User login
Researchers propose new risk groups for NK-AML
NEWPORT BEACH, CALIF. – New research suggests patients with normal karyotype acute myeloid leukemia (NK-AML) can be divided into four risk groups associated with overall survival.
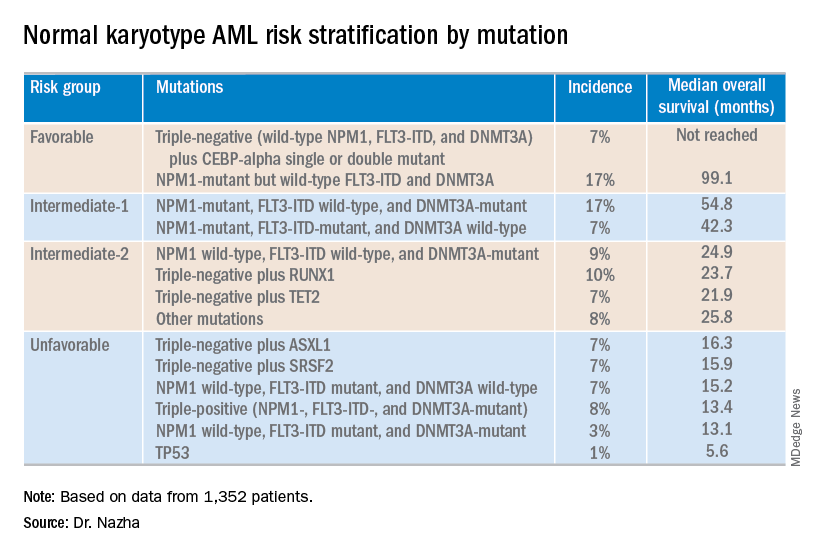
Investigators used machine learning algorithms to study the association between mutations and overall survival in 1,352 patients with NK-AML. The analysis revealed combinations of mutations that could be used to classify NK-AML patients into favorable, intermediate-1, intermediate-2, and unfavorable risk groups.
For example, patients who had NPM1 mutations but wild-type FLT3-ITD and DNMT3A, had a median overall survival of 99.1 months and could be classified as favorable risk. Conversely, patients who had NPM1, FLT3-ITD, and DNMT3A mutations, had a median overall survival of 13.4 months and could be classified as unfavorable risk.
Aziz Nazha, MD, of the Cleveland Clinic, and his colleagues conducted this research and presented the findings at the Acute Leukemia Forum of Hemedicus.
The investigators looked at genomic and clinical data from 1,352 patients with NK-AML. The patients were a median age of 55 years and had a median white blood cell count of 21.3 x 109/L, a median hemoglobin of 9.1 g/dL, and a median platelet count of 61 x 109/L. More than half of patients (57.3%) were male.
The patients were screened for 35 genes that are commonly mutated in AML and other myeloid malignancies. The investigators used machine learning algorithms, including random survival forest and recommender system algorithms, to study the association between mutations and overall survival in an “unbiased” way.
Dr. Nazha said there were a median of three mutations per patient sample, and “there are some competing interests between those mutations to impact the prognosis of the patient.”
The investigators used the mutations and their associations with overall survival to classify patients into the risk groups outlined in the table below.
These findings can improve the risk stratification of NK-AML and may aid physicians in making treatment decisions, according to Dr. Nazha and his colleagues. To move this work forward, the investigators are attempting to develop a personalized model that can make predictions specific to an individual patient based on that patient’s mutation information.
Dr. Nazha reported having no financial disclosures relevant to this research. Other investigators reported relationships with the Munich Leukemia Laboratory.
The Acute Leukemia Forum is held by Hemedicus, which is owned by the same company as this news organization.
NEWPORT BEACH, CALIF. – New research suggests patients with normal karyotype acute myeloid leukemia (NK-AML) can be divided into four risk groups associated with overall survival.
Investigators used machine learning algorithms to study the association between mutations and overall survival in 1,352 patients with NK-AML. The analysis revealed combinations of mutations that could be used to classify NK-AML patients into favorable, intermediate-1, intermediate-2, and unfavorable risk groups.
For example, patients who had NPM1 mutations but wild-type FLT3-ITD and DNMT3A, had a median overall survival of 99.1 months and could be classified as favorable risk. Conversely, patients who had NPM1, FLT3-ITD, and DNMT3A mutations, had a median overall survival of 13.4 months and could be classified as unfavorable risk.
Aziz Nazha, MD, of the Cleveland Clinic, and his colleagues conducted this research and presented the findings at the Acute Leukemia Forum of Hemedicus.
The investigators looked at genomic and clinical data from 1,352 patients with NK-AML. The patients were a median age of 55 years and had a median white blood cell count of 21.3 x 109/L, a median hemoglobin of 9.1 g/dL, and a median platelet count of 61 x 109/L. More than half of patients (57.3%) were male.
The patients were screened for 35 genes that are commonly mutated in AML and other myeloid malignancies. The investigators used machine learning algorithms, including random survival forest and recommender system algorithms, to study the association between mutations and overall survival in an “unbiased” way.
Dr. Nazha said there were a median of three mutations per patient sample, and “there are some competing interests between those mutations to impact the prognosis of the patient.”
The investigators used the mutations and their associations with overall survival to classify patients into the risk groups outlined in the table below.

These findings can improve the risk stratification of NK-AML and may aid physicians in making treatment decisions, according to Dr. Nazha and his colleagues. To move this work forward, the investigators are attempting to develop a personalized model that can make predictions specific to an individual patient based on that patient’s mutation information.
Dr. Nazha reported having no financial disclosures relevant to this research. Other investigators reported relationships with the Munich Leukemia Laboratory.
The Acute Leukemia Forum is held by Hemedicus, which is owned by the same company as this news organization.
NEWPORT BEACH, CALIF. – New research suggests patients with normal karyotype acute myeloid leukemia (NK-AML) can be divided into four risk groups associated with overall survival.
Investigators used machine learning algorithms to study the association between mutations and overall survival in 1,352 patients with NK-AML. The analysis revealed combinations of mutations that could be used to classify NK-AML patients into favorable, intermediate-1, intermediate-2, and unfavorable risk groups.
For example, patients who had NPM1 mutations but wild-type FLT3-ITD and DNMT3A, had a median overall survival of 99.1 months and could be classified as favorable risk. Conversely, patients who had NPM1, FLT3-ITD, and DNMT3A mutations, had a median overall survival of 13.4 months and could be classified as unfavorable risk.
Aziz Nazha, MD, of the Cleveland Clinic, and his colleagues conducted this research and presented the findings at the Acute Leukemia Forum of Hemedicus.
The investigators looked at genomic and clinical data from 1,352 patients with NK-AML. The patients were a median age of 55 years and had a median white blood cell count of 21.3 x 109/L, a median hemoglobin of 9.1 g/dL, and a median platelet count of 61 x 109/L. More than half of patients (57.3%) were male.
The patients were screened for 35 genes that are commonly mutated in AML and other myeloid malignancies. The investigators used machine learning algorithms, including random survival forest and recommender system algorithms, to study the association between mutations and overall survival in an “unbiased” way.
Dr. Nazha said there were a median of three mutations per patient sample, and “there are some competing interests between those mutations to impact the prognosis of the patient.”
The investigators used the mutations and their associations with overall survival to classify patients into the risk groups outlined in the table below.

These findings can improve the risk stratification of NK-AML and may aid physicians in making treatment decisions, according to Dr. Nazha and his colleagues. To move this work forward, the investigators are attempting to develop a personalized model that can make predictions specific to an individual patient based on that patient’s mutation information.
Dr. Nazha reported having no financial disclosures relevant to this research. Other investigators reported relationships with the Munich Leukemia Laboratory.
The Acute Leukemia Forum is held by Hemedicus, which is owned by the same company as this news organization.
REPORTING FROM ALF 2019
Combo proves most effective in HMA-naive, higher-risk MDS
NEWPORT BEACH, CALIF. – The combination of oral rigosertib and azacitidine is proceeding to a phase 3 trial in patients with myelodysplastic syndromes (MDS), but it isn’t clear if the combination will continue to be developed for acute myeloid leukemia (AML).
In a phase 1/2 trial, oral rigosertib plus azacitidine produced a 90% response rate in higher-risk MDS patients who were naive to hypomethylating agents (HMAs), a 54% response rate in higher-risk MDS patients who had failed HMA therapy, and a 50% response rate in patients with AML.
Genitourinary toxicities were initially a concern in this trial, but researchers found ways to mitigate the risk of these toxicities, according to Richard Woodman, MD, chief medical officer and senior vice president of research and development at Onconova Therapeutics, the company developing rigosertib.
Dr. Woodman and his colleagues presented results from the phase 1/2 trial in two posters at the Acute Leukemia Forum of Hemedicus.
Results in AML
The researchers reported phase 1 results in 17 patients with AML. Eleven patients had AML, according to investigator assessment, and six patients had refractory anemia with excess blasts in transformation, according to French American British criteria, as well as least 20% excess blasts at baseline.
The median age of the patients was 73 years, and 53% were men. Two patients had received no prior therapies, six patients had relapsed disease, and nine were refractory to their last therapy.
Patients received oral rigosertib at escalating doses twice daily on days 1-21 of a 28-day cycle. The recommended phase 2 dose was 840 mg daily (560 mg in the morning and 280 mg in the afternoon), but there were two expansion cohorts in which patients received 1,120 mg daily (560 mg twice a day or 840 mg in the morning and 280 mg in the afternoon). The patients also received azacitidine at 75 mg/m2 per day subcutaneously or intravenously for 7 days starting on day 8.
Patients received a median of three treatment cycles. Fifteen of the 17 patients (88%) discontinued treatment, most because of progressive disease (n = 5), toxicity (n = 4), or death (n = 3).
Twelve patients were evaluable for response, and six (50%) responded. One patient achieved a morphologic complete remission (CR), three achieved a morphologic leukemia-free state, and two had a partial response.
The most common treatment-emergent adverse events (TEAEs) were fatigue (53%), diarrhea (53%), nausea (53%), constipation (47%), back pain (41%), pyrexia (41%), and pneumonia (35%). Grade 3 or higher TEAEs included pneumonia (35%) and anemia (24%).
These results haven’t provided a clear way forward for oral rigosertib and azacitidine in AML. Dr. Woodman said the researchers will have to review past studies and evaluate how AML patients (with at least 20% blasts) have responded to intravenous rigosertib, consult experts in the field, and then decide how they will move forward with oral rigosertib and azacitidine in AML.
Results in MDS
Dr. Woodman and his colleagues presented data on 74 patients with higher-risk MDS. The median age was 69 years, and 59% were men. Most patients were high risk (n = 23) or very high risk (n = 33), according to the Revised International Prognostic Scoring System.
The patients received oral rigosertib at a dose of 840 mg/day or higher on days 1-21 of a 28-day cycle. They also received azacitidine at 75 mg/m2 per day subcutaneously or intravenously for 7 days starting on day 8.
The median duration of treatment was 7.8 months in patients who were HMA naive and 4.9 months in patients who failed HMA therapy. The most common reasons for treatment discontinuation in the HMA-naive patients were toxicity (n = 8), progression (n = 7), and patient request (n = 7). The most common reasons for discontinuation in patients who had failed HMA therapy were progression (n = 12), toxicity (n = 5), and investigator decision (n = 4).
In total, 55 patients were evaluable for response, 26 who had failed HMA therapy and 29 who were HMA naive.
“The best responses, not surprisingly, were in patients that were HMA naive,” Dr. Woodman said.
In the HMA-naive patients, the overall response rate was 90%. Ten patients had a CR, five had a marrow CR with hematologic improvement, three had hematologic improvement alone, eight had a marrow CR alone, and three patients had stable disease. None of the patients progressed.
In the patients who had failed HMA therapy, the overall response rate was 54%. One patient achieved a CR, one had a partial response, five had a marrow CR with hematologic improvement, two had hematologic improvement alone, five had a marrow CR alone, seven had stable disease, and five progressed.
The median duration of response was 10.8 months in patients who failed HMA therapy and 12.2 months in the HMA-naive patients.
The most common TEAEs in the entire MDS cohort were hematuria (45%), constipation (43%), diarrhea (42%), fatigue (42%), dysuria (38%), pyrexia (36%), nausea (35%), neutropenia (31%), and thrombocytopenia (30%).
Grade 3 or higher TEAEs were neutropenia (27%), thrombocytopenia (26%), hematuria (9%), dysuria (9%), diarrhea (5%), fatigue (4%), and pyrexia (1%).
Dr. Woodman said patients who were most likely to be at risk for genitourinary toxicities (hematuria and dysuria) were those who weren’t well hydrated, took rigosertib at night, and didn’t void their bladders before bedtime. He said the researchers’ hypothesis is that there is some local bladder irritation in that setting.
However, the researchers found ways to mitigate the risk of genitourinary toxicities, including:
- Requiring the second dose of rigosertib to be taken in the afternoon rather than evening (about 3 p.m.).
- Asking patients to consume at least 2 liters of fluid per day.
- Having patients empty their bladders before bedtime.
- Assessing urine pH roughly 2 hours after the morning dose of rigosertib and prescribing sodium bicarbonate if the pH is less than 7.5.
Dr. Woodman said the phase 2 results in MDS patients have prompted the development of a phase 3 trial in which researchers will compare oral rigosertib plus azacitidine to azacitidine plus placebo.
Dr. Woodman is employed by Onconova Therapeutics, which sponsored the phase 1/2 trial. The Acute Leukemia Forum is held by Hemedicus, which is owned by the same company as this news organization.
