In this Update, I outline important findings from several studies published in the past year. First and foremost, what are best practices for performing colposcopy in the United States? The American Society for Colposcopy and Cervical Pathology (ASCCP) released guidelines addressing such practices. Second, what are the implications of repeated negative screening and patients’ acceptance of extended screening intervals? A recent observational cohort study and a large study of Kaiser Permanente’s practices since 2003 shed light on these questions. Last, where do we stand with HPV vaccination? Two studies shed light on the efficacy of vaccination against human papillomavirus (HPV), and subsequent cervical intraepithelial neoplasia (CIN) and cervical cancer.
ASCCP releases updated quality guidelines for performing colposcopy
Khan MJ, Werner CL, Darragh TM, et al. ASCCP colposcopy standards: Role of colposcopy, benefits, potential harms, and terminology for colposcopic practice. J Low Genit Tract Dis. 2017;21(4):223-229.
Waxman AG, Conageski C, Silver MI, et al. ASCCP colposcopy standards: How do we perform colposcopy? Implications for establishing standards. J Low Genit Tract Dis. 2017;21(4):235-241.
Wentzensen N, Schiffman M, Silver MI, et al. ASCCP colposcopy standards: Risk-based colposcopy practice. J Low Genit Tract Dis. 2017;21(4):230-234.
In October 2017, the ASCCP released a set of standards on the role and performance of colposcopy that represents best practices in women's health care in the United States. The work of these groups comprised a literature search, a national survey of ASCCP members, public comment, and expert consensus, and addressed:
- establishment of a common understanding of 1) the benefits of colposcopy in health maintenance and risk prevention, 2) risks presented by the procedure, and 3) terminology and criteria for reporting results that reduce subjectivity in reporting
- the rationale for, approach to, and recommendations regarding assessment of cervical precancer at colposcopy
- both minimum and comprehensive guidelines for the colposcopic examination, from preprocedure evaluation to follow-up.
Each Working Group performed the analysis and produced its own report and recommendations, published sequentially in a 2017 issue of the Journal of Lower Urinary Tract Disease. The findings and standards that they produced 1) offer essential insight for high- and low-volume coloposcopists and 2) are intended to improve the quality of colposcopy, reduce subjectivity in reporting findings, and improve the sensitivity of the procedure. Aware of the concerns and objectives of payers and hospital credentialing committees, the ASCCP found it important to establish what would be considered US-based minimum quality standards and to present goals that providers and systems could strive to achieve.
Selected details of the 3 guideline reports
The past 6 years have brought us through a great deal of transition in the prevention of cervical precancer, with regard to screening intervals and types of screening (for example, see "HPV−cytology co-testing every 3 years lowers population rates of cervical precancer and cancer," in the 2017 "Cervical Disease Update," OBG Management, May 2017). The most significant change was in 2012, when American Cancer Society/ASCCP guidelines were revised to abandon screening with annual Pap testing on most patients--an effort to strike a balance between the lifesaving value of identifying precancer and the potential harm of excessive colposcopy.
If, as the US Preventive Services Task Force (USPSTF) has declared, excessive colposcopy is a harm of screening, then we should be adapting our practices, especially in terms of the frequency of screening, to 1) reduce the risk of unnecessarily screening and potentially triaging patients to colposcopy and 2) bring the highest standards of performance and reporting to colposcopic practice (see "Why aren't you doing a Pap on me?"). In other words, "This is the way I've always done it" shouldn't characterize efforts to detect disease, when the data are clear that doing less might be more beneficial for our patients. Adherence to extended screening intervals is not yet good enough to balance benefit and risk of harm, as Rendle and colleagues showed in an article this year in Preventive Medicine (discussed in the next section of this "Update"). We need to do better.
Adherence to extended screening intervals means fewer colposcopies and less exposure to risk of attendant harm. But adherence is not purely mechanical: It can be intertwined with how patients feel about the care we provide and about their safety. When a patient moves from years of annual Pap testing to less frequent screening, she might express her concern by challenging your expertise.
