User login
NGS comparable to FC for minimal residual disease assessment
NEW ORLEANS – Next-generation sequencing of peripheral blood is at least as effective as flow cytometry of bone marrow for assessing minimal residual disease, according to a new study.
Researchers compared bone marrow flow cytometry (FC) and peripheral blood next-generation sequencing (NGS) for minimal residual disease (MRD) assessment in pediatric and young adult patients with B-cell acute lymphoblastic leukemia (B-ALL) who received treatment with tisagenlecleucel. There was a high level of concordance between the assays, but the NGS assay detected more MRD-positive samples and NGS results provided a longer lead time to relapse.
Michael A. Pulsipher, MD, of the Children’s Hospital Los Angeles, presented these results at the annual meeting of the American Society of Pediatric Hematology/Oncology.
The researchers analyzed samples from pediatric and young adult patients aged 2-25 years who had relapsed or refractory B-ALL and received treatment with tisagenlecleucel on the ELIANA or ENSIGN trials.
The patients had received at least two prior lines of therapy and were ineligible for allogeneic transplant. They received a single dose of tisagenlecleucel. MRD was assessed before tisagenlecleucel infusion, at various time points after infusion, and at relapse.
Dr. Pulsipher and his colleagues compared MRD results from an NGS assay – Adaptive Biotechnologies’ clonoSEQ – using peripheral blood and results from FC of bone marrow. NGS and FC results were available for 237 samples from 83 patients.
After treatment, NGS detected more MRD-positive samples at each sensitivity level tested (10-4, 10-5, and 10-6). At 10-6, NGS detected 18% more MRD-positive samples than did FC – 50% and 32%, respectively.
Detection of MRD positivity prior to relapse was faster with NGS than with FC. In 17 of 34 patients with morphological relapse, NGS provided a median lead time of 67 days. FC provided a median lead time of 39 days in 11 of the 34 patients.
About 80% of patients who had an MRD status of zero by NGS at day 28 remained relapse-free for up to 3 years.
Among complete responders (n = 50), the duration of response was significantly longer in patients who had an MRD status of zero at day 28 by NGS than in patients who had an MRD status greater than zero (P = .0003). Overall survival was significantly better among patients with an MRD status of zero as well (P = .0004).
Dr. Pulsipher said additional studies are needed to confirm these findings and determine the best way to know if a patient has been cured or needs additional therapy after tisagenlecleucel.
Dr. Pulsipher reported relationships with Adaptive Biotech, Novartis, Incyte, Amgen, Bellicum Pharmaceuticals, Medac Pharma, and Miltenyi Biotec. ELIANA and ENSIGN were funded by Novartis, which markets tisagenlecleucel as Kymriah.
SOURCE: Pulsipher MA et al. ASPHO 2019, Abstract 2001.
NEW ORLEANS – Next-generation sequencing of peripheral blood is at least as effective as flow cytometry of bone marrow for assessing minimal residual disease, according to a new study.
Researchers compared bone marrow flow cytometry (FC) and peripheral blood next-generation sequencing (NGS) for minimal residual disease (MRD) assessment in pediatric and young adult patients with B-cell acute lymphoblastic leukemia (B-ALL) who received treatment with tisagenlecleucel. There was a high level of concordance between the assays, but the NGS assay detected more MRD-positive samples and NGS results provided a longer lead time to relapse.
Michael A. Pulsipher, MD, of the Children’s Hospital Los Angeles, presented these results at the annual meeting of the American Society of Pediatric Hematology/Oncology.
The researchers analyzed samples from pediatric and young adult patients aged 2-25 years who had relapsed or refractory B-ALL and received treatment with tisagenlecleucel on the ELIANA or ENSIGN trials.
The patients had received at least two prior lines of therapy and were ineligible for allogeneic transplant. They received a single dose of tisagenlecleucel. MRD was assessed before tisagenlecleucel infusion, at various time points after infusion, and at relapse.
Dr. Pulsipher and his colleagues compared MRD results from an NGS assay – Adaptive Biotechnologies’ clonoSEQ – using peripheral blood and results from FC of bone marrow. NGS and FC results were available for 237 samples from 83 patients.
After treatment, NGS detected more MRD-positive samples at each sensitivity level tested (10-4, 10-5, and 10-6). At 10-6, NGS detected 18% more MRD-positive samples than did FC – 50% and 32%, respectively.
Detection of MRD positivity prior to relapse was faster with NGS than with FC. In 17 of 34 patients with morphological relapse, NGS provided a median lead time of 67 days. FC provided a median lead time of 39 days in 11 of the 34 patients.
About 80% of patients who had an MRD status of zero by NGS at day 28 remained relapse-free for up to 3 years.
Among complete responders (n = 50), the duration of response was significantly longer in patients who had an MRD status of zero at day 28 by NGS than in patients who had an MRD status greater than zero (P = .0003). Overall survival was significantly better among patients with an MRD status of zero as well (P = .0004).
Dr. Pulsipher said additional studies are needed to confirm these findings and determine the best way to know if a patient has been cured or needs additional therapy after tisagenlecleucel.
Dr. Pulsipher reported relationships with Adaptive Biotech, Novartis, Incyte, Amgen, Bellicum Pharmaceuticals, Medac Pharma, and Miltenyi Biotec. ELIANA and ENSIGN were funded by Novartis, which markets tisagenlecleucel as Kymriah.
SOURCE: Pulsipher MA et al. ASPHO 2019, Abstract 2001.
NEW ORLEANS – Next-generation sequencing of peripheral blood is at least as effective as flow cytometry of bone marrow for assessing minimal residual disease, according to a new study.
Researchers compared bone marrow flow cytometry (FC) and peripheral blood next-generation sequencing (NGS) for minimal residual disease (MRD) assessment in pediatric and young adult patients with B-cell acute lymphoblastic leukemia (B-ALL) who received treatment with tisagenlecleucel. There was a high level of concordance between the assays, but the NGS assay detected more MRD-positive samples and NGS results provided a longer lead time to relapse.
Michael A. Pulsipher, MD, of the Children’s Hospital Los Angeles, presented these results at the annual meeting of the American Society of Pediatric Hematology/Oncology.
The researchers analyzed samples from pediatric and young adult patients aged 2-25 years who had relapsed or refractory B-ALL and received treatment with tisagenlecleucel on the ELIANA or ENSIGN trials.
The patients had received at least two prior lines of therapy and were ineligible for allogeneic transplant. They received a single dose of tisagenlecleucel. MRD was assessed before tisagenlecleucel infusion, at various time points after infusion, and at relapse.
Dr. Pulsipher and his colleagues compared MRD results from an NGS assay – Adaptive Biotechnologies’ clonoSEQ – using peripheral blood and results from FC of bone marrow. NGS and FC results were available for 237 samples from 83 patients.
After treatment, NGS detected more MRD-positive samples at each sensitivity level tested (10-4, 10-5, and 10-6). At 10-6, NGS detected 18% more MRD-positive samples than did FC – 50% and 32%, respectively.
Detection of MRD positivity prior to relapse was faster with NGS than with FC. In 17 of 34 patients with morphological relapse, NGS provided a median lead time of 67 days. FC provided a median lead time of 39 days in 11 of the 34 patients.
About 80% of patients who had an MRD status of zero by NGS at day 28 remained relapse-free for up to 3 years.
Among complete responders (n = 50), the duration of response was significantly longer in patients who had an MRD status of zero at day 28 by NGS than in patients who had an MRD status greater than zero (P = .0003). Overall survival was significantly better among patients with an MRD status of zero as well (P = .0004).
Dr. Pulsipher said additional studies are needed to confirm these findings and determine the best way to know if a patient has been cured or needs additional therapy after tisagenlecleucel.
Dr. Pulsipher reported relationships with Adaptive Biotech, Novartis, Incyte, Amgen, Bellicum Pharmaceuticals, Medac Pharma, and Miltenyi Biotec. ELIANA and ENSIGN were funded by Novartis, which markets tisagenlecleucel as Kymriah.
SOURCE: Pulsipher MA et al. ASPHO 2019, Abstract 2001.
REPORTING FROM 2019 ASPHO CONFERENCE
Key clinical point: Major finding: At the highest sensitivity level tested, next-generation sequencing detected 18% more minimal residual disease–positive samples than did flow cytometry – 50% and 32%, respectively.
