Conference Coverage
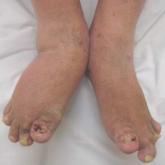
Psoriatic flare assessment tool in validation stage
MIAMI – Reaching consensus on measurement and assessment of flare in psoriatic arthritis remains challenging, especially with no widely accepted...
FROM JOURNAL OF RHEUMATOLOGY
A report on the largest cohort to date of patients with psoriatic arthritis who discontinued tumor necrosis factor inhibitor (TNFi) therapy during a period of low disease activity adds further evidence in support of keeping patients on the biologics during such periods.
The findings from the report on patients with psoriatic arthritis (PsA) from the U.S. Corrona registry found a rebound in disease activity in 69 (73%) of 94 patients. These results are in line with prior small studies, including a pilot, randomized, controlled trial, in PsA patients who have attempted to stop conventional synthetic disease-modifying antirheumatic drugs and biologic DMARDs, mostly TNFi agents, during periods of low disease activity (LDA).
Despite the findings from these studies, first author of the Corrona registry study, Leslie R. Harrold, MD, of the University of Massachusetts, Worcester, said in an interview that it would be premature to recommend to patients that they cannot stop TNFi therapy when they have LDA. The Corrona study provides a context for clinicians to have conversations with patients and to discuss the risks, she said.The 73% rate of disease activity rebound observed in the patients – defined by a Clinical Disease Activity Index (CDAI) score of more than 10, a reinitiation of their TNFi, or the start of a new agent – “offers an important perspective for physicians who are considering TNFi discontinuation for patients who have achieved remission/LDA and may provide a key piece of data in discussing the most likely potential outcome with their patients’ TNFi discontinuation,” Dr. Harrold and her associates wrote. “TNFi discontinuation after achieving LDA should be carefully considered.”
The 94 patients with PsA in the Corrona registry study all met study criteria for remission or LDA (CDAI score of 10 or less) and had discontinued their TNFi while in LDA and did not immediately switch to a different biologic. The mean age of the patients was 51; 57% were women and 92% were white. At the time of TNFi initiation, they had an average disease duration of 8.4 years and a mean CDAI score of 10.1, and 64% had LDA. Most patients had taken methotrexate (86%) or a conventional synthetic DMARD (10%) prior to starting a TNFi (J Rheumatol. 2017 Oct 1. doi: 10.3899/jrheum.161567).
Among the 69 patients with rebound of disease activity, 15 had a CDAI score of greater than 10, 24 restarted a biologic DMARD but did not have an elevated CDAI, and 30 had both an elevated CDAI and biologic initiation.
Close to three-quarters of the 59 rebounders who returned for a postrebound visit were in remission or LDA at that visit, and nearly half had resumed use of their original TNFi by the time of the visit.
The U.S.-based Corrona registry has registered about 6,000 PsA patients from around 500 rheumatologists since 2001. The conclusions that can be drawn from the current study, which included patients who started a TNFi between Oct. 1, 2002, and March 21, 2013, are limited by the use of the CDAI, a measure for disease activity in rheumatoid arthritis. The use of the CDAI means that disease features of PsA such as enthesitis, psoriasis, and dactylitis were not captured in the assessment of LDA. “That is a caveat,” Dr. Harrold said.
Because the CDAI doesn’t measure across the spectrum of PsA disease, some of the patients may have had active disease in entheses or skin or relapsed in those domains but not the joints, noted Philip Helliwell, MD, senior lecturer in rheumatology at the University of Leeds (England) and honorary consultant rheumatologist for the Bradford (England) Teaching Hospitals NHS Trust, who was not involved in the study.Other limitations of the study include its sporadic follow-up and the open-label, uncontrolled nature of the TNFi discontinuation, according to Dr. Helliwell.
“There could have been any number of reasons why the drug was stopped, not just because of low disease activity – because of financial reasons, perhaps, adverse events, or ... a switch to another drug,” he said in an interview.
Despite these limitations, Dr. Helliwell said that the study “provides useful data, and it will add to the number of publications on this subject that are already out there, and it is the biggest cohort that’s been published.”
Dr. Harrold and her coauthors said that their findings were consistent with those from other studies, including one that Dr. Helliwell’s group was a part of.
That was a pilot randomized, controlled trial to test the feasibility of drug withdrawal in PsA patients in a stable, low disease state, defined by meeting minimal disease activity criteria. Of the 17 patients randomized, 6 of 11 who were in the withdrawal arm experienced a flare (defined as not meeting minimal disease activity criteria). Of these six patients, two had been receiving methotrexate only and four had been treated with methotrexate plus a biologic, whereas none of the six patients who continued therapy experienced a flare (Clin Rheumatol. 2015;34[8]:1407-12). Dr. Helliwell noted that all patients in the withdrawal arm eventually relapsed.
Another study with similar results from investigators at the University of Erlangen-Nuremberg (Germany) found that out of 26 PsA patients on either methotrexate monotherapy (n = 14) or a TNFi (n = 12), with no musculoskeletal symptoms and minimal skin disease, 20 had disease recurrence at a mean of just 74 days after drug discontinuation (Ann Rheum Dis. 2015;74:655-60).
Investigators for another study from the Corrona registry looked at 325 discontinuations of TNFi therapy while in LDA in 302 PsA patients and found disease rebound in 45% at an estimated median time of about 29 months. This study included a physician’s global assessment of skin psoriasis of 20 or less out of 100 as part of the definition of LDA, in addition to a CDAI score of 10 or less. Current smoking was the only significant predictor of disease rebound in a multivariate analysis. Age, gender, the number of prior TNFis used, or overweight or obese status did not predict disease rebound. CDAI score of 3.2 or greater was just outside the range of statistical significance (RMD Open. 2017;3:e000395).
“I think the message here is: ‘Be very careful discontinuing anti-TNF drugs in people with PsA and low disease activity.’ It’s not the same as rheumatoid arthritis in that respect,” Dr. Helliwell said.
Ultimately, the goal is to determine the characteristics of patients who can either stop or taper drugs such as TNF inhibitors. “I think there are going to be patients who can be tapered off, and there are going to be patients who can’t be,” Dr. Harrold said. Going forward, it will be important to identify which subset of patients are going to flare when taken off medication, such as a TNF inhibitor, particularly because of the diversity of presentations that PsA patients can have, she said.
Dr. Helliwell agreed, saying that, when he is asked whether you can stop a TNFi in PsA patients with LDA, “I say you should taper the drug by increasing the interval between doses or lower the dose, but not discontinue it.”
The Corrona registry has been supported by numerous pharmaceutical companies that manufacture drugs for PsA and rheumatoid arthritis. Dr. Harrold and some of her coinvestigators are employees and shareholders of Corrona. Some authors are employees and shareholders of Amgen, and others reported financial relationships with various pharmaceutical companies. Dr. Helliwell has received grant or research support, consulted for, or served on the speakers bureau for many of the companies that support Corrona.

MIAMI – Reaching consensus on measurement and assessment of flare in psoriatic arthritis remains challenging, especially with no widely accepted...