NEWPORT BEACH, CALIF. – The combination of oral rigosertib and azacitidine is proceeding to a phase 3 trial in patients with myelodysplastic syndromes (MDS), but it isn’t clear if the combination will continue to be developed for acute myeloid leukemia (AML).
In a phase 1/2 trial, oral rigosertib plus azacitidine produced a 90% response rate in higher-risk MDS patients who were naive to hypomethylating agents (HMAs), a 54% response rate in higher-risk MDS patients who had failed HMA therapy, and a 50% response rate in patients with AML.
Genitourinary toxicities were initially a concern in this trial, but researchers found ways to mitigate the risk of these toxicities, according to Richard Woodman, MD, chief medical officer and senior vice president of research and development at Onconova Therapeutics, the company developing rigosertib.
Dr. Woodman and his colleagues presented results from the phase 1/2 trial in two posters at the Acute Leukemia Forum of Hemedicus.
Results in AML
The researchers reported phase 1 results in 17 patients with AML. Eleven patients had AML, according to investigator assessment, and six patients had refractory anemia with excess blasts in transformation, according to French American British criteria, as well as least 20% excess blasts at baseline.
The median age of the patients was 73 years, and 53% were men. Two patients had received no prior therapies, six patients had relapsed disease, and nine were refractory to their last therapy.
Patients received oral rigosertib at escalating doses twice daily on days 1-21 of a 28-day cycle. The recommended phase 2 dose was 840 mg daily (560 mg in the morning and 280 mg in the afternoon), but there were two expansion cohorts in which patients received 1,120 mg daily (560 mg twice a day or 840 mg in the morning and 280 mg in the afternoon). The patients also received azacitidine at 75 mg/m2 per day subcutaneously or intravenously for 7 days starting on day 8.
Patients received a median of three treatment cycles. Fifteen of the 17 patients (88%) discontinued treatment, most because of progressive disease (n = 5), toxicity (n = 4), or death (n = 3).
Twelve patients were evaluable for response, and six (50%) responded. One patient achieved a morphologic complete remission (CR), three achieved a morphologic leukemia-free state, and two had a partial response.
The most common treatment-emergent adverse events (TEAEs) were fatigue (53%), diarrhea (53%), nausea (53%), constipation (47%), back pain (41%), pyrexia (41%), and pneumonia (35%). Grade 3 or higher TEAEs included pneumonia (35%) and anemia (24%).
These results haven’t provided a clear way forward for oral rigosertib and azacitidine in AML. Dr. Woodman said the researchers will have to review past studies and evaluate how AML patients (with at least 20% blasts) have responded to intravenous rigosertib, consult experts in the field, and then decide how they will move forward with oral rigosertib and azacitidine in AML.
Results in MDS
Dr. Woodman and his colleagues presented data on 74 patients with higher-risk MDS. The median age was 69 years, and 59% were men. Most patients were high risk (n = 23) or very high risk (n = 33), according to the Revised International Prognostic Scoring System.
The patients received oral rigosertib at a dose of 840 mg/day or higher on days 1-21 of a 28-day cycle. They also received azacitidine at 75 mg/m2 per day subcutaneously or intravenously for 7 days starting on day 8.
The median duration of treatment was 7.8 months in patients who were HMA naive and 4.9 months in patients who failed HMA therapy. The most common reasons for treatment discontinuation in the HMA-naive patients were toxicity (n = 8), progression (n = 7), and patient request (n = 7). The most common reasons for discontinuation in patients who had failed HMA therapy were progression (n = 12), toxicity (n = 5), and investigator decision (n = 4).
In total, 55 patients were evaluable for response, 26 who had failed HMA therapy and 29 who were HMA naive.
“The best responses, not surprisingly, were in patients that were HMA naive,” Dr. Woodman said.
In the HMA-naive patients, the overall response rate was 90%. Ten patients had a CR, five had a marrow CR with hematologic improvement, three had hematologic improvement alone, eight had a marrow CR alone, and three patients had stable disease. None of the patients progressed.
In the patients who had failed HMA therapy, the overall response rate was 54%. One patient achieved a CR, one had a partial response, five had a marrow CR with hematologic improvement, two had hematologic improvement alone, five had a marrow CR alone, seven had stable disease, and five progressed.
The median duration of response was 10.8 months in patients who failed HMA therapy and 12.2 months in the HMA-naive patients.
The most common TEAEs in the entire MDS cohort were hematuria (45%), constipation (43%), diarrhea (42%), fatigue (42%), dysuria (38%), pyrexia (36%), nausea (35%), neutropenia (31%), and thrombocytopenia (30%).
Grade 3 or higher TEAEs were neutropenia (27%), thrombocytopenia (26%), hematuria (9%), dysuria (9%), diarrhea (5%), fatigue (4%), and pyrexia (1%).
Dr. Woodman said patients who were most likely to be at risk for genitourinary toxicities (hematuria and dysuria) were those who weren’t well hydrated, took rigosertib at night, and didn’t void their bladders before bedtime. He said the researchers’ hypothesis is that there is some local bladder irritation in that setting.
However, the researchers found ways to mitigate the risk of genitourinary toxicities, including:
- Requiring the second dose of rigosertib to be taken in the afternoon rather than evening (about 3 p.m.).
- Asking patients to consume at least 2 liters of fluid per day.
- Having patients empty their bladders before bedtime.
- Assessing urine pH roughly 2 hours after the morning dose of rigosertib and prescribing sodium bicarbonate if the pH is less than 7.5.
Dr. Woodman said the phase 2 results in MDS patients have prompted the development of a phase 3 trial in which researchers will compare oral rigosertib plus azacitidine to azacitidine plus placebo.
Dr. Woodman is employed by Onconova Therapeutics, which sponsored the phase 1/2 trial. The Acute Leukemia Forum is held by Hemedicus, which is owned by the same company as this news organization.
NEWPORT BEACH, CALIF. – The combination of oral rigosertib and azacitidine is proceeding to a phase 3 trial in patients with myelodysplastic syndromes (MDS), but it isn’t clear if the combination will continue to be developed for acute myeloid leukemia (AML).
In a phase 1/2 trial, oral rigosertib plus azacitidine produced a 90% response rate in higher-risk MDS patients who were naive to hypomethylating agents (HMAs), a 54% response rate in higher-risk MDS patients who had failed HMA therapy, and a 50% response rate in patients with AML.
Genitourinary toxicities were initially a concern in this trial, but researchers found ways to mitigate the risk of these toxicities, according to Richard Woodman, MD, chief medical officer and senior vice president of research and development at Onconova Therapeutics, the company developing rigosertib.
Dr. Woodman and his colleagues presented results from the phase 1/2 trial in two posters at the Acute Leukemia Forum of Hemedicus.
Results in AML
The researchers reported phase 1 results in 17 patients with AML. Eleven patients had AML, according to investigator assessment, and six patients had refractory anemia with excess blasts in transformation, according to French American British criteria, as well as least 20% excess blasts at baseline.
The median age of the patients was 73 years, and 53% were men. Two patients had received no prior therapies, six patients had relapsed disease, and nine were refractory to their last therapy.
Patients received oral rigosertib at escalating doses twice daily on days 1-21 of a 28-day cycle. The recommended phase 2 dose was 840 mg daily (560 mg in the morning and 280 mg in the afternoon), but there were two expansion cohorts in which patients received 1,120 mg daily (560 mg twice a day or 840 mg in the morning and 280 mg in the afternoon). The patients also received azacitidine at 75 mg/m2 per day subcutaneously or intravenously for 7 days starting on day 8.
Patients received a median of three treatment cycles. Fifteen of the 17 patients (88%) discontinued treatment, most because of progressive disease (n = 5), toxicity (n = 4), or death (n = 3).
Twelve patients were evaluable for response, and six (50%) responded. One patient achieved a morphologic complete remission (CR), three achieved a morphologic leukemia-free state, and two had a partial response.
The most common treatment-emergent adverse events (TEAEs) were fatigue (53%), diarrhea (53%), nausea (53%), constipation (47%), back pain (41%), pyrexia (41%), and pneumonia (35%). Grade 3 or higher TEAEs included pneumonia (35%) and anemia (24%).
These results haven’t provided a clear way forward for oral rigosertib and azacitidine in AML. Dr. Woodman said the researchers will have to review past studies and evaluate how AML patients (with at least 20% blasts) have responded to intravenous rigosertib, consult experts in the field, and then decide how they will move forward with oral rigosertib and azacitidine in AML.
Results in MDS
Dr. Woodman and his colleagues presented data on 74 patients with higher-risk MDS. The median age was 69 years, and 59% were men. Most patients were high risk (n = 23) or very high risk (n = 33), according to the Revised International Prognostic Scoring System.
The patients received oral rigosertib at a dose of 840 mg/day or higher on days 1-21 of a 28-day cycle. They also received azacitidine at 75 mg/m2 per day subcutaneously or intravenously for 7 days starting on day 8.
The median duration of treatment was 7.8 months in patients who were HMA naive and 4.9 months in patients who failed HMA therapy. The most common reasons for treatment discontinuation in the HMA-naive patients were toxicity (n = 8), progression (n = 7), and patient request (n = 7). The most common reasons for discontinuation in patients who had failed HMA therapy were progression (n = 12), toxicity (n = 5), and investigator decision (n = 4).
In total, 55 patients were evaluable for response, 26 who had failed HMA therapy and 29 who were HMA naive.
“The best responses, not surprisingly, were in patients that were HMA naive,” Dr. Woodman said.
In the HMA-naive patients, the overall response rate was 90%. Ten patients had a CR, five had a marrow CR with hematologic improvement, three had hematologic improvement alone, eight had a marrow CR alone, and three patients had stable disease. None of the patients progressed.
In the patients who had failed HMA therapy, the overall response rate was 54%. One patient achieved a CR, one had a partial response, five had a marrow CR with hematologic improvement, two had hematologic improvement alone, five had a marrow CR alone, seven had stable disease, and five progressed.
The median duration of response was 10.8 months in patients who failed HMA therapy and 12.2 months in the HMA-naive patients.
The most common TEAEs in the entire MDS cohort were hematuria (45%), constipation (43%), diarrhea (42%), fatigue (42%), dysuria (38%), pyrexia (36%), nausea (35%), neutropenia (31%), and thrombocytopenia (30%).
Grade 3 or higher TEAEs were neutropenia (27%), thrombocytopenia (26%), hematuria (9%), dysuria (9%), diarrhea (5%), fatigue (4%), and pyrexia (1%).
Dr. Woodman said patients who were most likely to be at risk for genitourinary toxicities (hematuria and dysuria) were those who weren’t well hydrated, took rigosertib at night, and didn’t void their bladders before bedtime. He said the researchers’ hypothesis is that there is some local bladder irritation in that setting.
However, the researchers found ways to mitigate the risk of genitourinary toxicities, including:
- Requiring the second dose of rigosertib to be taken in the afternoon rather than evening (about 3 p.m.).
- Asking patients to consume at least 2 liters of fluid per day.
- Having patients empty their bladders before bedtime.
- Assessing urine pH roughly 2 hours after the morning dose of rigosertib and prescribing sodium bicarbonate if the pH is less than 7.5.
Dr. Woodman said the phase 2 results in MDS patients have prompted the development of a phase 3 trial in which researchers will compare oral rigosertib plus azacitidine to azacitidine plus placebo.
Dr. Woodman is employed by Onconova Therapeutics, which sponsored the phase 1/2 trial. The Acute Leukemia Forum is held by Hemedicus, which is owned by the same company as this news organization.
REPORTING FROM ALF 2019
More abnormal cells linked to poorer ASCT outcomes in MDS
NEWPORT BEACH, CALIF. – Researchers say they’ve found an association between the percentage of cytogenetically abnormal cells at allogeneic stem cell transplant (ASCT) and posttransplant outcomes in patients with myelodysplastic syndromes (MDS).
Patients who had more than 60% cytogenetically abnormal cells at ASCT had significantly inferior overall survival (OS) and relapse-free survival (RFS), compared to patients with fewer abnormal cells.
Dipenkumar Modi, MD, of Barbara Ann Karmanos Cancer Institute at Wayne State University in Detroit, and his colleagues conducted this research and presented the results at the Acute Leukemia Forum of Hemedicus.
The researchers studied 109 adult MDS patients who underwent ASCT from January 2000 through December 2016. The patients were divided into three groups based on the percentage of cytogenetically abnormal cells at ASCT:
- Group 1 had less than 30% (n = 22)
- Group 2 had 30%-60% (n = 23)
- Group 3 had greater than 60% (n = 64).
Baseline characteristics were largely similar between the groups. However, patients in group 3 were significantly more likely than those in groups 1 and 2 to have del(5q) and monosomy 5+7 (P = .048).
Patients in group 1 had a significantly higher percentage of bone marrow transplants (as opposed to peripheral blood stem cell transplants) than patients in groups 2 and 3 (P = .039). And patients in group 1 had significantly fewer blasts at ASCT than patients in groups 2 and 3 (P = .011).
The researchers found no significant between-group differences in relapse and nonrelapse mortality, but there were significant differences in OS and RFS.
Patients in group 3 had inferior RFS compared to patients in group 1, which was the reference group. The hazard ratio (HR) was 2.503 (P = .013) in a univariable analysis and 2.196 (P = .049) in a multivariable analysis.
Group 3 also had inferior OS compared to group 1. The hazard ratio was 2.589 (P = .021) in a univariable analysis and 2.478 (P = .040) in a multivariable analysis.
There was no significant difference in RFS or OS between groups 1 and 2. The HR for RFS in group 2 was 1.879 (P = .148) in a univariable analysis and 1.365 (P = .506) in a multivariable analysis. The HR for OS was 1.997 (P = .155) and 1.413 (P = .511), respectively.