Study details: An analysis of samples from pediatric and young adult patients with B-cell acute lymphoblastic leukemia who received treatment with tisagenlecleucel on the ELIANA and ENSIGN trials.
Disclosures: The speaker reported relationships with Adaptive Biotech, Novartis, Incyte, Amgen, Bellicum Pharmaceuticals, Medac Pharma, and Miltenyi Biotec. The ELIANA and ENSIGN trials were funded by Novartis, which markets tisagenlecleucel as Kymriah.
Source: Pulsipher MA et al. ASPHO 2019, Abstract 2001.
QOL concerns prompt second-line therapy in children with ITP
NEW ORLEANS – In a survey of pediatric hematologists, quality of life was the most frequently cited reason for starting second-line therapy in children with immune thrombocytopenia.
Quality of life (QOL) was an indication for second-line treatment in nearly three-quarters of patients studied, and it ranked among the top three indications – along with bleeding frequency and bleeding severity – for treatment in more than half of patients.
Kristin A. Shimano, MD, of the department of pediatrics at the University of California, San Francisco, presented these results at the annual meeting of the American Society of Pediatric Hematology/Oncology.
Dr. Shimano and colleagues surveyed hematologists treating children in the ICON1 study (Am J Hematol. 2019 Apr 3. doi: 10.1002/ajh.25479).
The study enrolled 120 children receiving second-line immune thrombocytopenia (ITP) treatment at 21 centers. The median age at enrollment was 11.7 years (range, 1.2-17.8 years). About half of patients (53%) had chronic ITP, 31% had persistent ITP, and 16% had newly diagnosed ITP. The median number of prior treatments was three (range, zero to eight).
At study entry, the hematologists were asked to provide reasons that patients required second-line treatment. The list of 12 possible reasons included patient or parent QOL; bleeding severity; bleeding frequency; severity of thrombocytopenia; chronicity of ITP; high baseline activity level; involvement in sports; patient age; distance from medical center; and parent, patient, or physician anxiety. The hematologists were asked to choose all reasons that applied and to rank the top three reasons.
QOL was chosen as a reason to treat in 73% of patients (n = 88). QOL was among the top three reasons in 57% of patients (n = 68) and was the most important reason in 27% of patients (n = 32).
The severity and frequency of bleeding were ranked among the top three indications as well. Bleeding severity was a top indication in 29% of cases (n = 35), and bleeding frequency was a top indication in 40% of cases (n = 48).
Reasons for starting second-line treatment varied depending on patients’ phase of disease.
Bleeding severity was significantly more likely to be an indication for treatment among patients who had newly diagnosed or persistent ITP (69%), rather than chronic ITP (31%; P = .0025). Bleeding frequency was also significantly more likely to be an indication among patients with newly diagnosed or persistent ITP (63% vs. 37%; P = .0054).
Conversely, QOL was significantly more likely to be an indication for patients with chronic ITP (65%) rather than newly diagnosed or persistent ITP (35%, P = .0056). Sports participation was a more likely indication among patients with chronic ITP as well (75% vs. 26%, P = .017).
Indications for treatment also varied according to baseline platelet counts. For example, QOL was an indication for treatment in 42% of patients with baseline platelet counts less than 10 x 109/L and 78% of patients with platelet counts of 20 x 109/L or greater. So the higher the baseline platelet count, the more likely QOL was an indication for treatment (P = .006).
On the other hand, the importance hematologists placed on QOL did not appear to correlate with actual health-related QOL as assessed by the Kids ITP Tool. There was no difference reported in baseline health-related QOL, according to the tool, in children for whom QOL was ranked versus unranked by hematologists.
This finding suggests physicians may not be adequately assessing the impact of ITP on QOL, Dr. Shimano said.
“Better clinical measures of the impact of ITP on patient quality of life are needed to assess both need for treatment and treatment response,” she said. “Understanding the effects of individual second-line treatments on quality of life is critical for this patient population in order to best tailor therapy for each patient.”
Dr. Shimano reported involvement in an investigator-initiated trial for eltrombopag in children with ITP. The study, which has not yet opened, is funded by Novartis.
SOURCE: Shimano KA et al. ASPHO 2019, Abstract 2012.
NEW ORLEANS – In a survey of pediatric hematologists, quality of life was the most frequently cited reason for starting second-line therapy in children with immune thrombocytopenia.
Quality of life (QOL) was an indication for second-line treatment in nearly three-quarters of patients studied, and it ranked among the top three indications – along with bleeding frequency and bleeding severity – for treatment in more than half of patients.
Kristin A. Shimano, MD, of the department of pediatrics at the University of California, San Francisco, presented these results at the annual meeting of the American Society of Pediatric Hematology/Oncology.
Dr. Shimano and colleagues surveyed hematologists treating children in the ICON1 study (Am J Hematol. 2019 Apr 3. doi: 10.1002/ajh.25479).
The study enrolled 120 children receiving second-line immune thrombocytopenia (ITP) treatment at 21 centers. The median age at enrollment was 11.7 years (range, 1.2-17.8 years). About half of patients (53%) had chronic ITP, 31% had persistent ITP, and 16% had newly diagnosed ITP. The median number of prior treatments was three (range, zero to eight).
At study entry, the hematologists were asked to provide reasons that patients required second-line treatment. The list of 12 possible reasons included patient or parent QOL; bleeding severity; bleeding frequency; severity of thrombocytopenia; chronicity of ITP; high baseline activity level; involvement in sports; patient age; distance from medical center; and parent, patient, or physician anxiety. The hematologists were asked to choose all reasons that applied and to rank the top three reasons.
QOL was chosen as a reason to treat in 73% of patients (n = 88). QOL was among the top three reasons in 57% of patients (n = 68) and was the most important reason in 27% of patients (n = 32).
The severity and frequency of bleeding were ranked among the top three indications as well. Bleeding severity was a top indication in 29% of cases (n = 35), and bleeding frequency was a top indication in 40% of cases (n = 48).
Reasons for starting second-line treatment varied depending on patients’ phase of disease.
Bleeding severity was significantly more likely to be an indication for treatment among patients who had newly diagnosed or persistent ITP (69%), rather than chronic ITP (31%; P = .0025). Bleeding frequency was also significantly more likely to be an indication among patients with newly diagnosed or persistent ITP (63% vs. 37%; P = .0054).
Conversely, QOL was significantly more likely to be an indication for patients with chronic ITP (65%) rather than newly diagnosed or persistent ITP (35%, P = .0056). Sports participation was a more likely indication among patients with chronic ITP as well (75% vs. 26%, P = .017).
Indications for treatment also varied according to baseline platelet counts. For example, QOL was an indication for treatment in 42% of patients with baseline platelet counts less than 10 x 109/L and 78% of patients with platelet counts of 20 x 109/L or greater. So the higher the baseline platelet count, the more likely QOL was an indication for treatment (P = .006).
On the other hand, the importance hematologists placed on QOL did not appear to correlate with actual health-related QOL as assessed by the Kids ITP Tool. There was no difference reported in baseline health-related QOL, according to the tool, in children for whom QOL was ranked versus unranked by hematologists.
This finding suggests physicians may not be adequately assessing the impact of ITP on QOL, Dr. Shimano said.
“Better clinical measures of the impact of ITP on patient quality of life are needed to assess both need for treatment and treatment response,” she said. “Understanding the effects of individual second-line treatments on quality of life is critical for this patient population in order to best tailor therapy for each patient.”
Dr. Shimano reported involvement in an investigator-initiated trial for eltrombopag in children with ITP. The study, which has not yet opened, is funded by Novartis.
SOURCE: Shimano KA et al. ASPHO 2019, Abstract 2012.
NEW ORLEANS – In a survey of pediatric hematologists, quality of life was the most frequently cited reason for starting second-line therapy in children with immune thrombocytopenia.
Quality of life (QOL) was an indication for second-line treatment in nearly three-quarters of patients studied, and it ranked among the top three indications – along with bleeding frequency and bleeding severity – for treatment in more than half of patients.
Kristin A. Shimano, MD, of the department of pediatrics at the University of California, San Francisco, presented these results at the annual meeting of the American Society of Pediatric Hematology/Oncology.
Dr. Shimano and colleagues surveyed hematologists treating children in the ICON1 study (Am J Hematol. 2019 Apr 3. doi: 10.1002/ajh.25479).
