy engenders extramedullary disease,” Dr. Richardson said. “I think what we can say, though, is that, once CD38 treatment fails a patient, extramedullary disease … is a very real challenge. Therefore, we need rationally targeted approaches, ideally in combination, to meet that challenge.”
The overall response rate was similar for EMD and non-EMD patients – 23% and 27%, respectively. The median duration of response was 3.4 months in the EMD patients and 4.4 months in the non-EMD group. The clinical benefit rate was 30% and 45%, respectively.
In the EMD group, the overall response rate was 19% in patients with soft-tissue EMD and 28% in bone-related EMD. None of the patients with CNS disease responded.
The median progression-free survival was 2.9 months for patients with EMD and 4.6 months for those without EMD. The median overall survival was 5.8 month and 11.6 months, respectively.
The median overall survival was 18.5 months in EMD responders and 17.2 months in non-EMD responders. The median overall survival was 5.1 months in EMD nonresponders and 8.5 months in non-EMD nonresponders.
In all, 54% of patients received subsequent therapy. There were no significant differences in outcomes between EMD and non-EMD patients.
The safety profiles were similar for EMD and non-EMD patients, Dr. Richardson said. Melflufen-dexamethasone was considered well tolerated overall, and there were no treatment-related deaths.
The most common treatment-emergent adverse events (grade 3 and 4, respectively) were thrombocytopenia (22% and 46%), neutropenia (32% and 35%), anemia (35% and 1%), white blood cell count decrease (10% and 7%), and pneumonia (7% and 1%).
This trial is sponsored by Oncopeptides. Dr. Richardson reported an advisory role and research funding from Oncopeptides.
SOURCE: Richardson PG et al. IMW 2019, Abstract OAB-086.
REPORTING FROM IMW 2019

