Dr. Modi said these results suggest patients with greater than 60% cytogenetically abnormal cells at ASCT should be monitored more closely after transplant, and their immunosuppressive medication should be tapered as soon as possible.
Dr. Modi and his colleagues reported having no conflicts of interest relevant to this research.
The Acute Leukemia Forum is held by Hemedicus, which is owned by the same company as this news organization.
NEWPORT BEACH, CALIF. – Researchers say they’ve found an association between the percentage of cytogenetically abnormal cells at allogeneic stem cell transplant (ASCT) and posttransplant outcomes in patients with myelodysplastic syndromes (MDS).
Patients who had more than 60% cytogenetically abnormal cells at ASCT had significantly inferior overall survival (OS) and relapse-free survival (RFS), compared to patients with fewer abnormal cells.
Dipenkumar Modi, MD, of Barbara Ann Karmanos Cancer Institute at Wayne State University in Detroit, and his colleagues conducted this research and presented the results at the Acute Leukemia Forum of Hemedicus.
The researchers studied 109 adult MDS patients who underwent ASCT from January 2000 through December 2016. The patients were divided into three groups based on the percentage of cytogenetically abnormal cells at ASCT:
- Group 1 had less than 30% (n = 22)
- Group 2 had 30%-60% (n = 23)
- Group 3 had greater than 60% (n = 64).
Baseline characteristics were largely similar between the groups. However, patients in group 3 were significantly more likely than those in groups 1 and 2 to have del(5q) and monosomy 5+7 (P = .048).
Patients in group 1 had a significantly higher percentage of bone marrow transplants (as opposed to peripheral blood stem cell transplants) than patients in groups 2 and 3 (P = .039). And patients in group 1 had significantly fewer blasts at ASCT than patients in groups 2 and 3 (P = .011).
The researchers found no significant between-group differences in relapse and nonrelapse mortality, but there were significant differences in OS and RFS.
Patients in group 3 had inferior RFS compared to patients in group 1, which was the reference group. The hazard ratio (HR) was 2.503 (P = .013) in a univariable analysis and 2.196 (P = .049) in a multivariable analysis.
Group 3 also had inferior OS compared to group 1. The hazard ratio was 2.589 (P = .021) in a univariable analysis and 2.478 (P = .040) in a multivariable analysis.
There was no significant difference in RFS or OS between groups 1 and 2. The HR for RFS in group 2 was 1.879 (P = .148) in a univariable analysis and 1.365 (P = .506) in a multivariable analysis. The HR for OS was 1.997 (P = .155) and 1.413 (P = .511), respectively.
Dr. Modi said these results suggest patients with greater than 60% cytogenetically abnormal cells at ASCT should be monitored more closely after transplant, and their immunosuppressive medication should be tapered as soon as possible.
Dr. Modi and his colleagues reported having no conflicts of interest relevant to this research.
The Acute Leukemia Forum is held by Hemedicus, which is owned by the same company as this news organization.
NEWPORT BEACH, CALIF. – Researchers say they’ve found an association between the percentage of cytogenetically abnormal cells at allogeneic stem cell transplant (ASCT) and posttransplant outcomes in patients with myelodysplastic syndromes (MDS).
Patients who had more than 60% cytogenetically abnormal cells at ASCT had significantly inferior overall survival (OS) and relapse-free survival (RFS), compared to patients with fewer abnormal cells.
Dipenkumar Modi, MD, of Barbara Ann Karmanos Cancer Institute at Wayne State University in Detroit, and his colleagues conducted this research and presented the results at the Acute Leukemia Forum of Hemedicus.
The researchers studied 109 adult MDS patients who underwent ASCT from January 2000 through December 2016. The patients were divided into three groups based on the percentage of cytogenetically abnormal cells at ASCT:
- Group 1 had less than 30% (n = 22)
- Group 2 had 30%-60% (n = 23)
- Group 3 had greater than 60% (n = 64).
Baseline characteristics were largely similar between the groups. However, patients in group 3 were significantly more likely than those in groups 1 and 2 to have del(5q) and monosomy 5+7 (P = .048).
Patients in group 1 had a significantly higher percentage of bone marrow transplants (as opposed to peripheral blood stem cell transplants) than patients in groups 2 and 3 (P = .039). And patients in group 1 had significantly fewer blasts at ASCT than patients in groups 2 and 3 (P = .011).
The researchers found no significant between-group differences in relapse and nonrelapse mortality, but there were significant differences in OS and RFS.
Patients in group 3 had inferior RFS compared to patients in group 1, which was the reference group. The hazard ratio (HR) was 2.503 (P = .013) in a univariable analysis and 2.196 (P = .049) in a multivariable analysis.
Group 3 also had inferior OS compared to group 1. The hazard ratio was 2.589 (P = .021) in a univariable analysis and 2.478 (P = .040) in a multivariable analysis.
There was no significant difference in RFS or OS between groups 1 and 2. The HR for RFS in group 2 was 1.879 (P = .148) in a univariable analysis and 1.365 (P = .506) in a multivariable analysis. The HR for OS was 1.997 (P = .155) and 1.413 (P = .511), respectively.
Dr. Modi said these results suggest patients with greater than 60% cytogenetically abnormal cells at ASCT should be monitored more closely after transplant, and their immunosuppressive medication should be tapered as soon as possible.
Dr. Modi and his colleagues reported having no conflicts of interest relevant to this research.
The Acute Leukemia Forum is held by Hemedicus, which is owned by the same company as this news organization.
REPORTING FROM ALF 2019
Biomarker testing may transform treatment of acute GVHD
NEWPORT BEACH, CALIF. – Researchers say they have identified biomarkers that may help guide early treatment decisions in patients with acute graft-versus-host disease (GVHD).
The biomarkers, ST2 and REG3-alpha, were measured during the first month of GVHD treatment and proved more accurate than clinical response for predicting 6-month nonrelapse mortality (NRM). In fact, biomarker assessment revealed patients who responded to treatment but had a high risk of NRM and nonresponders who had a low risk of NRM.
The researchers also found that biomarkers changed over the first month of treatment but remained significant predictors of NRM. This suggests that modifying treatment according to biomarker findings at various time points could result in better outcomes for patients.
“We think this is going to transform the way we treat graft-versus-host disease,” said James L.M. Ferrara, MD, DSc, of the Icahn School of Medicine at Mount Sinai, New York.
Dr. Ferrara and Hrishikesh Srinagesh, along with their colleagues at Mount Sinai, have conducted extensive research with these biomarkers and presented some of their findings at the Acute Leukemia Forum of Hemedicus.
Comparing biomarkers and response
In one study, the researchers evaluated 355 patients who had undergone allogeneic hematopoietic stem cell transplant at 1 of 20 Mount Sinai Acute GVHD International Consortium (MAGIC) centers between January 2016 and February 2018. All patients developed acute GVHD and received systemic steroids as treatment.
Patients provided blood samples weekly for the first month of treatment, and concentrations of ST2 and REG3-alpha were measured in each sample. Both biomarker concentrations were used to calculate the biomarker probability of NRM.
“The concentration of those two biomarkers are put into a computer, and we get … a single number, and that gives us the probability of mortality,” Dr. Ferrara said. “[W]e call this the MAGIC algorithm probability, or MAP. And when a MAP is low, the patient has a very low chance of dying from graft-versus-host disease, when it’s intermediate, they have an intermediate risk, and when it’s high, they have a high risk.”
The researchers then compared the MAP and clinical response for their ability to predict 6-month NRM throughout the first month of therapy for acute GVHD.
MAP bests response
After 1 month of therapy, the MAP was more accurate than clinical response for predicting 6-month NRM. The area under the curve was 0.84 and 0.65, respectively (P less than .001).
Likewise, the MAP after 1 week of therapy was more accurate than clinical response at 1 month for predicting 6-month NRM. The area under the curve was 0.80 and 0.65, respectively (P less than .001).
“[T]he clinical responses were good, but not great, at predicting long-term outcome, where the biomarker, the MAP, was significantly better,” Dr. Ferrara said. “[A]t every time point we tested, the biomarkers were better than the clinical responses.”
The researchers also identified subgroups of clinical responders and nonresponders for whom MAP more accurately predicted 6-month NRM.
The team found that 61% of clinical nonresponders were actually low risk according to MAP. And the incidence of 6-month NRM was significantly lower in the MAP-designated low-risk patients than in MAP-designated high-risk patients – 22% and 56%, respectively (P less than .001).
On the other hand, 10% of clinical responders were high risk according to MAP. The incidence of 6-month NRM was significantly higher in the high-risk patients than in the low-risk patients – 40% and 13%, respectively (P less than .001).
Assessing changes over time
The researchers found that patients who were initially high risk by MAP but had not experienced NRM by 6 months had significant decreases in their MAP after 4 weeks of treatment (P = .003). Patients who did experience NRM had a significant increase in their MAP whether their initial MAP was low (P = .007) or high (P = .024).
“What we found was that patients who lived tended to either have low biomarkers at the start of treatment and stay low or start out with high biomarkers and have reductions over the first month of therapy,” Mr. Srinagesh said. “Conversely, patients who tended to do worse were those who had either increases in their biomarkers or stayed high at all time points.”
The researchers identified a threshold – 0.290 – for separating patients by mortality risk.
“Patients who started out above the threshold and then went below it had a 5-fold reduction in mortality, whereas patients who started out below the threshold and rose above it had a 5-fold increase in mortality,” Mr. Srinagesh said.
MAP in clinical trials and practice
Based on these findings and results from related studies, the researchers theorize that MAP would be a better endpoint for clinical trials than clinical response.
At present, there are three trials in which researchers are using MAP as an endpoint to assess the efficacy of treatment for GVHD (NCT02133924, NCT03459040, and NCT03846479). Dr. Ferrara said a fourth trial is set to begin this summer.
Additionally, MAP is being used in clinical practice. A company called Viracor Eurofins Clinical Diagnostics licensed the MAGIC algorithm and provides three related tests for consumer use.
Viracor’s aGVHD Pre-Symptomatic Algorithm assigns patients to high- and low-risk groups based on results from samples collected 7 days after transplant. The aGVHD Symptomatic Onset Algorithm assigns patients to high-, intermediate-, and low-risk groups. The aGVHD Post-Treatment Algorithm, which can be used 7 days or more after GVHD treatment initiation, stratifies steroid-resistant patients into high- or low-risk groups for both NRM and overall survival.
“We are still in early days of figuring out how to use [the biomarker tests], but … what I’ve heard is that people are finding them to be useful in their clinical practice,” Dr. Ferrara said.
Dr. Ferrara has an ownership interest in and receives royalties from Viracor. Mr. Srinagesh reported having no relevant conflicts of interest. The research was supported by grants from the National Cancer Institute and the American Cancer Society.
The Acute Leukemia Forum is held by Hemedicus, which is owned by the same company as this news organization.
NEWPORT BEACH, CALIF. – Researchers say they have identified biomarkers that may help guide early treatment decisions in patients with acute graft-versus-host disease (GVHD).
The biomarkers, ST2 and REG3-alpha, were measured during the first month of GVHD treatment and proved more accurate than clinical response for predicting 6-month nonrelapse mortality (NRM). In fact, biomarker assessment revealed patients who responded to treatment but had a high risk of NRM and nonresponders who had a low risk of NRM.
The researchers also found that biomarkers changed over the first month of treatment but remained significant predictors of NRM. This suggests that modifying treatment according to biomarker findings at various time points could result in better outcomes for patients.
“We think this is going to transform the way we treat graft-versus-host disease,” said James L.M. Ferrara, MD, DSc, of the Icahn School of Medicine at Mount Sinai, New York.
Dr. Ferrara and Hrishikesh Srinagesh, along with their colleagues at Mount Sinai, have conducted extensive research with these biomarkers and presented some of their findings at the Acute Leukemia Forum of Hemedicus.
Comparing biomarkers and response
In one study, the researchers evaluated 355 patients who had undergone allogeneic hematopoietic stem cell transplant at 1 of 20 Mount Sinai Acute GVHD International Consortium (MAGIC) centers between January 2016 and February 2018. All patients developed acute GVHD and received systemic steroids as treatment.
Patients provided blood samples weekly for the first month of treatment, and concentrations of ST2 and REG3-alpha were measured in each sample. Both biomarker concentrations were used to calculate the biomarker probability of NRM.
“The concentration of those two biomarkers are put into a computer, and we get … a single number, and that gives us the probability of mortality,” Dr. Ferrara said. “[W]e call this the MAGIC algorithm probability, or MAP. And when a MAP is low, the patient has a very low chance of dying from graft-versus-host disease, when it’s intermediate, they have an intermediate risk, and when it’s high, they have a high risk.”
The researchers then compared the MAP and clinical response for their ability to predict 6-month NRM throughout the first month of therapy for acute GVHD.
MAP bests response
After 1 month of therapy, the MAP was more accurate than clinical response for predicting 6-month NRM. The area under the curve was 0.84 and 0.65, respectively (P less than .001).
Likewise, the MAP after 1 week of therapy was more accurate than clinical response at 1 month for predicting 6-month NRM. The area under the curve was 0.80 and 0.65, respectively (P less than .001).
“[T]he clinical responses were good, but not great, at predicting long-term outcome, where the biomarker, the MAP, was significantly better,” Dr. Ferrara said. “[A]t every time point we tested, the biomarkers were better than the clinical responses.”
The researchers also identified subgroups of clinical responders and nonresponders for whom MAP more accurately predicted 6-month NRM.