The study enrolled 120 children receiving second-line immune thrombocytopenia (ITP) treatment at 21 centers. The median age at enrollment was 11.7 years (range, 1.2-17.8 years). About half of patients (53%) had chronic ITP, 31% had persistent ITP, and 16% had newly diagnosed ITP. The median number of prior treatments was three (range, zero to eight).
At study entry, the hematologists were asked to provide reasons that patients required second-line treatment. The list of 12 possible reasons included patient or parent QOL; bleeding severity; bleeding frequency; severity of thrombocytopenia; chronicity of ITP; high baseline activity level; involvement in sports; patient age; distance from medical center; and parent, patient, or physician anxiety. The hematologists were asked to choose all reasons that applied and to rank the top three reasons.
QOL was chosen as a reason to treat in 73% of patients (n = 88). QOL was among the top three reasons in 57% of patients (n = 68) and was the most important reason in 27% of patients (n = 32).
The severity and frequency of bleeding were ranked among the top three indications as well. Bleeding severity was a top indication in 29% of cases (n = 35), and bleeding frequency was a top indication in 40% of cases (n = 48).
Reasons for starting second-line treatment varied depending on patients’ phase of disease.
Bleeding severity was significantly more likely to be an indication for treatment among patients who had newly diagnosed or persistent ITP (69%), rather than chronic ITP (31%; P = .0025). Bleeding frequency was also significantly more likely to be an indication among patients with newly diagnosed or persistent ITP (63% vs. 37%; P = .0054).
Conversely, QOL was significantly more likely to be an indication for patients with chronic ITP (65%) rather than newly diagnosed or persistent ITP (35%, P = .0056). Sports participation was a more likely indication among patients with chronic ITP as well (75% vs. 26%, P = .017).
Indications for treatment also varied according to baseline platelet counts. For example, QOL was an indication for treatment in 42% of patients with baseline platelet counts less than 10 x 109/L and 78% of patients with platelet counts of 20 x 109/L or greater. So the higher the baseline platelet count, the more likely QOL was an indication for treatment (P = .006).
On the other hand, the importance hematologists placed on QOL did not appear to correlate with actual health-related QOL as assessed by the Kids ITP Tool. There was no difference reported in baseline health-related QOL, according to the tool, in children for whom QOL was ranked versus unranked by hematologists.
This finding suggests physicians may not be adequately assessing the impact of ITP on QOL, Dr. Shimano said.
“Better clinical measures of the impact of ITP on patient quality of life are needed to assess both need for treatment and treatment response,” she said. “Understanding the effects of individual second-line treatments on quality of life is critical for this patient population in order to best tailor therapy for each patient.”
Dr. Shimano reported involvement in an investigator-initiated trial for eltrombopag in children with ITP. The study, which has not yet opened, is funded by Novartis.
SOURCE: Shimano KA et al. ASPHO 2019, Abstract 2012.
REPORTING FROM THE 2019 ASPHO CONFERENCE
Key clinical point: Quality of life was the most frequently cited reason for starting second-line therapy in children with immune thrombocytopenia.
Major finding: Quality of life was chosen as a reason to treat in 73% of patients, it was among the top three reasons in 57% of patients, and it was the most important reason in 27%.
Study details: A survey of hematologists treating 120 children in an observational study.
Disclosures: The speaker reported involvement in an investigator-initiated trial for eltrombopag in children with ITP. The study, which has not yet opened, is funded by Novartis.
Source: Shimano KA et al. ASPHO 2019, Abstract 2012.
Master trial seeks to aid drug development for pediatric AML
NEW ORLEANS – Researchers are organizing a master trial in an attempt to improve the treatment of pediatric acute myeloid leukemia (AML).
The Pediatric Acute Leukemia (PedAL) trial is an effort to collect data on all pediatric AML patients. The plan is to use these data to match patients to clinical trials, better understand pediatric AML, and bring new treatments to this population.
E. Anders Kolb, MD, of Nemours Center for Cancer and Blood Disorders in Wilmington, Del., described the initiative at the annual meeting of the American Society of Pediatric Hematology/Oncology.
Dr. Kolb noted that several drugs have been approved to treat adult AML in the last 2 years, but most of them are not approved for use in children.
“What we see in childhood AML is a lot different than what we see in adult AML, and this challenges the paradigm that we have traditionally followed where we use the adult as the 'preclinical model' for pediatric AML,” he said. “I think we are learning more and more that children have a unique disease, unique targets, and need unique therapies.”
The PedAL initiative is an attempt to address these unique needs. PedAL is part of the Leukemia & Lymphoma Society’s Children’s Initiative, and it involves researchers from academic centers and the Children’s Oncology Group.
The PedAL initiative includes preclinical, biomarker, and informatics research, as well as the master clinical trial. The main goal of the master trial is to collect genomic, proteomic, metabolomic, flow cytometry, and clinical data from all children with AML and use these data to match patients to clinical trials.
The PedAL trial will leverage Project:EveryChild, an effort by the Children’s Oncology Group to study every child with cancer. Each child enrolled in this program has an identification number that follows the child through all clinical interventions.
The goal is that Project:EveryChild will capture all pediatric AML patients at the time of diagnosis, although patients can join the project at any time. Then, sequencing, clinical, and other data will be collected from these patients and stored in a data commons.
If patients relapse after standard or other therapies, the GEARBOX algorithm (genomic eligibility algorithm at relapse for better outcomes) can be used to match the patient’s information to clinical trial eligibility criteria and provide a list of appropriate trials.
Dr. Kolb said this process should reduce logistical barriers and get relapsed patients to trials more quickly. Additionally, the data collected through PedAL should help researchers design better trials for pediatric patients with relapsed AML.
“Ultimately, we’ll create the largest data set that will give us a better understanding of all the risks and benefits associated with postrelapse AML,” Dr. Kolb said. “No matter what happens to the patient, no matter where that patient enrolls, we’re going to have the capacity to collect data and present that data to the community for analysis for improved understanding of outcomes.”
Dr. Kolb and his colleagues are already working with researchers in Europe and Japan to make this a global effort and create an international data commons. In addition, the researchers are planning to collaborate with the pharmaceutical industry to unite efforts in pediatric AML drug development.
“We can’t just test drugs in kids because they worked in adults,” Dr. Kolb said. “We really need to maintain the integrity of the science and ask relevant questions in children but do so with the intent to make sure these drugs are licensed for use in kids.”
Dr. Kolb reported having no conflicts of interest. The PedAL trial is sponsored by the Leukemia & Lymphoma Society.
NEW ORLEANS – Researchers are organizing a master trial in an attempt to improve the treatment of pediatric acute myeloid leukemia (AML).
The Pediatric Acute Leukemia (PedAL) trial is an effort to collect data on all pediatric AML patients. The plan is to use these data to match patients to clinical trials, better understand pediatric AML, and bring new treatments to this population.
E. Anders Kolb, MD, of Nemours Center for Cancer and Blood Disorders in Wilmington, Del., described the initiative at the annual meeting of the American Society of Pediatric Hematology/Oncology.
Dr. Kolb noted that several drugs have been approved to treat adult AML in the last 2 years, but most of them are not approved for use in children.
“What we see in childhood AML is a lot different than what we see in adult AML, and this challenges the paradigm that we have traditionally followed where we use the adult as the 'preclinical model' for pediatric AML,” he said. “I think we are learning more and more that children have a unique disease, unique targets, and need unique therapies.”
The PedAL initiative is an attempt to address these unique needs. PedAL is part of the Leukemia & Lymphoma Society’s Children’s Initiative, and it involves researchers from academic centers and the Children’s Oncology Group.
The PedAL initiative includes preclinical, biomarker, and informatics research, as well as the master clinical trial. The main goal of the master trial is to collect genomic, proteomic, metabolomic, flow cytometry, and clinical data from all children with AML and use these data to match patients to clinical trials.
The PedAL trial will leverage Project:EveryChild, an effort by the Children’s Oncology Group to study every child with cancer. Each child enrolled in this program has an identification number that follows the child through all clinical interventions.
The goal is that Project:EveryChild will capture all pediatric AML patients at the time of diagnosis, although patients can join the project at any time. Then, sequencing, clinical, and other data will be collected from these patients and stored in a data commons.
