SAN ANTONIO – It’s still too soon to know whether neurostimulation will be the therapeutic advance that treatment-refractory epilepsy patients have been waiting for, but the possibility that it might be has the epilepsy community buzzing.
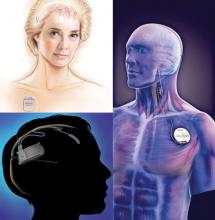 Photo credit: top left, © Medtronic Inc.; bottom left, © NeuroPace Inc.; right, © Cyberonics Inc.
Photo credit: top left, © Medtronic Inc.; bottom left, © NeuroPace Inc.; right, © Cyberonics Inc.
Medtronic's DBS system (top left) stimulates the anterior nucleus of the thalamus, whereas NeuroPace's RNS device (bottom left) responds to abnormal activity in targeted areas, and Cyberonics' VNS therapy system (right) periodically stimulates the left vagus nerve.
For approximately 30% of epilepsy patients, seizures cannot be controlled with antiepileptic drugs or surgery. New long-term safety and efficacy data for vagus nerve simulation (VNS) and the results of recent pivotal trials of two approaches to direct brain stimulation offer beacons of hope to these patients, Dr. Gregory K. Bergey said in a plenary session on neurostimulation at the annual meeting of the American Epilepsy Society.
"One of the frustrating things for those of us treating patients with epilepsy has been the fact that, although a number of new antiepileptic drugs have been developed over the past 10-15 years and most are better tolerated and have better pharmacokinetic profiles than earlier drugs, the number of patients with seizures that don’t respond to medical therapy has not been significantly reduced," said Dr. Bergey, director of the Johns Hopkins Epilepsy Center, Baltimore.
"So we’re stepping back and saying, ‘Is there some other way we can treat these patients?’ That has been the impetus for looking at neurostimulation, which has been around for well over a decade, and what we’re seeing is exciting."
Although the 40%-50% response rates observed in direct brain stimulation trials do not appear to be overwhelming, "this is just the beginning," Dr. Bergey stressed in an interview. "As opposed to a drug trial, where you go up to a certain dose and it either works or it doesn’t work, in the case of neurostimulation we don’t know the optimal stimulus parameters, and I think that’s what you’re going to begin to see over the next several years," he said.
"There’s going to be a lot of investigation into neurostimulation of the brain structures to try to figure out who are the best candidates and what the best stimulus parameters are. It’s easy to say we’re stimulating the brain, but do we stimulate 100 times per second, 50 times per second, 25 times per second, and what should the stimulus intensities be?"
Vagus Nerve Stimulation. Currently, Cyberonics’ VNS Therapy System is the only Food and Drug Administration–approved form of neurostimulation for the treatment of epilepsy. The technology was approved in 1997 for the treatment of medically refractory partial-onset seizures in patients 12 years or older. It consists of a stimulator that sends electric impulses to the left vagus nerve in the neck via a lead wire that is implanted under the skin. Studies since 1997 have indicated efficacy in generalized seizure disorders and children as well, according to Dr. Elinor Ben-Menachem, professor of neurology and epilepsy at the Institute for Clinical Neurosciences and Physiology, Göteborg (Sweden) University.
To date, more than 60,000 patients worldwide have been treated with VNS, and studies suggest that approximately 50% of patients who undergo the procedure experience a long-term decrease in mean seizure frequency of 50% or more. But fewer than 10% become seizure-free, Dr. Ben-Menachem said during the neurostimulation plenary presentation.
"[VNS] has a long history now, and what we know is that it does not cure or affect seizures immediately. We actually don’t notice a change in seizure activity until about 18 months or 2 years after starting."
For example, a recent long-term follow-up study of VNS patients in the Czech Republic showed that at 1 year post implantation, 44.4% of patients achieved more than 50% seizure reduction. The percentage of patients who reached that level of seizure reduction then increased from 58.7% at 2 years after implant to 64.4% at 5 years. At the 5-year mark, 15.5% of the patients had achieved a minimum 90% seizure reduction, and 5.5% were seizure free (Seizure 2009;18: 269-74).
The mechanism of action of VNS remains uncertain, but a number of possibilities have been suggested, including arousal of the reticular formation; stimulation of locus coeruleus and noradrenaline pathways; changes in a neurotransmitter, amino acid, or neuropeptide; or indirect thalamus stimulation, according to Dr. Ben-Menachem. "It’s also possible that there is long-term learning through synaptic structural changes," she said. "The more I work with this, the more I think it is a learning paradigm. It’s like learning to play the piano. You can’t just sit down and play, you have to redo and redo until the brain is trained."