The team found that 61% of clinical nonresponders were actually low risk according to MAP. And the incidence of 6-month NRM was significantly lower in the MAP-designated low-risk patients than in MAP-designated high-risk patients – 22% and 56%, respectively (P less than .001).
On the other hand, 10% of clinical responders were high risk according to MAP. The incidence of 6-month NRM was significantly higher in the high-risk patients than in the low-risk patients – 40% and 13%, respectively (P less than .001).
Assessing changes over time
The researchers found that patients who were initially high risk by MAP but had not experienced NRM by 6 months had significant decreases in their MAP after 4 weeks of treatment (P = .003). Patients who did experience NRM had a significant increase in their MAP whether their initial MAP was low (P = .007) or high (P = .024).
“What we found was that patients who lived tended to either have low biomarkers at the start of treatment and stay low or start out with high biomarkers and have reductions over the first month of therapy,” Mr. Srinagesh said. “Conversely, patients who tended to do worse were those who had either increases in their biomarkers or stayed high at all time points.”
The researchers identified a threshold – 0.290 – for separating patients by mortality risk.
“Patients who started out above the threshold and then went below it had a 5-fold reduction in mortality, whereas patients who started out below the threshold and rose above it had a 5-fold increase in mortality,” Mr. Srinagesh said.
MAP in clinical trials and practice
Based on these findings and results from related studies, the researchers theorize that MAP would be a better endpoint for clinical trials than clinical response.
At present, there are three trials in which researchers are using MAP as an endpoint to assess the efficacy of treatment for GVHD (NCT02133924, NCT03459040, and NCT03846479). Dr. Ferrara said a fourth trial is set to begin this summer.
Additionally, MAP is being used in clinical practice. A company called Viracor Eurofins Clinical Diagnostics licensed the MAGIC algorithm and provides three related tests for consumer use.
Viracor’s aGVHD Pre-Symptomatic Algorithm assigns patients to high- and low-risk groups based on results from samples collected 7 days after transplant. The aGVHD Symptomatic Onset Algorithm assigns patients to high-, intermediate-, and low-risk groups. The aGVHD Post-Treatment Algorithm, which can be used 7 days or more after GVHD treatment initiation, stratifies steroid-resistant patients into high- or low-risk groups for both NRM and overall survival.
“We are still in early days of figuring out how to use [the biomarker tests], but … what I’ve heard is that people are finding them to be useful in their clinical practice,” Dr. Ferrara said.
Dr. Ferrara has an ownership interest in and receives royalties from Viracor. Mr. Srinagesh reported having no relevant conflicts of interest. The research was supported by grants from the National Cancer Institute and the American Cancer Society.
The Acute Leukemia Forum is held by Hemedicus, which is owned by the same company as this news organization.
NEWPORT BEACH, CALIF. – Researchers say they have identified biomarkers that may help guide early treatment decisions in patients with acute graft-versus-host disease (GVHD).
The biomarkers, ST2 and REG3-alpha, were measured during the first month of GVHD treatment and proved more accurate than clinical response for predicting 6-month nonrelapse mortality (NRM). In fact, biomarker assessment revealed patients who responded to treatment but had a high risk of NRM and nonresponders who had a low risk of NRM.
The researchers also found that biomarkers changed over the first month of treatment but remained significant predictors of NRM. This suggests that modifying treatment according to biomarker findings at various time points could result in better outcomes for patients.
“We think this is going to transform the way we treat graft-versus-host disease,” said James L.M. Ferrara, MD, DSc, of the Icahn School of Medicine at Mount Sinai, New York.
Dr. Ferrara and Hrishikesh Srinagesh, along with their colleagues at Mount Sinai, have conducted extensive research with these biomarkers and presented some of their findings at the Acute Leukemia Forum of Hemedicus.
Comparing biomarkers and response
In one study, the researchers evaluated 355 patients who had undergone allogeneic hematopoietic stem cell transplant at 1 of 20 Mount Sinai Acute GVHD International Consortium (MAGIC) centers between January 2016 and February 2018. All patients developed acute GVHD and received systemic steroids as treatment.
Patients provided blood samples weekly for the first month of treatment, and concentrations of ST2 and REG3-alpha were measured in each sample. Both biomarker concentrations were used to calculate the biomarker probability of NRM.
“The concentration of those two biomarkers are put into a computer, and we get … a single number, and that gives us the probability of mortality,” Dr. Ferrara said. “[W]e call this the MAGIC algorithm probability, or MAP. And when a MAP is low, the patient has a very low chance of dying from graft-versus-host disease, when it’s intermediate, they have an intermediate risk, and when it’s high, they have a high risk.”
The researchers then compared the MAP and clinical response for their ability to predict 6-month NRM throughout the first month of therapy for acute GVHD.
MAP bests response
After 1 month of therapy, the MAP was more accurate than clinical response for predicting 6-month NRM. The area under the curve was 0.84 and 0.65, respectively (P less than .001).
Likewise, the MAP after 1 week of therapy was more accurate than clinical response at 1 month for predicting 6-month NRM. The area under the curve was 0.80 and 0.65, respectively (P less than .001).
“[T]he clinical responses were good, but not great, at predicting long-term outcome, where the biomarker, the MAP, was significantly better,” Dr. Ferrara said. “[A]t every time point we tested, the biomarkers were better than the clinical responses.”
The researchers also identified subgroups of clinical responders and nonresponders for whom MAP more accurately predicted 6-month NRM.
The team found that 61% of clinical nonresponders were actually low risk according to MAP. And the incidence of 6-month NRM was significantly lower in the MAP-designated low-risk patients than in MAP-designated high-risk patients – 22% and 56%, respectively (P less than .001).
On the other hand, 10% of clinical responders were high risk according to MAP. The incidence of 6-month NRM was significantly higher in the high-risk patients than in the low-risk patients – 40% and 13%, respectively (P less than .001).
Assessing changes over time
The researchers found that patients who were initially high risk by MAP but had not experienced NRM by 6 months had significant decreases in their MAP after 4 weeks of treatment (P = .003). Patients who did experience NRM had a significant increase in their MAP whether their initial MAP was low (P = .007) or high (P = .024).
“What we found was that patients who lived tended to either have low biomarkers at the start of treatment and stay low or start out with high biomarkers and have reductions over the first month of therapy,” Mr. Srinagesh said. “Conversely, patients who tended to do worse were those who had either increases in their biomarkers or stayed high at all time points.”
The researchers identified a threshold – 0.290 – for separating patients by mortality risk.
“Patients who started out above the threshold and then went below it had a 5-fold reduction in mortality, whereas patients who started out below the threshold and rose above it had a 5-fold increase in mortality,” Mr. Srinagesh said.
MAP in clinical trials and practice
Based on these findings and results from related studies, the researchers theorize that MAP would be a better endpoint for clinical trials than clinical response.
At present, there are three trials in which researchers are using MAP as an endpoint to assess the efficacy of treatment for GVHD (NCT02133924, NCT03459040, and NCT03846479). Dr. Ferrara said a fourth trial is set to begin this summer.
Additionally, MAP is being used in clinical practice. A company called Viracor Eurofins Clinical Diagnostics licensed the MAGIC algorithm and provides three related tests for consumer use.
Viracor’s aGVHD Pre-Symptomatic Algorithm assigns patients to high- and low-risk groups based on results from samples collected 7 days after transplant. The aGVHD Symptomatic Onset Algorithm assigns patients to high-, intermediate-, and low-risk groups. The aGVHD Post-Treatment Algorithm, which can be used 7 days or more after GVHD treatment initiation, stratifies steroid-resistant patients into high- or low-risk groups for both NRM and overall survival.
“We are still in early days of figuring out how to use [the biomarker tests], but … what I’ve heard is that people are finding them to be useful in their clinical practice,” Dr. Ferrara said.
Dr. Ferrara has an ownership interest in and receives royalties from Viracor. Mr. Srinagesh reported having no relevant conflicts of interest. The research was supported by grants from the National Cancer Institute and the American Cancer Society.
The Acute Leukemia Forum is held by Hemedicus, which is owned by the same company as this news organization.
REPORTING FROM ALF 2019
Model inspired by Netflix, Amazon may help guide MDS treatment
NEWPORT BEACH, CALIF. — A model that mimics the recommender system used by Netflix and Amazon can help predict outcomes of lenalidomide treatment in patients with non–deletion 5q (non-del[5q]) myelodysplastic syndromes (MDS), according to new research.
The model was used to identify genomic biomarkers that were associated with resistance or response to lenalidomide. Researchers found these associations in 39% of patients with non-del(5q) MDS, and the model predicted response or resistance with 82% accuracy.
Yazan Madanat, MD, of the Cleveland Clinic, and his colleagues presented these findings at the Acute Leukemia Forum of Hemedicus.
Dr. Madanat explained that his group’s model is similar to the recommender system used by Netflix and Amazon, which makes suggestions for new products based on customers’ past behavior. Dr. Madanat and his colleagues used their model to show that patients with certain molecular or cytogenetic abnormalities are likely to respond or not respond to lenalidomide.
The researchers began by looking at 139 patients who had received at least two cycles of lenalidomide treatment. There were 118 patients with MDS, and 108 who had received lenalidomide monotherapy. However, the team focused on the 100 patients who had non-del(5q) MDS, 58 of whom had normal karyotype (NK) and 19 of whom had complex karyotype (CK).
The model revealed several combinations of genomic/cytogenetic abnormalities that could predict resistance to lenalidomide, including the following:
- DNMT3A and SF3B1
- EZH2 and NK
- ASXL1, TET2, and NK
- STAG2, IDH1/2, and NK
- TP53, del(5q), and CK
- BCOR/BCORL1 and NK
- JAK2, TET2, and NK
- U2AF1, +/– ETV6, and NK
However, only the following two combinations could predict response to lenalidomide:
- DDX41 and NK
- MECOM and KDM6A/B
These combinations could be applied to 39% of the patients with non-del(5q) MDS, and the model predicted response or resistance to lenalidomide with 82% accuracy.
Although the biomarkers were found in only a subset of patients, Dr. Madanat said these findings may help physicians tailor therapy for MDS patients, given the high level of accuracy the researchers observed.
“It’s really important to validate the results in a prospective manner and to ensure that we’re able to apply them clinically and potentially change the way we’re treating our patients,” he added.
Dr. Madanat and his colleagues reported having no relevant conflicts of interest.
The Acute Leukemia Forum is held by Hemedicus, which is owned by the same company as this news organization.
NEWPORT BEACH, CALIF. — A model that mimics the recommender system used by Netflix and Amazon can help predict outcomes of lenalidomide treatment in patients with non–deletion 5q (non-del[5q]) myelodysplastic syndromes (MDS), according to new research.
The model was used to identify genomic biomarkers that were associated with resistance or response to lenalidomide. Researchers found these associations in 39% of patients with non-del(5q) MDS, and the model predicted response or resistance with 82% accuracy.
Yazan Madanat, MD, of the Cleveland Clinic, and his colleagues presented these findings at the Acute Leukemia Forum of Hemedicus.
Dr. Madanat explained that his group’s model is similar to the recommender system used by Netflix and Amazon, which makes suggestions for new products based on customers’ past behavior. Dr. Madanat and his colleagues used their model to show that patients with certain molecular or cytogenetic abnormalities are likely to respond or not respond to lenalidomide.
The researchers began by looking at 139 patients who had received at least two cycles of lenalidomide treatment. There were 118 patients with MDS, and 108 who had received lenalidomide monotherapy. However, the team focused on the 100 patients who had non-del(5q) MDS, 58 of whom had normal karyotype (NK) and 19 of whom had complex karyotype (CK).
The model revealed several combinations of genomic/cytogenetic abnormalities that could predict resistance to lenalidomide, including the following:
- DNMT3A and SF3B1
- EZH2 and NK
- ASXL1, TET2, and NK
- STAG2, IDH1/2, and NK
- TP53, del(5q), and CK
- BCOR/BCORL1 and NK
- JAK2, TET2, and NK
- U2AF1, +/– ETV6, and NK
However, only the following two combinations could predict response to lenalidomide:
- DDX41 and NK
- MECOM and KDM6A/B
These combinations could be applied to 39% of the patients with non-del(5q) MDS, and the model predicted response or resistance to lenalidomide with 82% accuracy.
Although the biomarkers were found in only a subset of patients, Dr. Madanat said these findings may help physicians tailor therapy for MDS patients, given the high level of accuracy the researchers observed.
“It’s really important to validate the results in a prospective manner and to ensure that we’re able to apply them clinically and potentially change the way we’re treating our patients,” he added.
Dr. Madanat and his colleagues reported having no relevant conflicts of interest.
The Acute Leukemia Forum is held by Hemedicus, which is owned by the same company as this news organization.
NEWPORT BEACH, CALIF. — A model that mimics the recommender system used by Netflix and Amazon can help predict outcomes of lenalidomide treatment in patients with non–deletion 5q (non-del[5q]) myelodysplastic syndromes (MDS), according to new research.
The model was used to identify genomic biomarkers that were associated with resistance or response to lenalidomide. Researchers found these associations in 39% of patients with non-del(5q) MDS, and the model predicted response or resistance with 82% accuracy.
Yazan Madanat, MD, of the Cleveland Clinic, and his colleagues presented these findings at the Acute Leukemia Forum of Hemedicus.
Dr. Madanat explained that his group’s model is similar to the recommender system used by Netflix and Amazon, which makes suggestions for new products based on customers’ past behavior. Dr. Madanat and his colleagues used their model to show that patients with certain molecular or cytogenetic abnormalities are likely to respond or not respond to lenalidomide.