If patients relapse after standard or other therapies, the GEARBOX algorithm (genomic eligibility algorithm at relapse for better outcomes) can be used to match the patient’s information to clinical trial eligibility criteria and provide a list of appropriate trials.
Dr. Kolb said this process should reduce logistical barriers and get relapsed patients to trials more quickly. Additionally, the data collected through PedAL should help researchers design better trials for pediatric patients with relapsed AML.
“Ultimately, we’ll create the largest data set that will give us a better understanding of all the risks and benefits associated with postrelapse AML,” Dr. Kolb said. “No matter what happens to the patient, no matter where that patient enrolls, we’re going to have the capacity to collect data and present that data to the community for analysis for improved understanding of outcomes.”
Dr. Kolb and his colleagues are already working with researchers in Europe and Japan to make this a global effort and create an international data commons. In addition, the researchers are planning to collaborate with the pharmaceutical industry to unite efforts in pediatric AML drug development.
“We can’t just test drugs in kids because they worked in adults,” Dr. Kolb said. “We really need to maintain the integrity of the science and ask relevant questions in children but do so with the intent to make sure these drugs are licensed for use in kids.”
Dr. Kolb reported having no conflicts of interest. The PedAL trial is sponsored by the Leukemia & Lymphoma Society.
NEW ORLEANS – Researchers are organizing a master trial in an attempt to improve the treatment of pediatric acute myeloid leukemia (AML).
The Pediatric Acute Leukemia (PedAL) trial is an effort to collect data on all pediatric AML patients. The plan is to use these data to match patients to clinical trials, better understand pediatric AML, and bring new treatments to this population.
E. Anders Kolb, MD, of Nemours Center for Cancer and Blood Disorders in Wilmington, Del., described the initiative at the annual meeting of the American Society of Pediatric Hematology/Oncology.
Dr. Kolb noted that several drugs have been approved to treat adult AML in the last 2 years, but most of them are not approved for use in children.
“What we see in childhood AML is a lot different than what we see in adult AML, and this challenges the paradigm that we have traditionally followed where we use the adult as the 'preclinical model' for pediatric AML,” he said. “I think we are learning more and more that children have a unique disease, unique targets, and need unique therapies.”
The PedAL initiative is an attempt to address these unique needs. PedAL is part of the Leukemia & Lymphoma Society’s Children’s Initiative, and it involves researchers from academic centers and the Children’s Oncology Group.
The PedAL initiative includes preclinical, biomarker, and informatics research, as well as the master clinical trial. The main goal of the master trial is to collect genomic, proteomic, metabolomic, flow cytometry, and clinical data from all children with AML and use these data to match patients to clinical trials.
The PedAL trial will leverage Project:EveryChild, an effort by the Children’s Oncology Group to study every child with cancer. Each child enrolled in this program has an identification number that follows the child through all clinical interventions.
The goal is that Project:EveryChild will capture all pediatric AML patients at the time of diagnosis, although patients can join the project at any time. Then, sequencing, clinical, and other data will be collected from these patients and stored in a data commons.
If patients relapse after standard or other therapies, the GEARBOX algorithm (genomic eligibility algorithm at relapse for better outcomes) can be used to match the patient’s information to clinical trial eligibility criteria and provide a list of appropriate trials.
Dr. Kolb said this process should reduce logistical barriers and get relapsed patients to trials more quickly. Additionally, the data collected through PedAL should help researchers design better trials for pediatric patients with relapsed AML.
“Ultimately, we’ll create the largest data set that will give us a better understanding of all the risks and benefits associated with postrelapse AML,” Dr. Kolb said. “No matter what happens to the patient, no matter where that patient enrolls, we’re going to have the capacity to collect data and present that data to the community for analysis for improved understanding of outcomes.”
Dr. Kolb and his colleagues are already working with researchers in Europe and Japan to make this a global effort and create an international data commons. In addition, the researchers are planning to collaborate with the pharmaceutical industry to unite efforts in pediatric AML drug development.
“We can’t just test drugs in kids because they worked in adults,” Dr. Kolb said. “We really need to maintain the integrity of the science and ask relevant questions in children but do so with the intent to make sure these drugs are licensed for use in kids.”
Dr. Kolb reported having no conflicts of interest. The PedAL trial is sponsored by the Leukemia & Lymphoma Society.
REPORTING FROM 2019 ASPHO CONFERENCE
Next-generation sequencing test detects pathogens with high sensitivity
NEW ORLEANS – A next-generation sequencing (NGS) test for pathogen detection demonstrated higher sensitivity than conventional testing methods in a cohort of diverse pediatric patients, according to researchers.
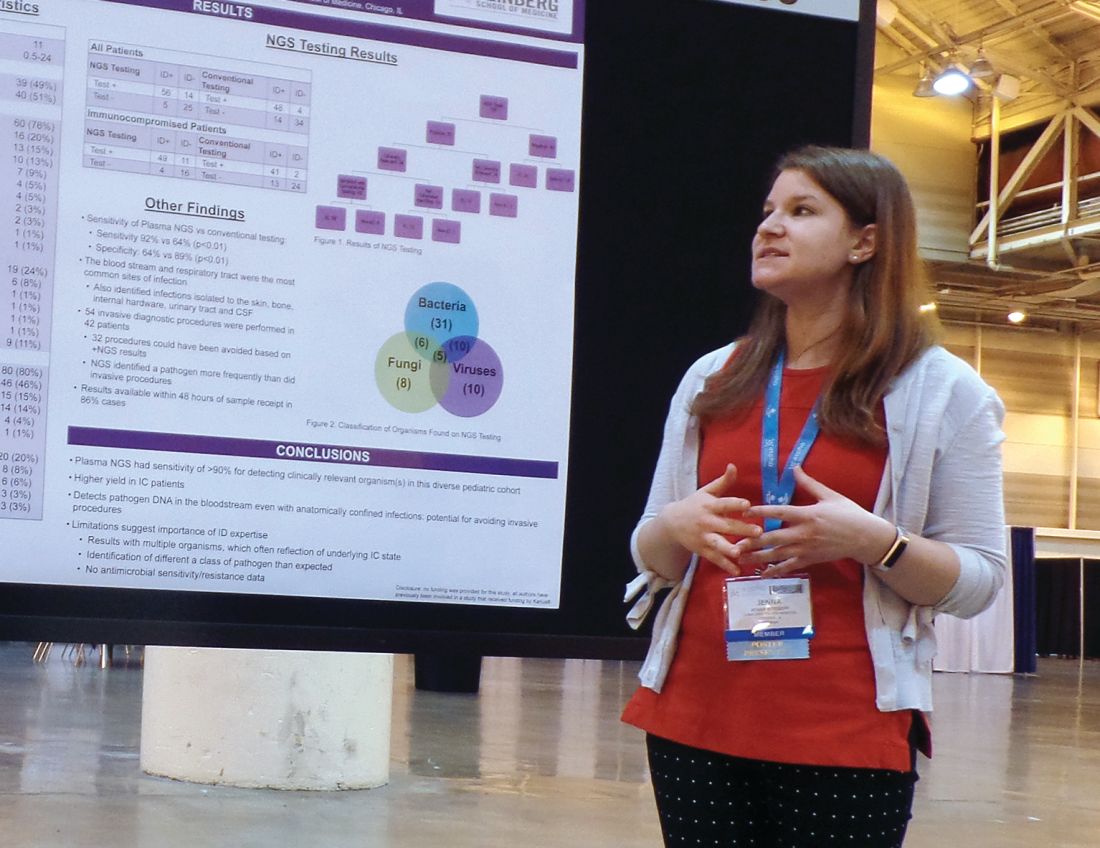
The NGS test, which detects sequences of circulating cell-free DNA in plasma, detected pathogens with 92% sensitivity, compared with 64% sensitivity for all conventional testing methods combined (P less than .01).
“While I think we can all recognize that specificity is important, I think sensitivity is more important to be able to get at sources of infection,” said Jenna Rossoff, MD, of Ann & Robert H. Lurie Children’s Hospital of Chicago.
Dr. Rossoff and her colleagues conducted this study and presented the results in a poster at the annual meeting of the American Society of Pediatric Hematology/Oncology.
Lurie Children’s Hospital began using a commercially available NGS pathogen test, the Karius test, in 2016. Dr. Rossoff and her colleagues set out to evaluate how the test affected patient care by conducting a retrospective analysis of tests performed from December 2016 through August 2018.