In my practice, I have a simple, 1-minute conversation with the patient that is important to wedge into our discussion of her care. I explain that increasing the frequency of screening is only going to increase the chance that I will perform a colposcopy but not increase the chance that I will identify cancer. I conclude by reassuring her that I do not want to harm her, or to cause her anxiety, pain, cramping, or bleeding--or require her to spend time away from work or show her family that she is suffering. Patients are reassured and happy after that, I find. This is a patient-centered discussion that providers need to have if they hope to establish and maintain adherence to recommended screening intervals.
-- Mark H. Einstein, MD, MS
Here is a limited encapsulation of the 3 wide-ranging reports on the ASCCP colposcopy recommendations:
Role of colposcopy; benefits, potential harms, terminology (Khan et al; Working Group 1). The authors provide reinforcement: The strategic benefit of colposcopy is clear--a "drastic" reduction in excisional procedures by limiting them to patients in whom cervical cancer precursors have been confirmed or who present a high risk of occult invasive cervical cancer. Furthermore, the rate of adverse events for colposcopy−including significant bleeding and infection−is low.
Nevertheless, the potential for harm exists when an unskilled provider performs colposcopy; the Working Group emphasizes that proficiency comes with training and experience. Even in skilled hands, however, anxiety and the discomfort of a speculum examination and from acetic acid, as well as cramping and pain, might dissuade some women from receiving regular cervical screening subsequently. The authors cite research showing that educational interventions can help soothe anxiety about colposcopy and potential findings,1,2 although consensus is lacking on the value of such interventions.
The Working Group 1) developed recommended terminology for reporting findings in colposcopy practice in the United States and 2) defined the comprehensive documentation of the procedure as comprising cervix and squamocolumnar junction visibility; acetowhitening; presence of a lesion; lesion visibility, size and location of lesion(s); vascular changes; other features; and colposcopic impression (TABLE 1).3 Minimum criteria for reporting colposcopy results were also proposed, extracted from the comprehensive standards.
Risk-based colposcopy practice (Wentzensen et al). Women referred to colposcopy present with a range of underlying risk of precancer. Assessing that risk at the colposcopy visit allows the provider to modify and individualize the procedure. Risk can be estimated by referral screening tests (eg, cytology, HPV testing) performed in conjunction with the colposcopic impression. As opposed to a lack of standards for a minimum number of biopsies, the Working Group recommends that, as a standard, multiple targeted biopsies (≥2, as many as 4) are needed to improve detection of prevalent precancers. Colposcopic impression alone is not enough to diagnose precancerous cells. Let's face it: Our eyes with a colposcopic magnification of 15X do not make a microscope.
Implementing the Working Group's recommendations is expected to lead to improved detection of cervical precancers at colposcopy and to provide stronger reassurance of negative colposcopy results. Regarding biopsy of lesions, ASCCP did not find added benefit to taking random (nondirected) biopsies for women at low risk for precancer. The sensitivity of biopsy is increased by taking multiple biopsies of suspicious lesions, based on a risk-based approach detailed in the ASCCP guidelines. So, depending on underlying risk (estimated from screening and triage tests), colposcopy practice can be adapted in a useful manner to account for differences in risk:
- When risk of precancer is very high, for example, immediate treatment might reduce cost and prevent the patient from being lost to follow-up. When risk is very low, consider expectant management (serial cytology and HPV testing) with limited need for biopsy. In a setting of intermediate risk, the Working Group proposes, "multiple biopsies of acetowhite lesions lead to increased detection of precancer."
- Perform multiple biopsies that target all areas characterized by 1) acetowhitening, 2) metaplasia, and 3) higher abnormalities.
- Do not perform nontargeted biopsies on patients at the lowest end of risk who have been referred to colposcopy−ie, those with cytology that is less than HSIL; no evidence of HPV types 16/18; and a normal colposcopic impression (ie, no acetowhitening or metaplasia, or other visible abnormality).