The researchers began by looking at 139 patients who had received at least two cycles of lenalidomide treatment. There were 118 patients with MDS, and 108 who had received lenalidomide monotherapy. However, the team focused on the 100 patients who had non-del(5q) MDS, 58 of whom had normal karyotype (NK) and 19 of whom had complex karyotype (CK).
The model revealed several combinations of genomic/cytogenetic abnormalities that could predict resistance to lenalidomide, including the following:
- DNMT3A and SF3B1
- EZH2 and NK
- ASXL1, TET2, and NK
- STAG2, IDH1/2, and NK
- TP53, del(5q), and CK
- BCOR/BCORL1 and NK
- JAK2, TET2, and NK
- U2AF1, +/– ETV6, and NK
However, only the following two combinations could predict response to lenalidomide:
- DDX41 and NK
- MECOM and KDM6A/B
These combinations could be applied to 39% of the patients with non-del(5q) MDS, and the model predicted response or resistance to lenalidomide with 82% accuracy.
Although the biomarkers were found in only a subset of patients, Dr. Madanat said these findings may help physicians tailor therapy for MDS patients, given the high level of accuracy the researchers observed.
“It’s really important to validate the results in a prospective manner and to ensure that we’re able to apply them clinically and potentially change the way we’re treating our patients,” he added.
Dr. Madanat and his colleagues reported having no relevant conflicts of interest.
The Acute Leukemia Forum is held by Hemedicus, which is owned by the same company as this news organization.
REPORTING FROM ALF 2019
Sorafenib plus GCLAM held safe in AML, MDS phase-1 study
NEWPORT BEACH, CALIF. – A five-drug regimen was deemed safe in patients with newly diagnosed acute myeloid leukemia (AML) or high-risk myelodysplastic syndromes (MDS), and it appeared to be effective regardless of patients’ FLT3 status.
Researchers tested this regimen – sorafenib plus granulocyte colony–stimulating factor (G-CSF), cladribine, high-dose cytarabine, and mitoxantrone (GCLAM) – in a phase 1 trial.
Kelsey-Leigh Garcia, a clinical research coordinator at Seattle Cancer Care Alliance, and her colleagues presented the results at the Acute Leukemia Forum of Hemedicus.
“The background for doing this study was our institutional results of GCLAM [Leukemia. 2018 Nov;32(11):2352-62] that showed a higher minimal residual disease–negative complete response rate than 7+3 [cytarabine continuously for 7 days, along with short infusions of an anthracycline on each of the first 3 days] and an international study by Röllig that showed the addition of sorafenib to 7+3 increased event-free survival versus [7+3 and] placebo [Lancet Oncol. 2015 Dec;16(16):1691-9],” Ms. Garcia said.
“GCLAM is the standard backbone at our institution, and we wanted to ask the question, ‘If we add sorafenib, can this improve upon the results of GCLAM?’ ” said Anna Halpern, MD, a hematologist-oncologist at the University of Washington, Seattle and principal investigator of the phase 1 trial.
The trial (NCT02728050) included 47 patients, 39 with AML and 8 with MDS. Patients were aged 60 years or younger and had a median age of 48. They had a median treatment-related mortality score of 1.76 (range, 0.19-12.26). A total of 11 patients (23%) had FLT3-ITD, and 4 (9%) had FLT3-TKD.
Treatment and toxicity
For induction, patients received G-CSF at 5 mcg/kg on days 0-5, cladribine at 5 mg/m2 on days 1-5, and cytarabine at 2 g/m2 on days 1-5. Mitoxantrone was given at 10 mg/m2, 12 mg/m2, 15 mg/m2, or 18 mg/m2 on days 1-3. Sorafenib was given at 200 mg twice daily, 400 mg in the morning and 200 mg in the afternoon, or 400 mg b.i.d. on days 10-19.
For consolidation, patients could receive up to four cycles of G-CSF, cladribine, and cytarabine plus sorafenib on days 8-27. Patients who did not proceed to transplant could receive 12 months of sorafenib as maintenance therapy.
There were four dose-limiting toxicities.
- Grade 4 intracranial hemorrhage with mitoxantrone at 12 mg/m2 and sorafenib at 200 mg b.i.d.
- Grade 4 prolonged count recovery with mitoxantrone at 15 mg/m2 and sorafenib at 200 mg b.i.d.
- Grade 4 sepsis, Sweet syndrome, and Bell’s palsy with mitoxantrone at 18 mg/m2 and sorafenib at 200 mg b.i.d.
- Grade 3 cardiomyopathy and acute pericarditis with mitoxantrone at 18 mg/m2 and sorafenib at 400 mg b.i.d.
However, these toxicities did not define the maximum-tolerated dose. Therefore, the recommended phase 2 dose of mitoxantrone is 18 mg/m2, and the recommended phase 2 dose of sorafenib is 400 mg b.i.d.
There were no grade 5 treatment-related adverse events. Grade 3 events included febrile neutropenia (90%), maculopapular rash (20%), infections (10%), hand-foot syndrome (2%), and diarrhea (1%). Grade 4 events included sepsis, intracranial hemorrhage, and oral mucositis (all 1%).
Response and survival
Among the 46 evaluable patients, 83% achieved a complete response, 78% had a minimal residual disease–negative complete response, and 4% had a minimal residual disease–negative complete response with incomplete count recovery. A morphological leukemia-free state was achieved by 4% of patients, and 8% had resistant disease.
Fifty-nine percent of patients went on to transplant. The median overall survival had not been reached at a median follow-up of 10 months.
The researchers compared outcomes in this trial with outcomes in a cohort of patients who had received GCLAM alone, and there were no significant differences in overall survival or event-free survival.
“The trial wasn’t powered, necessarily, for efficacy, but we compared these results to our historical cohort of medically matched and age-matched patients treated with GCLAM alone and, so far, found no differences in survival between the two groups,” Dr. Halpern said.
She noted, however, that follow-up was short in the sorafenib trial, and it included patients treated with all dose levels of sorafenib and mitoxantrone.
A phase 2 study of sorafenib plus GCLAM in newly diagnosed AML or high-risk MDS is now underway.
Dr. Halpern and Ms. Garcia reported that they had no conflicts of interest. The phase 1 trial was sponsored by the University of Washington in collaboration with the National Cancer Institute, and funding was provided by Bayer.
The Acute Leukemia Forum is held by Hemedicus, which is owned by the same company as this news organization.
NEWPORT BEACH, CALIF. – A five-drug regimen was deemed safe in patients with newly diagnosed acute myeloid leukemia (AML) or high-risk myelodysplastic syndromes (MDS), and it appeared to be effective regardless of patients’ FLT3 status.
Researchers tested this regimen – sorafenib plus granulocyte colony–stimulating factor (G-CSF), cladribine, high-dose cytarabine, and mitoxantrone (GCLAM) – in a phase 1 trial.
Kelsey-Leigh Garcia, a clinical research coordinator at Seattle Cancer Care Alliance, and her colleagues presented the results at the Acute Leukemia Forum of Hemedicus.
“The background for doing this study was our institutional results of GCLAM [Leukemia. 2018 Nov;32(11):2352-62] that showed a higher minimal residual disease–negative complete response rate than 7+3 [cytarabine continuously for 7 days, along with short infusions of an anthracycline on each of the first 3 days] and an international study by Röllig that showed the addition of sorafenib to 7+3 increased event-free survival versus [7+3 and] placebo [Lancet Oncol. 2015 Dec;16(16):1691-9],” Ms. Garcia said.
“GCLAM is the standard backbone at our institution, and we wanted to ask the question, ‘If we add sorafenib, can this improve upon the results of GCLAM?’ ” said Anna Halpern, MD, a hematologist-oncologist at the University of Washington, Seattle and principal investigator of the phase 1 trial.
The trial (NCT02728050) included 47 patients, 39 with AML and 8 with MDS. Patients were aged 60 years or younger and had a median age of 48. They had a median treatment-related mortality score of 1.76 (range, 0.19-12.26). A total of 11 patients (23%) had FLT3-ITD, and 4 (9%) had FLT3-TKD.
Treatment and toxicity
For induction, patients received G-CSF at 5 mcg/kg on days 0-5, cladribine at 5 mg/m2 on days 1-5, and cytarabine at 2 g/m2 on days 1-5. Mitoxantrone was given at 10 mg/m2, 12 mg/m2, 15 mg/m2, or 18 mg/m2 on days 1-3. Sorafenib was given at 200 mg twice daily, 400 mg in the morning and 200 mg in the afternoon, or 400 mg b.i.d. on days 10-19.
For consolidation, patients could receive up to four cycles of G-CSF, cladribine, and cytarabine plus sorafenib on days 8-27. Patients who did not proceed to transplant could receive 12 months of sorafenib as maintenance therapy.
There were four dose-limiting toxicities.
- Grade 4 intracranial hemorrhage with mitoxantrone at 12 mg/m2 and sorafenib at 200 mg b.i.d.
- Grade 4 prolonged count recovery with mitoxantrone at 15 mg/m2 and sorafenib at 200 mg b.i.d.
- Grade 4 sepsis, Sweet syndrome, and Bell’s palsy with mitoxantrone at 18 mg/m2 and sorafenib at 200 mg b.i.d.
- Grade 3 cardiomyopathy and acute pericarditis with mitoxantrone at 18 mg/m2 and sorafenib at 400 mg b.i.d.
However, these toxicities did not define the maximum-tolerated dose. Therefore, the recommended phase 2 dose of mitoxantrone is 18 mg/m2, and the recommended phase 2 dose of sorafenib is 400 mg b.i.d.
There were no grade 5 treatment-related adverse events. Grade 3 events included febrile neutropenia (90%), maculopapular rash (20%), infections (10%), hand-foot syndrome (2%), and diarrhea (1%). Grade 4 events included sepsis, intracranial hemorrhage, and oral mucositis (all 1%).
Response and survival
Among the 46 evaluable patients, 83% achieved a complete response, 78% had a minimal residual disease–negative complete response, and 4% had a minimal residual disease–negative complete response with incomplete count recovery. A morphological leukemia-free state was achieved by 4% of patients, and 8% had resistant disease.
Fifty-nine percent of patients went on to transplant. The median overall survival had not been reached at a median follow-up of 10 months.
The researchers compared outcomes in this trial with outcomes in a cohort of patients who had received GCLAM alone, and there were no significant differences in overall survival or event-free survival.
“The trial wasn’t powered, necessarily, for efficacy, but we compared these results to our historical cohort of medically matched and age-matched patients treated with GCLAM alone and, so far, found no differences in survival between the two groups,” Dr. Halpern said.
She noted, however, that follow-up was short in the sorafenib trial, and it included patients treated with all dose levels of sorafenib and mitoxantrone.
A phase 2 study of sorafenib plus GCLAM in newly diagnosed AML or high-risk MDS is now underway.
Dr. Halpern and Ms. Garcia reported that they had no conflicts of interest. The phase 1 trial was sponsored by the University of Washington in collaboration with the National Cancer Institute, and funding was provided by Bayer.
The Acute Leukemia Forum is held by Hemedicus, which is owned by the same company as this news organization.
NEWPORT BEACH, CALIF. – A five-drug regimen was deemed safe in patients with newly diagnosed acute myeloid leukemia (AML) or high-risk myelodysplastic syndromes (MDS), and it appeared to be effective regardless of patients’ FLT3 status.
Researchers tested this regimen – sorafenib plus granulocyte colony–stimulating factor (G-CSF), cladribine, high-dose cytarabine, and mitoxantrone (GCLAM) – in a phase 1 trial.
Kelsey-Leigh Garcia, a clinical research coordinator at Seattle Cancer Care Alliance, and her colleagues presented the results at the Acute Leukemia Forum of Hemedicus.
“The background for doing this study was our institutional results of GCLAM [Leukemia. 2018 Nov;32(11):2352-62] that showed a higher minimal residual disease–negative complete response rate than 7+3 [cytarabine continuously for 7 days, along with short infusions of an anthracycline on each of the first 3 days] and an international study by Röllig that showed the addition of sorafenib to 7+3 increased event-free survival versus [7+3 and] placebo [Lancet Oncol. 2015 Dec;16(16):1691-9],” Ms. Garcia said.
“GCLAM is the standard backbone at our institution, and we wanted to ask the question, ‘If we add sorafenib, can this improve upon the results of GCLAM?’ ” said Anna Halpern, MD, a hematologist-oncologist at the University of Washington, Seattle and principal investigator of the phase 1 trial.
The trial (NCT02728050) included 47 patients, 39 with AML and 8 with MDS. Patients were aged 60 years or younger and had a median age of 48. They had a median treatment-related mortality score of 1.76 (range, 0.19-12.26). A total of 11 patients (23%) had FLT3-ITD, and 4 (9%) had FLT3-TKD.
Treatment and toxicity
For induction, patients received G-CSF at 5 mcg/kg on days 0-5, cladribine at 5 mg/m2 on days 1-5, and cytarabine at 2 g/m2 on days 1-5. Mitoxantrone was given at 10 mg/m2, 12 mg/m2, 15 mg/m2, or 18 mg/m2 on days 1-3. Sorafenib was given at 200 mg twice daily, 400 mg in the morning and 200 mg in the afternoon, or 400 mg b.i.d. on days 10-19.
For consolidation, patients could receive up to four cycles of G-CSF, cladribine, and cytarabine plus sorafenib on days 8-27. Patients who did not proceed to transplant could receive 12 months of sorafenib as maintenance therapy.
There were four dose-limiting toxicities.
- Grade 4 intracranial hemorrhage with mitoxantrone at 12 mg/m2 and sorafenib at 200 mg b.i.d.
- Grade 4 prolonged count recovery with mitoxantrone at 15 mg/m2 and sorafenib at 200 mg b.i.d.