The researchers studied 100 NGS tests performed for 79 pediatric patients. The patients had a median age of 11 years (range, 0.5-24 years).
Most patients (n = 60) were immunocompromised, largely due to a hematologic malignancy (n = 16), primary immune deficiency (n = 13), hematopoietic cell transplant (n = 10), or solid organ transplant (n = 7).
The remaining 19 patients were immunocompetent, and 9 of them had no underlying diagnosis. The most common diagnosis for this group was neurologic disorder (n = 6).
Results
Of the 100 NGS tests evaluated, 70 were positive for any organism, and 56 of these were deemed clinically relevant.
“What I think is quite remarkable is that, of those clinically relevant organisms, tests on 14, which is 25% of those, were able to identify clinically relevant or pathogenic organisms when no other conventional testing modality was able to identify them,” Dr. Rossoff said. “And these were often in patients who underwent invasive procedures to try to get at the source of their infectious disease.”
In fact, the study included 42 patients who underwent 54 invasive diagnostic procedures, and 32 of those procedures could have been avoided based on positive NGS results, according to Dr. Rossoff and her colleagues.
Dr. Rossoff noted that the most common sites of infection were the bloodstream and respiratory tract, but the NGS test was able to identify pathogens in the bone, skin, cerebrospinal fluid, and urinary tract. She also pointed out that NGS results were available “in a fairly timely manner,” as 86% of test results were available within 48 hours of sample receipt.
Dr. Rossoff and her colleagues did not receive any funding for this study, but they were previously involved in a study funded by Karius, the company that commercialized the NGS test.
SOURCE: Rossoff J et al. ASPHO 2019. Abstract 439.
NEW ORLEANS – A next-generation sequencing (NGS) test for pathogen detection demonstrated higher sensitivity than conventional testing methods in a cohort of diverse pediatric patients, according to researchers.
The NGS test, which detects sequences of circulating cell-free DNA in plasma, detected pathogens with 92% sensitivity, compared with 64% sensitivity for all conventional testing methods combined (P less than .01).
“While I think we can all recognize that specificity is important, I think sensitivity is more important to be able to get at sources of infection,” said Jenna Rossoff, MD, of Ann & Robert H. Lurie Children’s Hospital of Chicago.
Dr. Rossoff and her colleagues conducted this study and presented the results in a poster at the annual meeting of the American Society of Pediatric Hematology/Oncology.
Lurie Children’s Hospital began using a commercially available NGS pathogen test, the Karius test, in 2016. Dr. Rossoff and her colleagues set out to evaluate how the test affected patient care by conducting a retrospective analysis of tests performed from December 2016 through August 2018.
The researchers studied 100 NGS tests performed for 79 pediatric patients. The patients had a median age of 11 years (range, 0.5-24 years).
Most patients (n = 60) were immunocompromised, largely due to a hematologic malignancy (n = 16), primary immune deficiency (n = 13), hematopoietic cell transplant (n = 10), or solid organ transplant (n = 7).
The remaining 19 patients were immunocompetent, and 9 of them had no underlying diagnosis. The most common diagnosis for this group was neurologic disorder (n = 6).
Results
Of the 100 NGS tests evaluated, 70 were positive for any organism, and 56 of these were deemed clinically relevant.
“What I think is quite remarkable is that, of those clinically relevant organisms, tests on 14, which is 25% of those, were able to identify clinically relevant or pathogenic organisms when no other conventional testing modality was able to identify them,” Dr. Rossoff said. “And these were often in patients who underwent invasive procedures to try to get at the source of their infectious disease.”
In fact, the study included 42 patients who underwent 54 invasive diagnostic procedures, and 32 of those procedures could have been avoided based on positive NGS results, according to Dr. Rossoff and her colleagues.
Dr. Rossoff noted that the most common sites of infection were the bloodstream and respiratory tract, but the NGS test was able to identify pathogens in the bone, skin, cerebrospinal fluid, and urinary tract. She also pointed out that NGS results were available “in a fairly timely manner,” as 86% of test results were available within 48 hours of sample receipt.
Dr. Rossoff and her colleagues did not receive any funding for this study, but they were previously involved in a study funded by Karius, the company that commercialized the NGS test.
SOURCE: Rossoff J et al. ASPHO 2019. Abstract 439.
NEW ORLEANS – A next-generation sequencing (NGS) test for pathogen detection demonstrated higher sensitivity than conventional testing methods in a cohort of diverse pediatric patients, according to researchers.
The NGS test, which detects sequences of circulating cell-free DNA in plasma, detected pathogens with 92% sensitivity, compared with 64% sensitivity for all conventional testing methods combined (P less than .01).
“While I think we can all recognize that specificity is important, I think sensitivity is more important to be able to get at sources of infection,” said Jenna Rossoff, MD, of Ann & Robert H. Lurie Children’s Hospital of Chicago.
Dr. Rossoff and her colleagues conducted this study and presented the results in a poster at the annual meeting of the American Society of Pediatric Hematology/Oncology.
Lurie Children’s Hospital began using a commercially available NGS pathogen test, the Karius test, in 2016. Dr. Rossoff and her colleagues set out to evaluate how the test affected patient care by conducting a retrospective analysis of tests performed from December 2016 through August 2018.
The researchers studied 100 NGS tests performed for 79 pediatric patients. The patients had a median age of 11 years (range, 0.5-24 years).
Most patients (n = 60) were immunocompromised, largely due to a hematologic malignancy (n = 16), primary immune deficiency (n = 13), hematopoietic cell transplant (n = 10), or solid organ transplant (n = 7).
The remaining 19 patients were immunocompetent, and 9 of them had no underlying diagnosis. The most common diagnosis for this group was neurologic disorder (n = 6).
Results
Of the 100 NGS tests evaluated, 70 were positive for any organism, and 56 of these were deemed clinically relevant.
“What I think is quite remarkable is that, of those clinically relevant organisms, tests on 14, which is 25% of those, were able to identify clinically relevant or pathogenic organisms when no other conventional testing modality was able to identify them,” Dr. Rossoff said. “And these were often in patients who underwent invasive procedures to try to get at the source of their infectious disease.”
In fact, the study included 42 patients who underwent 54 invasive diagnostic procedures, and 32 of those procedures could have been avoided based on positive NGS results, according to Dr. Rossoff and her colleagues.
Dr. Rossoff noted that the most common sites of infection were the bloodstream and respiratory tract, but the NGS test was able to identify pathogens in the bone, skin, cerebrospinal fluid, and urinary tract. She also pointed out that NGS results were available “in a fairly timely manner,” as 86% of test results were available within 48 hours of sample receipt.
Dr. Rossoff and her colleagues did not receive any funding for this study, but they were previously involved in a study funded by Karius, the company that commercialized the NGS test.
SOURCE: Rossoff J et al. ASPHO 2019. Abstract 439.
REPORTING FROM 2019 ASPHO CONFERENCE
Gabapentin falls short in treating sickle cell pain
NEW ORLEANS – Adding gabapentin to standard therapy did not significantly reduce vaso-occlusive pain in most patients with sickle cell disease enrolled in a phase 2 trial.
In the entire cohort, there were no significant differences in pain response between patients who received gabapentin and those who received placebo. However, patients with the HbSS genotype had a significantly greater decrease in pain score from baseline to discharge if they received gabapentin rather than placebo.
Additional studies are needed to confirm these findings because this trial was limited by a small sample size, according to study investigator Latika Puri, MD, of St. Jude Children’s Research Hospital in Memphis. Dr. Puri presented the trial at the annual meeting of the American Society of Pediatric Hematology/Oncology.
The trial included 86 evaluable patients who had vaso-occlusive pain and a pain score of at least 4. All patients received standard therapy for vaso-occlusive pain and were randomized to receive placebo (n = 44) or a single oral dose of gabapentin at 15 mg/kg (n = 42).
Baseline characteristics were similar between the treatment arms. For the entire cohort, the mean age was 11.8 years (range, 1-21 years), and 51% of patients were male. Forty-four patients had the HbSS genotype, 25 had the HbSC genotype, 8 had HbS/beta0-thalassemia, and 9 had other genotypes.
The mean pain score at baseline was 7.8 for the entire cohort, 8.0 for the gabapentin arm, and 7.7 for the placebo arm.
For the entire cohort, there was no significant difference in pain response between the gabapentin and placebo arms.
