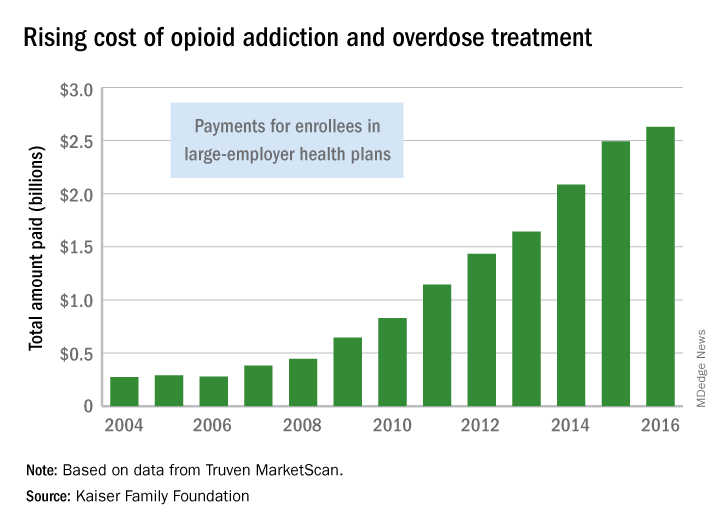
Federal agencies and leaders are taking a multipronged approach to the opioid crisis.
Efforts by the Centers for Medicare & Medicaid Services focus on changes to the Medicare Part D program and Medicare Advantage, via two policy changes issued April 2: The Part D/Medicare Advantage annual update and final calendar year 2019 guidance to insurers who provide coverage under those two programs.
The annual Part D/Medicare Advantage update implements provisions of the Comprehensive Addiction and Recovery Act of 2016 (S. 524), requiring “CMS to establish through regulation a framework that allows Part D sponsors to implement drug management programs,” according to a CMS fact sheet. “Under such programs, a sponsor can limit at-risk beneficiaries’ access to coverage for frequently abused drugs beginning with the 2019 plan year.” The update designates opioids and benzodiazepines as frequently abused drugs.
If a Part D beneficiary is determined to be at-risk, his or her insurer will be able to “lock in” specific prescribers and pharmacies from which he or she can obtain opioids or benzodiazepines.The agency is taking the further step of limiting special enrollment periods for dual eligibles – those beneficiaries eligible for both Medicare and Medicaid – if they are identified as at-risk or potentially at-risk for prescription drug abuse under the programs. Beneficiaries maintain the right to challenge the determination.
Patients who are being treated for cancer-related pain and those receiving hospice/palliative/end-of-life care are exempted from the drug management programs.
The policies also update opioid prescription limits. CMS is calling on “all Part D sponsors to implement a hard safety edit to limit initial opioid prescription fills for the treatment of acute pain to no more than a 7-day supply,” according to the fact sheet.