- Grade 4 sepsis, Sweet syndrome, and Bell’s palsy with mitoxantrone at 18 mg/m2 and sorafenib at 200 mg b.i.d.
- Grade 3 cardiomyopathy and acute pericarditis with mitoxantrone at 18 mg/m2 and sorafenib at 400 mg b.i.d.
However, these toxicities did not define the maximum-tolerated dose. Therefore, the recommended phase 2 dose of mitoxantrone is 18 mg/m2, and the recommended phase 2 dose of sorafenib is 400 mg b.i.d.
There were no grade 5 treatment-related adverse events. Grade 3 events included febrile neutropenia (90%), maculopapular rash (20%), infections (10%), hand-foot syndrome (2%), and diarrhea (1%). Grade 4 events included sepsis, intracranial hemorrhage, and oral mucositis (all 1%).
Response and survival
Among the 46 evaluable patients, 83% achieved a complete response, 78% had a minimal residual disease–negative complete response, and 4% had a minimal residual disease–negative complete response with incomplete count recovery. A morphological leukemia-free state was achieved by 4% of patients, and 8% had resistant disease.
Fifty-nine percent of patients went on to transplant. The median overall survival had not been reached at a median follow-up of 10 months.
The researchers compared outcomes in this trial with outcomes in a cohort of patients who had received GCLAM alone, and there were no significant differences in overall survival or event-free survival.
“The trial wasn’t powered, necessarily, for efficacy, but we compared these results to our historical cohort of medically matched and age-matched patients treated with GCLAM alone and, so far, found no differences in survival between the two groups,” Dr. Halpern said.
She noted, however, that follow-up was short in the sorafenib trial, and it included patients treated with all dose levels of sorafenib and mitoxantrone.
A phase 2 study of sorafenib plus GCLAM in newly diagnosed AML or high-risk MDS is now underway.
Dr. Halpern and Ms. Garcia reported that they had no conflicts of interest. The phase 1 trial was sponsored by the University of Washington in collaboration with the National Cancer Institute, and funding was provided by Bayer.
The Acute Leukemia Forum is held by Hemedicus, which is owned by the same company as this news organization.
REPORTING FROM ALF 2019
Study highlights lack of data on transgender leukemia patients
NEWPORT BEACH, CALIF. – Researchers have shown they can identify transgender leukemia patients by detecting gender-karyotype mismatches, but some transgender patients may be overlooked with this method.
The researchers’ work also highlights how little we know about transgender patients with leukemia and other cancers.
Alison Alpert, MD, of the University of Rochester (N.Y.) Medical Center, and her colleagues conducted this research and presented their findings in a poster at the Acute Leukemia Forum of Hemedicus.
“There’s almost no data about transgender people with cancer ... in terms of prevalence or anything else,” Dr. Alpert noted. “And because we don’t know which patients with cancer are transgender, we can’t begin to answer any of the other big questions for patients.”
Specifically, it’s unclear what kinds of cancer transgender patients have, if there are health disparities among transgender patients, if it is safe to continue hormone therapy during cancer treatment, and if it is possible to do transition-related surgeries in the context of cancer care.
With this in mind, Dr. Alpert and her colleagues set out to identify transgender patients by detecting gender-karyotype mismatches. The team analyzed data on patients with acute myeloid leukemia (AML) or myelodysplastic syndromes enrolled in five Southwest Oncology Group (SWOG) trials.
Of the 1,748 patients analyzed, six (0.3%) had a gender-karyotype mismatch. Five patients had a 46,XY karyotype and identified as female, and one patient had a 46,XX karyotype and identified as male.
“Some transgender patients have their gender identity accurately reflected in the electronic medical record, [but] some transgender patients probably don’t,” Dr. Alpert noted. “So we identified some, but probably not all, and probably not even most, transgender patients with leukemia in this cohort.”
All six of the transgender patients identified had AML, and all were white. They ranged in age from 18 to 57 years. Four patients had achieved a complete response to therapy, and two had refractory disease.
Four patients, including one who was refractory, were still alive at last follow-up. The remaining two patients, including one who had achieved a complete response, had died.
The transgender patients identified in this analysis represent a very small percentage of the population studied, Dr. Alpert noted. Therefore, the researchers could not draw any conclusions about transgender patients with AML.
“Mostly, what we did was, we pointed out how little information we have,” Dr. Alpert said. “Oncologists don’t routinely collect gender identity information, and this information doesn’t exist in cooperative group databases either.”
“But going forward, what probably really needs to happen is that oncologists need to ask their patients whether they are transgender or not. And then, ideally, consent forms for large cooperative groups like SWOG would include gender identity data, and then we would be able to answer some of our other questions and better counsel our patients.”
Dr. Alpert and her colleagues are hoping to gain insights regarding transgender patients with lymphoma as well. The researchers are analyzing the lymphoma database at the University of Rochester Medical Center, which includes about 2,200 patients.
The team is attempting to identify transgender lymphoma patients using gender-karyotype mismatch as well as other methods, including assessing patients’ medication and surgical histories, determining whether patients have any aliases, and looking for the word “transgender” in patient charts.
“Given that the country is finally starting to talk about transgender patients, their health disparities, and their needs and experiences, it’s really time that we start collecting this data,” Dr. Alpert said.
“[I]f we are able to start to collect this data, it can help us build relationships with our patients, improve their care and outcomes, and, hopefully, be able to better counsel them about hormones and surgery.”
Dr. Alpert and her colleagues did not disclose any conflicts of interest.
The Acute Leukemia Forum is organized by Hemedicus, which is owned by the same company as this news organization.
NEWPORT BEACH, CALIF. – Researchers have shown they can identify transgender leukemia patients by detecting gender-karyotype mismatches, but some transgender patients may be overlooked with this method.
The researchers’ work also highlights how little we know about transgender patients with leukemia and other cancers.
Alison Alpert, MD, of the University of Rochester (N.Y.) Medical Center, and her colleagues conducted this research and presented their findings in a poster at the Acute Leukemia Forum of Hemedicus.
“There’s almost no data about transgender people with cancer ... in terms of prevalence or anything else,” Dr. Alpert noted. “And because we don’t know which patients with cancer are transgender, we can’t begin to answer any of the other big questions for patients.”
Specifically, it’s unclear what kinds of cancer transgender patients have, if there are health disparities among transgender patients, if it is safe to continue hormone therapy during cancer treatment, and if it is possible to do transition-related surgeries in the context of cancer care.
With this in mind, Dr. Alpert and her colleagues set out to identify transgender patients by detecting gender-karyotype mismatches. The team analyzed data on patients with acute myeloid leukemia (AML) or myelodysplastic syndromes enrolled in five Southwest Oncology Group (SWOG) trials.
Of the 1,748 patients analyzed, six (0.3%) had a gender-karyotype mismatch. Five patients had a 46,XY karyotype and identified as female, and one patient had a 46,XX karyotype and identified as male.
“Some transgender patients have their gender identity accurately reflected in the electronic medical record, [but] some transgender patients probably don’t,” Dr. Alpert noted. “So we identified some, but probably not all, and probably not even most, transgender patients with leukemia in this cohort.”
All six of the transgender patients identified had AML, and all were white. They ranged in age from 18 to 57 years. Four patients had achieved a complete response to therapy, and two had refractory disease.
Four patients, including one who was refractory, were still alive at last follow-up. The remaining two patients, including one who had achieved a complete response, had died.
The transgender patients identified in this analysis represent a very small percentage of the population studied, Dr. Alpert noted. Therefore, the researchers could not draw any conclusions about transgender patients with AML.
“Mostly, what we did was, we pointed out how little information we have,” Dr. Alpert said. “Oncologists don’t routinely collect gender identity information, and this information doesn’t exist in cooperative group databases either.”
“But going forward, what probably really needs to happen is that oncologists need to ask their patients whether they are transgender or not. And then, ideally, consent forms for large cooperative groups like SWOG would include gender identity data, and then we would be able to answer some of our other questions and better counsel our patients.”
Dr. Alpert and her colleagues are hoping to gain insights regarding transgender patients with lymphoma as well. The researchers are analyzing the lymphoma database at the University of Rochester Medical Center, which includes about 2,200 patients.
The team is attempting to identify transgender lymphoma patients using gender-karyotype mismatch as well as other methods, including assessing patients’ medication and surgical histories, determining whether patients have any aliases, and looking for the word “transgender” in patient charts.
“Given that the country is finally starting to talk about transgender patients, their health disparities, and their needs and experiences, it’s really time that we start collecting this data,” Dr. Alpert said.
“[I]f we are able to start to collect this data, it can help us build relationships with our patients, improve their care and outcomes, and, hopefully, be able to better counsel them about hormones and surgery.”
Dr. Alpert and her colleagues did not disclose any conflicts of interest.
The Acute Leukemia Forum is organized by Hemedicus, which is owned by the same company as this news organization.
NEWPORT BEACH, CALIF. – Researchers have shown they can identify transgender leukemia patients by detecting gender-karyotype mismatches, but some transgender patients may be overlooked with this method.
The researchers’ work also highlights how little we know about transgender patients with leukemia and other cancers.
Alison Alpert, MD, of the University of Rochester (N.Y.) Medical Center, and her colleagues conducted this research and presented their findings in a poster at the Acute Leukemia Forum of Hemedicus.
“There’s almost no data about transgender people with cancer ... in terms of prevalence or anything else,” Dr. Alpert noted. “And because we don’t know which patients with cancer are transgender, we can’t begin to answer any of the other big questions for patients.”
Specifically, it’s unclear what kinds of cancer transgender patients have, if there are health disparities among transgender patients, if it is safe to continue hormone therapy during cancer treatment, and if it is possible to do transition-related surgeries in the context of cancer care.
With this in mind, Dr. Alpert and her colleagues set out to identify transgender patients by detecting gender-karyotype mismatches. The team analyzed data on patients with acute myeloid leukemia (AML) or myelodysplastic syndromes enrolled in five Southwest Oncology Group (SWOG) trials.
Of the 1,748 patients analyzed, six (0.3%) had a gender-karyotype mismatch. Five patients had a 46,XY karyotype and identified as female, and one patient had a 46,XX karyotype and identified as male.
“Some transgender patients have their gender identity accurately reflected in the electronic medical record, [but] some transgender patients probably don’t,” Dr. Alpert noted. “So we identified some, but probably not all, and probably not even most, transgender patients with leukemia in this cohort.”
All six of the transgender patients identified had AML, and all were white. They ranged in age from 18 to 57 years. Four patients had achieved a complete response to therapy, and two had refractory disease.
Four patients, including one who was refractory, were still alive at last follow-up. The remaining two patients, including one who had achieved a complete response, had died.
The transgender patients identified in this analysis represent a very small percentage of the population studied, Dr. Alpert noted. Therefore, the researchers could not draw any conclusions about transgender patients with AML.
“Mostly, what we did was, we pointed out how little information we have,” Dr. Alpert said. “Oncologists don’t routinely collect gender identity information, and this information doesn’t exist in cooperative group databases either.”
“But going forward, what probably really needs to happen is that oncologists need to ask their patients whether they are transgender or not. And then, ideally, consent forms for large cooperative groups like SWOG would include gender identity data, and then we would be able to answer some of our other questions and better counsel our patients.”
Dr. Alpert and her colleagues are hoping to gain insights regarding transgender patients with lymphoma as well. The researchers are analyzing the lymphoma database at the University of Rochester Medical Center, which includes about 2,200 patients.
The team is attempting to identify transgender lymphoma patients using gender-karyotype mismatch as well as other methods, including assessing patients’ medication and surgical histories, determining whether patients have any aliases, and looking for the word “transgender” in patient charts.
“Given that the country is finally starting to talk about transgender patients, their health disparities, and their needs and experiences, it’s really time that we start collecting this data,” Dr. Alpert said.
“[I]f we are able to start to collect this data, it can help us build relationships with our patients, improve their care and outcomes, and, hopefully, be able to better counsel them about hormones and surgery.”
Dr. Alpert and her colleagues did not disclose any conflicts of interest.
The Acute Leukemia Forum is organized by Hemedicus, which is owned by the same company as this news organization.
REPORTING FROM ALF 2019
ALF 2019 showcases evolving treatment of AML
NEWPORT BEACH, CALIF. – The evolving treatment of acute myeloid leukemia (AML) was highlighted at the Acute Leukemia Forum of Hemedicus.
In a video interview, Martin Tallman, MD, of Memorial Sloan Kettering Cancer Center in New York, discussed several meeting presentations on the treatment of AML.
In his presentation, Craig Jordan, PhD, of the University of Colorado at Denver, Aurora, explained how the combination of venetoclax and azacitidine appears to target leukemic stem cells in AML.
Courtney DiNardo, MD, of the University of Texas MD Anderson Cancer Center, Houston, presented information on novel agents for AML, including antibody-drug conjugates; bispecific therapies; checkpoint inhibitors; and inhibitors of IDH1/2, MCL1, and MDM2.
Richard Larson, MD, of the University of Chicago, explored the possibility of an individualized approach to postremission therapy in AML.
Frederick Appelbaum, MD, of Fred Hutchinson Cancer Research Center in Seattle, showed that various maintenance therapies given after allogeneic hematopoietic stem cell transplant (HSCT) have not proven beneficial for AML patients.
Richard Jones, MD, of Johns Hopkins Medicine in Baltimore, presented data showing that post-HSCT cyclophosphamide has made haploidentical transplants safer and more effective for AML patients.
And James Ferrara, MD, of the Icahn School of Medicine at Mount Sinai, New York, detailed research showing that biomarkers of graft-versus-host disease can predict nonrelapse mortality after HSCT.