The proportion of patients who experienced a greater than 33% decrease in pain from baseline to 3 hours posttreatment was 67% in the gabapentin arm and 59% in the placebo arm (P = .23). The proportion of patients who experienced a greater than 33% decrease from baseline to discharge from the acute care clinic was 75% and 61%, respectively (P = .18).
In the entire cohort, decreases in pain scores from baseline to 3 hours posttreatment were not significantly different between the gabapentin and placebo arms, at 1.3 and 0.7, respectively (P = .74). Likewise, decreases in pain scores from baseline to discharge were not significantly different, at 1.6 and 0.8 (P = .38).
Among patients who had the HbSS genotype, there was a significantly greater decrease in pain score from baseline to discharge in the gabapentin arm than in the placebo arm, 5.9 versus 3.6 (P = .03). However, there were no other significant differences in pain response for the HbSS subgroup.
There were no significant differences in opioid consumption or hospitalization for the HbSS subgroup or the entire cohort. For the entire cohort, the mean morphine equivalent dose from baseline to 3 hours posttreatment was 0.16 mg/kg in the gabapentin arm and 0.17 mg/kg in the placebo arm (P = .89). For the HbSS subgroup, the mean dose was 0.16 mg/kg and 0.15 mg/kg, respectively (P = .93).
In the entire cohort, 24% of patients in the gabapentin arm and 27% of those in the placebo arm were hospitalized (P = .71). In the HbSS subgroup, hospitalizations occurred in 11% and 35% (P = .15).
Dr. Puri pointed out several challenges that led to limitations in this study. Specifically, the investigators had to obtain patient consent while delivering standard treatment, while patients were in pain and distress, and from patients who had already received opioids and were sleepy. Additionally, gabapentin had to be delivered within 1 hour of opioid administration, and a lack of after-hours staff limited enrollment.
“These challenges led to one of our biggest limitations, which was a small sample size, leading to a limited power to observe real differences,” Dr. Puri said. “We also defined a very short time period of evaluation for the primary outcomes; that was 3 hours from the gabapentin dose or placebo dose. This limited our capability to see real differences if they existed.”
Dr. Puri said additional studies with larger sample sizes are needed to confirm these findings. She added that efforts to better characterize pain in sickle cell disease could reveal patients who may benefit from gabapentin because they have a neuropathic component to their pain.
The trial was sponsored by St. Jude Children’s Research Hospital in collaboration with Scan|Design Foundation. Dr. Puri did not provide disclosure information at the meeting.
SOURCE: Puri L et al. ASPHO 2019, Abstract 2011.
NEW ORLEANS – Adding gabapentin to standard therapy did not significantly reduce vaso-occlusive pain in most patients with sickle cell disease enrolled in a phase 2 trial.
In the entire cohort, there were no significant differences in pain response between patients who received gabapentin and those who received placebo. However, patients with the HbSS genotype had a significantly greater decrease in pain score from baseline to discharge if they received gabapentin rather than placebo.
Additional studies are needed to confirm these findings because this trial was limited by a small sample size, according to study investigator Latika Puri, MD, of St. Jude Children’s Research Hospital in Memphis. Dr. Puri presented the trial at the annual meeting of the American Society of Pediatric Hematology/Oncology.
The trial included 86 evaluable patients who had vaso-occlusive pain and a pain score of at least 4. All patients received standard therapy for vaso-occlusive pain and were randomized to receive placebo (n = 44) or a single oral dose of gabapentin at 15 mg/kg (n = 42).
Baseline characteristics were similar between the treatment arms. For the entire cohort, the mean age was 11.8 years (range, 1-21 years), and 51% of patients were male. Forty-four patients had the HbSS genotype, 25 had the HbSC genotype, 8 had HbS/beta0-thalassemia, and 9 had other genotypes.
The mean pain score at baseline was 7.8 for the entire cohort, 8.0 for the gabapentin arm, and 7.7 for the placebo arm.
For the entire cohort, there was no significant difference in pain response between the gabapentin and placebo arms.
The proportion of patients who experienced a greater than 33% decrease in pain from baseline to 3 hours posttreatment was 67% in the gabapentin arm and 59% in the placebo arm (P = .23). The proportion of patients who experienced a greater than 33% decrease from baseline to discharge from the acute care clinic was 75% and 61%, respectively (P = .18).
In the entire cohort, decreases in pain scores from baseline to 3 hours posttreatment were not significantly different between the gabapentin and placebo arms, at 1.3 and 0.7, respectively (P = .74). Likewise, decreases in pain scores from baseline to discharge were not significantly different, at 1.6 and 0.8 (P = .38).
Among patients who had the HbSS genotype, there was a significantly greater decrease in pain score from baseline to discharge in the gabapentin arm than in the placebo arm, 5.9 versus 3.6 (P = .03). However, there were no other significant differences in pain response for the HbSS subgroup.
There were no significant differences in opioid consumption or hospitalization for the HbSS subgroup or the entire cohort. For the entire cohort, the mean morphine equivalent dose from baseline to 3 hours posttreatment was 0.16 mg/kg in the gabapentin arm and 0.17 mg/kg in the placebo arm (P = .89). For the HbSS subgroup, the mean dose was 0.16 mg/kg and 0.15 mg/kg, respectively (P = .93).
In the entire cohort, 24% of patients in the gabapentin arm and 27% of those in the placebo arm were hospitalized (P = .71). In the HbSS subgroup, hospitalizations occurred in 11% and 35% (P = .15).
Dr. Puri pointed out several challenges that led to limitations in this study. Specifically, the investigators had to obtain patient consent while delivering standard treatment, while patients were in pain and distress, and from patients who had already received opioids and were sleepy. Additionally, gabapentin had to be delivered within 1 hour of opioid administration, and a lack of after-hours staff limited enrollment.
“These challenges led to one of our biggest limitations, which was a small sample size, leading to a limited power to observe real differences,” Dr. Puri said. “We also defined a very short time period of evaluation for the primary outcomes; that was 3 hours from the gabapentin dose or placebo dose. This limited our capability to see real differences if they existed.”
Dr. Puri said additional studies with larger sample sizes are needed to confirm these findings. She added that efforts to better characterize pain in sickle cell disease could reveal patients who may benefit from gabapentin because they have a neuropathic component to their pain.
The trial was sponsored by St. Jude Children’s Research Hospital in collaboration with Scan|Design Foundation. Dr. Puri did not provide disclosure information at the meeting.
SOURCE: Puri L et al. ASPHO 2019, Abstract 2011.
NEW ORLEANS – Adding gabapentin to standard therapy did not significantly reduce vaso-occlusive pain in most patients with sickle cell disease enrolled in a phase 2 trial.
In the entire cohort, there were no significant differences in pain response between patients who received gabapentin and those who received placebo. However, patients with the HbSS genotype had a significantly greater decrease in pain score from baseline to discharge if they received gabapentin rather than placebo.
Additional studies are needed to confirm these findings because this trial was limited by a small sample size, according to study investigator Latika Puri, MD, of St. Jude Children’s Research Hospital in Memphis. Dr. Puri presented the trial at the annual meeting of the American Society of Pediatric Hematology/Oncology.
The trial included 86 evaluable patients who had vaso-occlusive pain and a pain score of at least 4. All patients received standard therapy for vaso-occlusive pain and were randomized to receive placebo (n = 44) or a single oral dose of gabapentin at 15 mg/kg (n = 42).
Baseline characteristics were similar between the treatment arms. For the entire cohort, the mean age was 11.8 years (range, 1-21 years), and 51% of patients were male. Forty-four patients had the HbSS genotype, 25 had the HbSC genotype, 8 had HbS/beta0-thalassemia, and 9 had other genotypes.
The mean pain score at baseline was 7.8 for the entire cohort, 8.0 for the gabapentin arm, and 7.7 for the placebo arm.
For the entire cohort, there was no significant difference in pain response between the gabapentin and placebo arms.
The proportion of patients who experienced a greater than 33% decrease in pain from baseline to 3 hours posttreatment was 67% in the gabapentin arm and 59% in the placebo arm (P = .23). The proportion of patients who experienced a greater than 33% decrease from baseline to discharge from the acute care clinic was 75% and 61%, respectively (P = .18).