- Immediate excision without biopsy confirmation or colposcopy with multiple targeted biopsies is acceptable in nonpregnant women 25 years and older whose risk of precancer is very high (≥2 of the following: HSIL cytology, HPV 16- or HPV 18-positive(or both), and high-grade colposcopy impression). Endocervical sampling should be conducted according to ASCCP's 2012 management guidelines. If biopsies do not show precancer, manage the patient using ASCCP's 2012 management guidelines, the Working Group recommends.
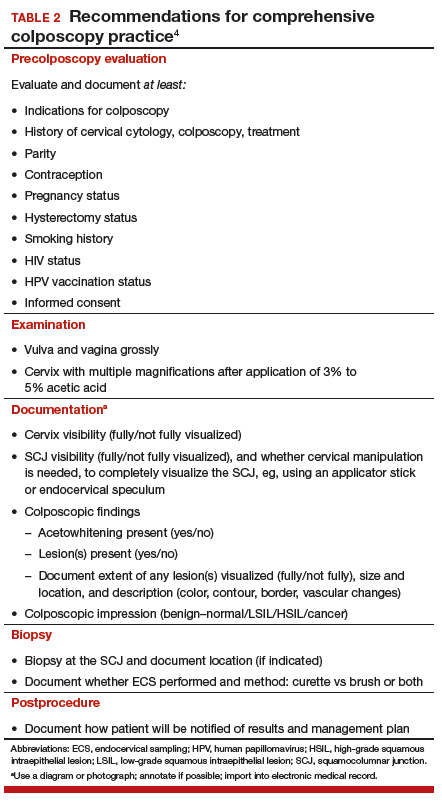
How do we perform colposcopy? Implications for establishing standards (Waxman et al; Working Group 3). To serve as a guide to standardizing colposcopy across the United States, the authors defined and delineated 6 major components (and their constituent parts) of a comprehensive colposcopy:
- precolposcopy evaluation
- the examination
- use of colposcopy adjuncts
- documentation
- biopsy sampling
- postcolposcopy procedures.
The constituent parts of these components are laid out in TABLE 2.4 A set of components for a minimum colposcopy procedure is drawn mostly from the comprehensive protocol.
The Working Group acknowledges that, in the United States, "the accuracy and reproducibility of colposcopy with biopsy as a diagnostic tool are limited." Why? Three contributing factors, the authors write, might be the absence of practice recommendations for colposcopy-biopsy procedures; of measures of quality assurance; and of standardized terminology.
Standards arrive for practice
Minimum quality standards are becoming part of almost everything US health care providers do−whether it is documentation, billing practices, or good care. Our work in gynecology, including colposcopy, is now being assessed as it is in much of the world, where minimum standards are already in place and guidelines must be followed. (In some countries standards require performing a minimum number of colposcopies per year to be identified as a "certified" colposcopist.)
What should be considered "minimum standards" for colposcopy in the United States? These ASCCP reports ask, and deliver answers to that question, bringing a broad range of concerns about high-quality practice into focus. Physicians and advanced-practice clinicians in this country who perform colposcopies have been trained to do so, but they have never had minimum standards by which to model and assess their performance. A procedure that has the potential to lead to additional testing for either cervical cancer, or to surveillance, should have minimum standards by which it is performed and documented in the United States as it is for much of the world that has widespread cervical cancer screening.
Guidance and recommendations developed by ASCCP offer women's health care providers a set of comprehensive and, alternatively, minimum quality standards that should be incorporated into practice across all aspects of the colposcopic exam, including precolposcopy evaluation, how to perform the procedure, how to document and report findings (TABLE 2), biopsy practice, establish quality control and assurance, as well as postprocedure follow-up. In taking the initiative to draw up these standards, ASCCP encourages providers to exceed the minimum requirements.