The Acute Leukemia Forum is held by Hemedicus, which is owned by the same company as this news organization.
NEWPORT BEACH, CALIF. – The evolving treatment of acute myeloid leukemia (AML) was highlighted at the Acute Leukemia Forum of Hemedicus.
In a video interview, Martin Tallman, MD, of Memorial Sloan Kettering Cancer Center in New York, discussed several meeting presentations on the treatment of AML.
In his presentation, Craig Jordan, PhD, of the University of Colorado at Denver, Aurora, explained how the combination of venetoclax and azacitidine appears to target leukemic stem cells in AML.
Courtney DiNardo, MD, of the University of Texas MD Anderson Cancer Center, Houston, presented information on novel agents for AML, including antibody-drug conjugates; bispecific therapies; checkpoint inhibitors; and inhibitors of IDH1/2, MCL1, and MDM2.
Richard Larson, MD, of the University of Chicago, explored the possibility of an individualized approach to postremission therapy in AML.
Frederick Appelbaum, MD, of Fred Hutchinson Cancer Research Center in Seattle, showed that various maintenance therapies given after allogeneic hematopoietic stem cell transplant (HSCT) have not proven beneficial for AML patients.
Richard Jones, MD, of Johns Hopkins Medicine in Baltimore, presented data showing that post-HSCT cyclophosphamide has made haploidentical transplants safer and more effective for AML patients.
And James Ferrara, MD, of the Icahn School of Medicine at Mount Sinai, New York, detailed research showing that biomarkers of graft-versus-host disease can predict nonrelapse mortality after HSCT.
The Acute Leukemia Forum is held by Hemedicus, which is owned by the same company as this news organization.
NEWPORT BEACH, CALIF. – The evolving treatment of acute myeloid leukemia (AML) was highlighted at the Acute Leukemia Forum of Hemedicus.
In a video interview, Martin Tallman, MD, of Memorial Sloan Kettering Cancer Center in New York, discussed several meeting presentations on the treatment of AML.
In his presentation, Craig Jordan, PhD, of the University of Colorado at Denver, Aurora, explained how the combination of venetoclax and azacitidine appears to target leukemic stem cells in AML.
Courtney DiNardo, MD, of the University of Texas MD Anderson Cancer Center, Houston, presented information on novel agents for AML, including antibody-drug conjugates; bispecific therapies; checkpoint inhibitors; and inhibitors of IDH1/2, MCL1, and MDM2.
Richard Larson, MD, of the University of Chicago, explored the possibility of an individualized approach to postremission therapy in AML.
Frederick Appelbaum, MD, of Fred Hutchinson Cancer Research Center in Seattle, showed that various maintenance therapies given after allogeneic hematopoietic stem cell transplant (HSCT) have not proven beneficial for AML patients.
Richard Jones, MD, of Johns Hopkins Medicine in Baltimore, presented data showing that post-HSCT cyclophosphamide has made haploidentical transplants safer and more effective for AML patients.
And James Ferrara, MD, of the Icahn School of Medicine at Mount Sinai, New York, detailed research showing that biomarkers of graft-versus-host disease can predict nonrelapse mortality after HSCT.
The Acute Leukemia Forum is held by Hemedicus, which is owned by the same company as this news organization.
REPORTING FROM ALF 2019
Back to the drawing board for MPN combo
NEWPORT BEACH, CALIF. – The combination of ruxolitinib and decitabine will not proceed to a phase 3 trial in patients with accelerated or blast phase myeloproliferative neoplasms (MPNs).
The combination demonstrated activity and tolerability in a phase 2 trial, but outcomes were not optimal, according to Raajit K. Rampal, MD, PhD, of Memorial Sloan Kettering Cancer Center in New York.
“[P]erhaps the outcomes might be favorable compared to standard induction chemotherapy regimens,” Dr. Rampal said. “Nonetheless, it’s clear that we still have a lot of work to do, and the outcomes are not optimal in these patients.”
However, Dr. Rampal and his colleagues are investigating the possibility of combining ruxolitinib and decitabine with other agents to treat patients with accelerated or blast phase MPNs.
Dr. Rampal and his colleagues presented results from the phase 2 trial in a poster at the Acute Leukemia Forum of Hemedicus.
The trial (NCT02076191) enrolled 25 patients, 10 with accelerated phase MPN (10%-19% blasts) and 15 with blast phase MPN (at least 20% blasts). The patients’ median age was 71 years.
Patients had a median disease duration of 72.9 months. Six patients (25%) had received prior ruxolitinib, and two (8.3%) had received prior decitabine.
Treatment and safety
For the first cycle, patients received decitabine at 20 mg/m2 per day on days 8-12 and ruxolitinib at 25 mg twice a day on days 1-35. For subsequent cycles, patients received the same dose of decitabine on days 1-5 and ruxolitinib at 10 mg twice a day on days 6-28. Patients were treated until progression, withdrawal, or unacceptable toxicity.
“The adverse events we saw in this study were typical for this population, including fevers, mostly neutropenic fevers, as well as anemia and thrombocytopenia,” Dr. Rampal said.
Nonhematologic adverse events (AEs) included fatigue, abdominal pain, pneumonia, diarrhea, dizziness, and constipation. Hematologic AEs included anemia, neutropenia, febrile neutropenia, and thrombocytopenia.
Response and survival
Eighteen patients were evaluable for response. Four patients were not evaluable because they withdrew from the study due to secondary AEs and completed one cycle of therapy or less, two patients did not have circulating blasts at baseline, and one patient refused further treatment.
Among the evaluable patients, nine (50%) achieved a partial response, including four patients with accelerated phase MPN and five with blast phase MPN.
Two patients (11.1%), one with accelerated phase MPN and one with blast phase MPN, achieved a complete response with incomplete count recovery.
The remaining seven patients (38.9%), five with blast phase MPN and two with accelerated phase MPN, did not respond.
The median overall survival was 7.6 months for the entire cohort, 9.7 months for patients with blast phase MPN, and 5.8 months for patients with accelerated phase MPN.
Based on these results, Dr. Rampal and his colleagues theorized that ruxolitinib plus decitabine might be improved by the addition of other agents. The researchers are currently investigating this possibility.
“The work for this trial really came out of preclinical work in the laboratory where we combined these drugs and saw efficacy in murine models,” Dr. Rampal said. “So we’re going back to the drawing board and looking at those again to see, ‘Can we come up with new rational combinations?’ ”
Dr. Rampal and his colleagues reported having no conflicts of interest. Their study was supported by the National Institutes of Health, the National Cancer Institute, and Incyte Corporation.
The Acute Leukemia Forum is held by Hemedicus, which is owned by the same company as this news organization.
NEWPORT BEACH, CALIF. – The combination of ruxolitinib and decitabine will not proceed to a phase 3 trial in patients with accelerated or blast phase myeloproliferative neoplasms (MPNs).
The combination demonstrated activity and tolerability in a phase 2 trial, but outcomes were not optimal, according to Raajit K. Rampal, MD, PhD, of Memorial Sloan Kettering Cancer Center in New York.
“[P]erhaps the outcomes might be favorable compared to standard induction chemotherapy regimens,” Dr. Rampal said. “Nonetheless, it’s clear that we still have a lot of work to do, and the outcomes are not optimal in these patients.”
However, Dr. Rampal and his colleagues are investigating the possibility of combining ruxolitinib and decitabine with other agents to treat patients with accelerated or blast phase MPNs.
Dr. Rampal and his colleagues presented results from the phase 2 trial in a poster at the Acute Leukemia Forum of Hemedicus.
The trial (NCT02076191) enrolled 25 patients, 10 with accelerated phase MPN (10%-19% blasts) and 15 with blast phase MPN (at least 20% blasts). The patients’ median age was 71 years.
Patients had a median disease duration of 72.9 months. Six patients (25%) had received prior ruxolitinib, and two (8.3%) had received prior decitabine.
Treatment and safety
For the first cycle, patients received decitabine at 20 mg/m2 per day on days 8-12 and ruxolitinib at 25 mg twice a day on days 1-35. For subsequent cycles, patients received the same dose of decitabine on days 1-5 and ruxolitinib at 10 mg twice a day on days 6-28. Patients were treated until progression, withdrawal, or unacceptable toxicity.
“The adverse events we saw in this study were typical for this population, including fevers, mostly neutropenic fevers, as well as anemia and thrombocytopenia,” Dr. Rampal said.
Nonhematologic adverse events (AEs) included fatigue, abdominal pain, pneumonia, diarrhea, dizziness, and constipation. Hematologic AEs included anemia, neutropenia, febrile neutropenia, and thrombocytopenia.
Response and survival
Eighteen patients were evaluable for response. Four patients were not evaluable because they withdrew from the study due to secondary AEs and completed one cycle of therapy or less, two patients did not have circulating blasts at baseline, and one patient refused further treatment.
Among the evaluable patients, nine (50%) achieved a partial response, including four patients with accelerated phase MPN and five with blast phase MPN.
Two patients (11.1%), one with accelerated phase MPN and one with blast phase MPN, achieved a complete response with incomplete count recovery.
The remaining seven patients (38.9%), five with blast phase MPN and two with accelerated phase MPN, did not respond.
The median overall survival was 7.6 months for the entire cohort, 9.7 months for patients with blast phase MPN, and 5.8 months for patients with accelerated phase MPN.
Based on these results, Dr. Rampal and his colleagues theorized that ruxolitinib plus decitabine might be improved by the addition of other agents. The researchers are currently investigating this possibility.
“The work for this trial really came out of preclinical work in the laboratory where we combined these drugs and saw efficacy in murine models,” Dr. Rampal said. “So we’re going back to the drawing board and looking at those again to see, ‘Can we come up with new rational combinations?’ ”
Dr. Rampal and his colleagues reported having no conflicts of interest. Their study was supported by the National Institutes of Health, the National Cancer Institute, and Incyte Corporation.
The Acute Leukemia Forum is held by Hemedicus, which is owned by the same company as this news organization.
NEWPORT BEACH, CALIF. – The combination of ruxolitinib and decitabine will not proceed to a phase 3 trial in patients with accelerated or blast phase myeloproliferative neoplasms (MPNs).
The combination demonstrated activity and tolerability in a phase 2 trial, but outcomes were not optimal, according to Raajit K. Rampal, MD, PhD, of Memorial Sloan Kettering Cancer Center in New York.
“[P]erhaps the outcomes might be favorable compared to standard induction chemotherapy regimens,” Dr. Rampal said. “Nonetheless, it’s clear that we still have a lot of work to do, and the outcomes are not optimal in these patients.”
However, Dr. Rampal and his colleagues are investigating the possibility of combining ruxolitinib and decitabine with other agents to treat patients with accelerated or blast phase MPNs.
Dr. Rampal and his colleagues presented results from the phase 2 trial in a poster at the Acute Leukemia Forum of Hemedicus.
The trial (NCT02076191) enrolled 25 patients, 10 with accelerated phase MPN (10%-19% blasts) and 15 with blast phase MPN (at least 20% blasts). The patients’ median age was 71 years.
Patients had a median disease duration of 72.9 months. Six patients (25%) had received prior ruxolitinib, and two (8.3%) had received prior decitabine.
Treatment and safety
For the first cycle, patients received decitabine at 20 mg/m2 per day on days 8-12 and ruxolitinib at 25 mg twice a day on days 1-35. For subsequent cycles, patients received the same dose of decitabine on days 1-5 and ruxolitinib at 10 mg twice a day on days 6-28. Patients were treated until progression, withdrawal, or unacceptable toxicity.
“The adverse events we saw in this study were typical for this population, including fevers, mostly neutropenic fevers, as well as anemia and thrombocytopenia,” Dr. Rampal said.
Nonhematologic adverse events (AEs) included fatigue, abdominal pain, pneumonia, diarrhea, dizziness, and constipation. Hematologic AEs included anemia, neutropenia, febrile neutropenia, and thrombocytopenia.
Response and survival
Eighteen patients were evaluable for response. Four patients were not evaluable because they withdrew from the study due to secondary AEs and completed one cycle of therapy or less, two patients did not have circulating blasts at baseline, and one patient refused further treatment.
Among the evaluable patients, nine (50%) achieved a partial response, including four patients with accelerated phase MPN and five with blast phase MPN.
Two patients (11.1%), one with accelerated phase MPN and one with blast phase MPN, achieved a complete response with incomplete count recovery.
The remaining seven patients (38.9%), five with blast phase MPN and two with accelerated phase MPN, did not respond.
The median overall survival was 7.6 months for the entire cohort, 9.7 months for patients with blast phase MPN, and 5.8 months for patients with accelerated phase MPN.
Based on these results, Dr. Rampal and his colleagues theorized that ruxolitinib plus decitabine might be improved by the addition of other agents. The researchers are currently investigating this possibility.
“The work for this trial really came out of preclinical work in the laboratory where we combined these drugs and saw efficacy in murine models,” Dr. Rampal said. “So we’re going back to the drawing board and looking at those again to see, ‘Can we come up with new rational combinations?’ ”
Dr. Rampal and his colleagues reported having no conflicts of interest. Their study was supported by the National Institutes of Health, the National Cancer Institute, and Incyte Corporation.
The Acute Leukemia Forum is held by Hemedicus, which is owned by the same company as this news organization.
REPORTING FROM ALF 2019
Creating CAR T-cell therapies for T-cell malignancies
NEWPORT BEACH, CALIF. – Preclinical research has revealed workarounds that may make chimeric antigen receptor (CAR) T-cell therapy feasible for patients with T-cell malignancies.