In the entire cohort, decreases in pain scores from baseline to 3 hours posttreatment were not significantly different between the gabapentin and placebo arms, at 1.3 and 0.7, respectively (P = .74). Likewise, decreases in pain scores from baseline to discharge were not significantly different, at 1.6 and 0.8 (P = .38).
Among patients who had the HbSS genotype, there was a significantly greater decrease in pain score from baseline to discharge in the gabapentin arm than in the placebo arm, 5.9 versus 3.6 (P = .03). However, there were no other significant differences in pain response for the HbSS subgroup.
There were no significant differences in opioid consumption or hospitalization for the HbSS subgroup or the entire cohort. For the entire cohort, the mean morphine equivalent dose from baseline to 3 hours posttreatment was 0.16 mg/kg in the gabapentin arm and 0.17 mg/kg in the placebo arm (P = .89). For the HbSS subgroup, the mean dose was 0.16 mg/kg and 0.15 mg/kg, respectively (P = .93).
In the entire cohort, 24% of patients in the gabapentin arm and 27% of those in the placebo arm were hospitalized (P = .71). In the HbSS subgroup, hospitalizations occurred in 11% and 35% (P = .15).
Dr. Puri pointed out several challenges that led to limitations in this study. Specifically, the investigators had to obtain patient consent while delivering standard treatment, while patients were in pain and distress, and from patients who had already received opioids and were sleepy. Additionally, gabapentin had to be delivered within 1 hour of opioid administration, and a lack of after-hours staff limited enrollment.
“These challenges led to one of our biggest limitations, which was a small sample size, leading to a limited power to observe real differences,” Dr. Puri said. “We also defined a very short time period of evaluation for the primary outcomes; that was 3 hours from the gabapentin dose or placebo dose. This limited our capability to see real differences if they existed.”
Dr. Puri said additional studies with larger sample sizes are needed to confirm these findings. She added that efforts to better characterize pain in sickle cell disease could reveal patients who may benefit from gabapentin because they have a neuropathic component to their pain.
The trial was sponsored by St. Jude Children’s Research Hospital in collaboration with Scan|Design Foundation. Dr. Puri did not provide disclosure information at the meeting.
SOURCE: Puri L et al. ASPHO 2019, Abstract 2011.
REPORTING FROM THE 2019 ASPHO CONFERENCE
Key clinical point:
Major finding: The proportion of patients who experienced a greater than 33% decrease in pain from baseline to 3 hours posttreatment was 67% in the gabapentin arm and 59% in the placebo arm (P = .23).
Study details: A phase 2 trial of 86 evaluable patients.
Disclosures: The trial was sponsored by St. Jude Children’s Research Hospital in collaboration with Scan|Design Foundation. The speaker did not provide disclosure information at the meeting.
Source: Puri L et al. 2019 ASPHO Conference, Abstract 2011.
LentiGlobin reduces transfusion dependence in young thalassemia patients
NEW ORLEANS – The gene therapy LentiGlobin can reduce transfusion dependence in children and young adults with non-beta0/beta0 thalassemia, according to two trials.
In a phase 1/2 trial, 8 of 10 of patients achieved transfusion independence at a median follow-up of 36.0 months. In a phase 3 trial, transfusion independence was achieved by 2 of 3 patients with follow-up of at least 12 months.
Timothy S. Olson, MD, PhD, of Children’s Hospital of Philadelphia, presented results from the phase 1/2 HGB-204 trial and the phase 3 HGB-207 trial at the annual meeting of the American Society of Pediatric Hematology/Oncology.
Treatment
In both trials, patients received granulocyte colony-stimulating factor and plerixafor for hematopoietic stem cell mobilization. Their cells were collected via apheresis and transduced with the betibeglogene darolentivec (BB305) lentiviral vector. The patients received busulfan (for an average of 4 days) as conditioning and were infused with the transduced cells.
The manufacturing process for LentiGlobin was refined in the HGB-207 trial, which translated to a product with a higher vector copy number and higher proportion of CD34+ cells transduced, Dr. Olson said.
The median vector copy number was 3.1 in the HGB-207 trial and 0.7 in the HGB-204 trial. The median proportion of CD34+ cells transfused was 81% and 29%, respectively. The median cell dose was 7.7 x 106 CD34+ cells/kg and 7.1 x 106 CD34+ cells/kg, respectively.
HGB-204 patients and efficacy
The HGB-204 trial included 10 patients with non-beta0/beta0 genotypes – 6 with betaE/beta0, 1 with beta+/beta0, 2 with beta+/beta+, and 1 with an “other” genotype.
The patients’ median age at consent was 19.5 years (range, 16-34). The annualized median prestudy red blood cell (RBC) transfusion volume was 151 mL/kg per year.
At a median follow-up of 36 months, 8 of the 10 patients achieved transfusion independence. The median duration of transfusion independence was 38 months. The median weighted average hemoglobin during transfusion independence was 10.2 g/dL.
“Two patients did not achieve transfusion independence, and both patients were on the lower end of the spectrum both in terms of vector copy number per cell and the percentage of CD34+ cells that were successfully transduced,” Dr. Olson said. “Both patients actually experienced a reduction in the annualized transfusion volume requirements of between 43% and 77%.”
HGB-207 patients and efficacy
The HGB-207 trial included 16 patients with non-beta0/beta0 genotypes – 6 with betaE/beta0, 7 with beta+/beta0, and 3 with the beta+/beta+ genotype.
The patients’ median age at consent was 19 years . The annualized median prestudy RBC transfusion volume was 192 mL/kg per year.
The median follow-up in this trial is 9.3 months. Ten of 11 patients with at least 3 months of follow-up are transfusion-free with hemoglobin levels greater than 11 g/dL.
Two patients have achieved transfusion independence according to the protocol definition, which is weighted average hemoglobin of 9 g/dL or greater without any RBC transfusions for at least 12 months.
“In the one patient in this study who did not achieve transfusion independence, the vector-derived hemoglobin was quite low, and this correlated with a very low vector copy number seen in circulating peripheral blood mononuclear cells,” Dr. Olson said.
It isn’t clear why this occurred, however, as the vector copy number wasn’t especially low in the LentiGlobin product the patient received. Therefore, the researchers are still investigating why this patient failed to achieve transfusion independence.
Safety in both trials
“Very importantly, there were no deaths, there were no engraftment failures, there was no evidence of vector-mediated replication-competent lentivirus, and integration site analysis revealed no evidence of clonal dominance,” Dr. Olson said.
He added that most of the grade 3 or greater adverse events seen in both trials were directly attributable to busulfan-based myeloablative conditioning, including four episodes of veno-occlusive disease.
Nonhematologic grade 3 or higher adverse events in HGB-204 included stomatitis (n = 8), febrile neutropenia (n = 6), irregular menstruation (n = 3), pharyngeal inflammation (n = 2), and veno-occlusive liver disease (n = 1).
Nonhematologic grade 3 or higher adverse events in HGB-207 included stomatitis (n = 9), febrile neutropenia (n = 4), pharyngeal inflammation (n = 2), epistaxis (n = 3), pyrexia (n = 3), veno-occlusive liver disease (n = 3), ALT increase (n = 2), bilirubin increase (n = 2), and hypoxia (n = 2).
One patient in HGB-207 had grade 3 thrombocytopenia considered possibly related to LentiGlobin.
Dr. Olson reported advisory board engagement with bluebird bio, which sponsored both trials.
SOURCE: Olson TS et al. ASPHO 2019. Abstract 2002.
NEW ORLEANS – The gene therapy LentiGlobin can reduce transfusion dependence in children and young adults with non-beta0/beta0 thalassemia, according to two trials.
In a phase 1/2 trial, 8 of 10 of patients achieved transfusion independence at a median follow-up of 36.0 months. In a phase 3 trial, transfusion independence was achieved by 2 of 3 patients with follow-up of at least 12 months.
Timothy S. Olson, MD, PhD, of Children’s Hospital of Philadelphia, presented results from the phase 1/2 HGB-204 trial and the phase 3 HGB-207 trial at the annual meeting of the American Society of Pediatric Hematology/Oncology.
Treatment
In both trials, patients received granulocyte colony-stimulating factor and plerixafor for hematopoietic stem cell mobilization. Their cells were collected via apheresis and transduced with the betibeglogene darolentivec (BB305) lentiviral vector. The patients received busulfan (for an average of 4 days) as conditioning and were infused with the transduced cells.