Researchers have found that using allogeneic cells for CAR T-cell therapy can eliminate contamination by malignant T cells, and editing those allogeneic T cells to delete the target antigen and the T-cell receptor alpha chain (TRAC) can prevent fratricide and graft-versus-host disease (GVHD).
Additionally, an interleukin-7 molecule called NT-I7 has been shown to enhance CAR T-cell proliferation, differentiation, and tumor killing in a mouse model of a T-cell malignancy.
John F. DiPersio, MD, PhD, of Washington University in St. Louis, described this work in a presentation at the Acute Leukemia Forum of Hemedicus.
Obstacles to development
“The primary obstacle for targeting T-cell malignancies with a T cell is that all of the targets that are on the [malignant] T cells are also expressed on the normal T cells,” Dr. DiPersio said. “So when you put a CAR into a normal T cell, it just kills itself. It’s called fratricide.”
A second issue that has limited development is that the phenotype of the malignant T cell in the blood is similar to a normal T cell, so they can’t be separated, he explained.
“So if you were to do anything to a normal T cell, you would also be doing it, in theory, to the malignant T cell – in theory, making it resistant to therapy,” he said.
A third obstacle, which has been seen in patients with B-cell malignancies as well, is the inability to harvest enough T cells to generate effective CAR T-cell therapy.
And a fourth obstacle is that T cells from patients with malignancies may not function normally because they have been exposed to prior therapies.
Dr. DiPersio and his colleagues believe these obstacles can be overcome by creating CAR T-cell therapies using T cells derived from healthy donors or cord blood, using gene editing to remove the target antigen and TRAC, and using NT-I7 to enhance the efficacy of these universal, “off-the-shelf” CAR T cells.
The researchers have tested these theories, and achieved successes, in preclinical models. The team is now planning a clinical trial in patients at Washington University. Dr. DiPersio and his colleagues also created a company called WUGEN that will develop the universal CAR T-cell therapies if the initial proof-of-principle trial proves successful.
UCART7
One of the universal CAR T-cell therapies Dr. DiPersio and his colleagues have tested is UCART7, which targets CD7. Dr. DiPersio noted that CD7 is expressed on 98% of T-cell acute lymphoblastic leukemias (T-ALLs), 24% of acute myeloid leukemias, natural killer (NK) cells, and T cells.
The researchers created UCART7 by using CRISPR/Cas9 to delete CD7 and TRAC from allogeneic T cells and following this with lentiviral transduction with a third-generation CD7-CAR. The team found a way to delete both TRAC and CD7 in a single day with 95% efficiency, Dr. DiPersio noted.
“Knocking out CD7 doesn’t seem to have any impact on the expansion or trafficking of these T cells in vivo,” Dr. DiPersio said. “So we think that deleting that target in a normal T cell will not affect its overall ability to kill a target when we put a CAR into those T cells.”
In fact, the researchers’ experiments showed that UCART7 can kill T-ALL cells in vitro and target primary T-ALL in vivo without inducing GVHD (Leukemia. 2018 Sep;32[9]:1970-83.)
UCART2 and NT-I7
Dr. DiPersio and his colleagues have also tested UCART2, an allogeneic CAR T-cell therapy in which CD2 and TRAC are deleted. The therapy targets CD2 because this antigen is expressed on T-ALL and other T-cell and NK-cell malignancies. Experiments showed that UCART2 targets T-cell malignancies, including T-ALL and cutaneous T-cell lymphoma, in vitro.
The researchers also tested UCART2 in a mouse model of Sézary syndrome. In these experiments, UCART2 was combined with NT-I7.
NT-I7 enhanced the proliferation, persistence, and tumor killing ability of UCART2. Sézary mice that received UCART2 and NT-I7 had “virtually no tumor burden,” according to researchers, and survived longer than mice treated with UCART2 alone (Blood. 2018;132:340).
Dr. DiPersio noted that there was no cytokine release syndrome because these were immunodeficient mice. However, cytokine release syndrome may be a side effect of NT-I7 in patients as NT-I7 induces rapid expansion of CAR T cells.
Dr. DiPersio reported ownership and investment in WUGEN and Magenta Therapeutics. He also has relationships with Cellworks Group, Tioma Therapeutics, RiverVest Venture Partners, Bioline, Asterias Biotherapeutics, Amphivena Therapeutics, Bluebird Bio, Celgene, Incyte, NeoImuneTech, and MacroGenics.
The Acute Leukemia Forum is organized by Hemedicus, which is owned by the same company as this news organization.
NEWPORT BEACH, CALIF. – Preclinical research has revealed workarounds that may make chimeric antigen receptor (CAR) T-cell therapy feasible for patients with T-cell malignancies.
Researchers have found that using allogeneic cells for CAR T-cell therapy can eliminate contamination by malignant T cells, and editing those allogeneic T cells to delete the target antigen and the T-cell receptor alpha chain (TRAC) can prevent fratricide and graft-versus-host disease (GVHD).
Additionally, an interleukin-7 molecule called NT-I7 has been shown to enhance CAR T-cell proliferation, differentiation, and tumor killing in a mouse model of a T-cell malignancy.
John F. DiPersio, MD, PhD, of Washington University in St. Louis, described this work in a presentation at the Acute Leukemia Forum of Hemedicus.
Obstacles to development
“The primary obstacle for targeting T-cell malignancies with a T cell is that all of the targets that are on the [malignant] T cells are also expressed on the normal T cells,” Dr. DiPersio said. “So when you put a CAR into a normal T cell, it just kills itself. It’s called fratricide.”
A second issue that has limited development is that the phenotype of the malignant T cell in the blood is similar to a normal T cell, so they can’t be separated, he explained.
“So if you were to do anything to a normal T cell, you would also be doing it, in theory, to the malignant T cell – in theory, making it resistant to therapy,” he said.
A third obstacle, which has been seen in patients with B-cell malignancies as well, is the inability to harvest enough T cells to generate effective CAR T-cell therapy.
And a fourth obstacle is that T cells from patients with malignancies may not function normally because they have been exposed to prior therapies.
Dr. DiPersio and his colleagues believe these obstacles can be overcome by creating CAR T-cell therapies using T cells derived from healthy donors or cord blood, using gene editing to remove the target antigen and TRAC, and using NT-I7 to enhance the efficacy of these universal, “off-the-shelf” CAR T cells.
The researchers have tested these theories, and achieved successes, in preclinical models. The team is now planning a clinical trial in patients at Washington University. Dr. DiPersio and his colleagues also created a company called WUGEN that will develop the universal CAR T-cell therapies if the initial proof-of-principle trial proves successful.
UCART7
One of the universal CAR T-cell therapies Dr. DiPersio and his colleagues have tested is UCART7, which targets CD7. Dr. DiPersio noted that CD7 is expressed on 98% of T-cell acute lymphoblastic leukemias (T-ALLs), 24% of acute myeloid leukemias, natural killer (NK) cells, and T cells.
The researchers created UCART7 by using CRISPR/Cas9 to delete CD7 and TRAC from allogeneic T cells and following this with lentiviral transduction with a third-generation CD7-CAR. The team found a way to delete both TRAC and CD7 in a single day with 95% efficiency, Dr. DiPersio noted.
“Knocking out CD7 doesn’t seem to have any impact on the expansion or trafficking of these T cells in vivo,” Dr. DiPersio said. “So we think that deleting that target in a normal T cell will not affect its overall ability to kill a target when we put a CAR into those T cells.”
In fact, the researchers’ experiments showed that UCART7 can kill T-ALL cells in vitro and target primary T-ALL in vivo without inducing GVHD (Leukemia. 2018 Sep;32[9]:1970-83.)
UCART2 and NT-I7
Dr. DiPersio and his colleagues have also tested UCART2, an allogeneic CAR T-cell therapy in which CD2 and TRAC are deleted. The therapy targets CD2 because this antigen is expressed on T-ALL and other T-cell and NK-cell malignancies. Experiments showed that UCART2 targets T-cell malignancies, including T-ALL and cutaneous T-cell lymphoma, in vitro.
The researchers also tested UCART2 in a mouse model of Sézary syndrome. In these experiments, UCART2 was combined with NT-I7.
NT-I7 enhanced the proliferation, persistence, and tumor killing ability of UCART2. Sézary mice that received UCART2 and NT-I7 had “virtually no tumor burden,” according to researchers, and survived longer than mice treated with UCART2 alone (Blood. 2018;132:340).
Dr. DiPersio noted that there was no cytokine release syndrome because these were immunodeficient mice. However, cytokine release syndrome may be a side effect of NT-I7 in patients as NT-I7 induces rapid expansion of CAR T cells.
Dr. DiPersio reported ownership and investment in WUGEN and Magenta Therapeutics. He also has relationships with Cellworks Group, Tioma Therapeutics, RiverVest Venture Partners, Bioline, Asterias Biotherapeutics, Amphivena Therapeutics, Bluebird Bio, Celgene, Incyte, NeoImuneTech, and MacroGenics.
The Acute Leukemia Forum is organized by Hemedicus, which is owned by the same company as this news organization.
NEWPORT BEACH, CALIF. – Preclinical research has revealed workarounds that may make chimeric antigen receptor (CAR) T-cell therapy feasible for patients with T-cell malignancies.
Researchers have found that using allogeneic cells for CAR T-cell therapy can eliminate contamination by malignant T cells, and editing those allogeneic T cells to delete the target antigen and the T-cell receptor alpha chain (TRAC) can prevent fratricide and graft-versus-host disease (GVHD).
Additionally, an interleukin-7 molecule called NT-I7 has been shown to enhance CAR T-cell proliferation, differentiation, and tumor killing in a mouse model of a T-cell malignancy.
John F. DiPersio, MD, PhD, of Washington University in St. Louis, described this work in a presentation at the Acute Leukemia Forum of Hemedicus.
Obstacles to development
“The primary obstacle for targeting T-cell malignancies with a T cell is that all of the targets that are on the [malignant] T cells are also expressed on the normal T cells,” Dr. DiPersio said. “So when you put a CAR into a normal T cell, it just kills itself. It’s called fratricide.”
A second issue that has limited development is that the phenotype of the malignant T cell in the blood is similar to a normal T cell, so they can’t be separated, he explained.
“So if you were to do anything to a normal T cell, you would also be doing it, in theory, to the malignant T cell – in theory, making it resistant to therapy,” he said.
A third obstacle, which has been seen in patients with B-cell malignancies as well, is the inability to harvest enough T cells to generate effective CAR T-cell therapy.
And a fourth obstacle is that T cells from patients with malignancies may not function normally because they have been exposed to prior therapies.
Dr. DiPersio and his colleagues believe these obstacles can be overcome by creating CAR T-cell therapies using T cells derived from healthy donors or cord blood, using gene editing to remove the target antigen and TRAC, and using NT-I7 to enhance the efficacy of these universal, “off-the-shelf” CAR T cells.
The researchers have tested these theories, and achieved successes, in preclinical models. The team is now planning a clinical trial in patients at Washington University. Dr. DiPersio and his colleagues also created a company called WUGEN that will develop the universal CAR T-cell therapies if the initial proof-of-principle trial proves successful.
UCART7
One of the universal CAR T-cell therapies Dr. DiPersio and his colleagues have tested is UCART7, which targets CD7. Dr. DiPersio noted that CD7 is expressed on 98% of T-cell acute lymphoblastic leukemias (T-ALLs), 24% of acute myeloid leukemias, natural killer (NK) cells, and T cells.
The researchers created UCART7 by using CRISPR/Cas9 to delete CD7 and TRAC from allogeneic T cells and following this with lentiviral transduction with a third-generation CD7-CAR. The team found a way to delete both TRAC and CD7 in a single day with 95% efficiency, Dr. DiPersio noted.
“Knocking out CD7 doesn’t seem to have any impact on the expansion or trafficking of these T cells in vivo,” Dr. DiPersio said. “So we think that deleting that target in a normal T cell will not affect its overall ability to kill a target when we put a CAR into those T cells.”
In fact, the researchers’ experiments showed that UCART7 can kill T-ALL cells in vitro and target primary T-ALL in vivo without inducing GVHD (Leukemia. 2018 Sep;32[9]:1970-83.)
UCART2 and NT-I7
Dr. DiPersio and his colleagues have also tested UCART2, an allogeneic CAR T-cell therapy in which CD2 and TRAC are deleted. The therapy targets CD2 because this antigen is expressed on T-ALL and other T-cell and NK-cell malignancies. Experiments showed that UCART2 targets T-cell malignancies, including T-ALL and cutaneous T-cell lymphoma, in vitro.
The researchers also tested UCART2 in a mouse model of Sézary syndrome. In these experiments, UCART2 was combined with NT-I7.
NT-I7 enhanced the proliferation, persistence, and tumor killing ability of UCART2. Sézary mice that received UCART2 and NT-I7 had “virtually no tumor burden,” according to researchers, and survived longer than mice treated with UCART2 alone (Blood. 2018;132:340).
Dr. DiPersio noted that there was no cytokine release syndrome because these were immunodeficient mice. However, cytokine release syndrome may be a side effect of NT-I7 in patients as NT-I7 induces rapid expansion of CAR T cells.
Dr. DiPersio reported ownership and investment in WUGEN and Magenta Therapeutics. He also has relationships with Cellworks Group, Tioma Therapeutics, RiverVest Venture Partners, Bioline, Asterias Biotherapeutics, Amphivena Therapeutics, Bluebird Bio, Celgene, Incyte, NeoImuneTech, and MacroGenics.
The Acute Leukemia Forum is organized by Hemedicus, which is owned by the same company as this news organization.
EXPERT ANALYSIS FROM ALF 2019