The manufacturing process for LentiGlobin was refined in the HGB-207 trial, which translated to a product with a higher vector copy number and higher proportion of CD34+ cells transduced, Dr. Olson said.
The median vector copy number was 3.1 in the HGB-207 trial and 0.7 in the HGB-204 trial. The median proportion of CD34+ cells transfused was 81% and 29%, respectively. The median cell dose was 7.7 x 106 CD34+ cells/kg and 7.1 x 106 CD34+ cells/kg, respectively.
HGB-204 patients and efficacy
The HGB-204 trial included 10 patients with non-beta0/beta0 genotypes – 6 with betaE/beta0, 1 with beta+/beta0, 2 with beta+/beta+, and 1 with an “other” genotype.
The patients’ median age at consent was 19.5 years (range, 16-34). The annualized median prestudy red blood cell (RBC) transfusion volume was 151 mL/kg per year.
At a median follow-up of 36 months, 8 of the 10 patients achieved transfusion independence. The median duration of transfusion independence was 38 months. The median weighted average hemoglobin during transfusion independence was 10.2 g/dL.
“Two patients did not achieve transfusion independence, and both patients were on the lower end of the spectrum both in terms of vector copy number per cell and the percentage of CD34+ cells that were successfully transduced,” Dr. Olson said. “Both patients actually experienced a reduction in the annualized transfusion volume requirements of between 43% and 77%.”
HGB-207 patients and efficacy
The HGB-207 trial included 16 patients with non-beta0/beta0 genotypes – 6 with betaE/beta0, 7 with beta+/beta0, and 3 with the beta+/beta+ genotype.
The patients’ median age at consent was 19 years . The annualized median prestudy RBC transfusion volume was 192 mL/kg per year.
The median follow-up in this trial is 9.3 months. Ten of 11 patients with at least 3 months of follow-up are transfusion-free with hemoglobin levels greater than 11 g/dL.
Two patients have achieved transfusion independence according to the protocol definition, which is weighted average hemoglobin of 9 g/dL or greater without any RBC transfusions for at least 12 months.
“In the one patient in this study who did not achieve transfusion independence, the vector-derived hemoglobin was quite low, and this correlated with a very low vector copy number seen in circulating peripheral blood mononuclear cells,” Dr. Olson said.
It isn’t clear why this occurred, however, as the vector copy number wasn’t especially low in the LentiGlobin product the patient received. Therefore, the researchers are still investigating why this patient failed to achieve transfusion independence.
Safety in both trials
“Very importantly, there were no deaths, there were no engraftment failures, there was no evidence of vector-mediated replication-competent lentivirus, and integration site analysis revealed no evidence of clonal dominance,” Dr. Olson said.
He added that most of the grade 3 or greater adverse events seen in both trials were directly attributable to busulfan-based myeloablative conditioning, including four episodes of veno-occlusive disease.
Nonhematologic grade 3 or higher adverse events in HGB-204 included stomatitis (n = 8), febrile neutropenia (n = 6), irregular menstruation (n = 3), pharyngeal inflammation (n = 2), and veno-occlusive liver disease (n = 1).
Nonhematologic grade 3 or higher adverse events in HGB-207 included stomatitis (n = 9), febrile neutropenia (n = 4), pharyngeal inflammation (n = 2), epistaxis (n = 3), pyrexia (n = 3), veno-occlusive liver disease (n = 3), ALT increase (n = 2), bilirubin increase (n = 2), and hypoxia (n = 2).
One patient in HGB-207 had grade 3 thrombocytopenia considered possibly related to LentiGlobin.
Dr. Olson reported advisory board engagement with bluebird bio, which sponsored both trials.
SOURCE: Olson TS et al. ASPHO 2019. Abstract 2002.
NEW ORLEANS – The gene therapy LentiGlobin can reduce transfusion dependence in children and young adults with non-beta0/beta0 thalassemia, according to two trials.
In a phase 1/2 trial, 8 of 10 of patients achieved transfusion independence at a median follow-up of 36.0 months. In a phase 3 trial, transfusion independence was achieved by 2 of 3 patients with follow-up of at least 12 months.
Timothy S. Olson, MD, PhD, of Children’s Hospital of Philadelphia, presented results from the phase 1/2 HGB-204 trial and the phase 3 HGB-207 trial at the annual meeting of the American Society of Pediatric Hematology/Oncology.
Treatment
In both trials, patients received granulocyte colony-stimulating factor and plerixafor for hematopoietic stem cell mobilization. Their cells were collected via apheresis and transduced with the betibeglogene darolentivec (BB305) lentiviral vector. The patients received busulfan (for an average of 4 days) as conditioning and were infused with the transduced cells.
The manufacturing process for LentiGlobin was refined in the HGB-207 trial, which translated to a product with a higher vector copy number and higher proportion of CD34+ cells transduced, Dr. Olson said.
The median vector copy number was 3.1 in the HGB-207 trial and 0.7 in the HGB-204 trial. The median proportion of CD34+ cells transfused was 81% and 29%, respectively. The median cell dose was 7.7 x 106 CD34+ cells/kg and 7.1 x 106 CD34+ cells/kg, respectively.
HGB-204 patients and efficacy
The HGB-204 trial included 10 patients with non-beta0/beta0 genotypes – 6 with betaE/beta0, 1 with beta+/beta0, 2 with beta+/beta+, and 1 with an “other” genotype.
The patients’ median age at consent was 19.5 years (range, 16-34). The annualized median prestudy red blood cell (RBC) transfusion volume was 151 mL/kg per year.
At a median follow-up of 36 months, 8 of the 10 patients achieved transfusion independence. The median duration of transfusion independence was 38 months. The median weighted average hemoglobin during transfusion independence was 10.2 g/dL.
“Two patients did not achieve transfusion independence, and both patients were on the lower end of the spectrum both in terms of vector copy number per cell and the percentage of CD34+ cells that were successfully transduced,” Dr. Olson said. “Both patients actually experienced a reduction in the annualized transfusion volume requirements of between 43% and 77%.”
HGB-207 patients and efficacy
The HGB-207 trial included 16 patients with non-beta0/beta0 genotypes – 6 with betaE/beta0, 7 with beta+/beta0, and 3 with the beta+/beta+ genotype.
The patients’ median age at consent was 19 years . The annualized median prestudy RBC transfusion volume was 192 mL/kg per year.
The median follow-up in this trial is 9.3 months. Ten of 11 patients with at least 3 months of follow-up are transfusion-free with hemoglobin levels greater than 11 g/dL.
Two patients have achieved transfusion independence according to the protocol definition, which is weighted average hemoglobin of 9 g/dL or greater without any RBC transfusions for at least 12 months.
“In the one patient in this study who did not achieve transfusion independence, the vector-derived hemoglobin was quite low, and this correlated with a very low vector copy number seen in circulating peripheral blood mononuclear cells,” Dr. Olson said.
It isn’t clear why this occurred, however, as the vector copy number wasn’t especially low in the LentiGlobin product the patient received. Therefore, the researchers are still investigating why this patient failed to achieve transfusion independence.
Safety in both trials
“Very importantly, there were no deaths, there were no engraftment failures, there was no evidence of vector-mediated replication-competent lentivirus, and integration site analysis revealed no evidence of clonal dominance,” Dr. Olson said.
He added that most of the grade 3 or greater adverse events seen in both trials were directly attributable to busulfan-based myeloablative conditioning, including four episodes of veno-occlusive disease.
Nonhematologic grade 3 or higher adverse events in HGB-204 included stomatitis (n = 8), febrile neutropenia (n = 6), irregular menstruation (n = 3), pharyngeal inflammation (n = 2), and veno-occlusive liver disease (n = 1).
Nonhematologic grade 3 or higher adverse events in HGB-207 included stomatitis (n = 9), febrile neutropenia (n = 4), pharyngeal inflammation (n = 2), epistaxis (n = 3), pyrexia (n = 3), veno-occlusive liver disease (n = 3), ALT increase (n = 2), bilirubin increase (n = 2), and hypoxia (n = 2).
One patient in HGB-207 had grade 3 thrombocytopenia considered possibly related to LentiGlobin.
Dr. Olson reported advisory board engagement with bluebird bio, which sponsored both trials.
SOURCE: Olson TS et al. ASPHO 2019. Abstract 2002.
REPORTING FROM 2019 ASPHO CONFERENCE