To the Editor:
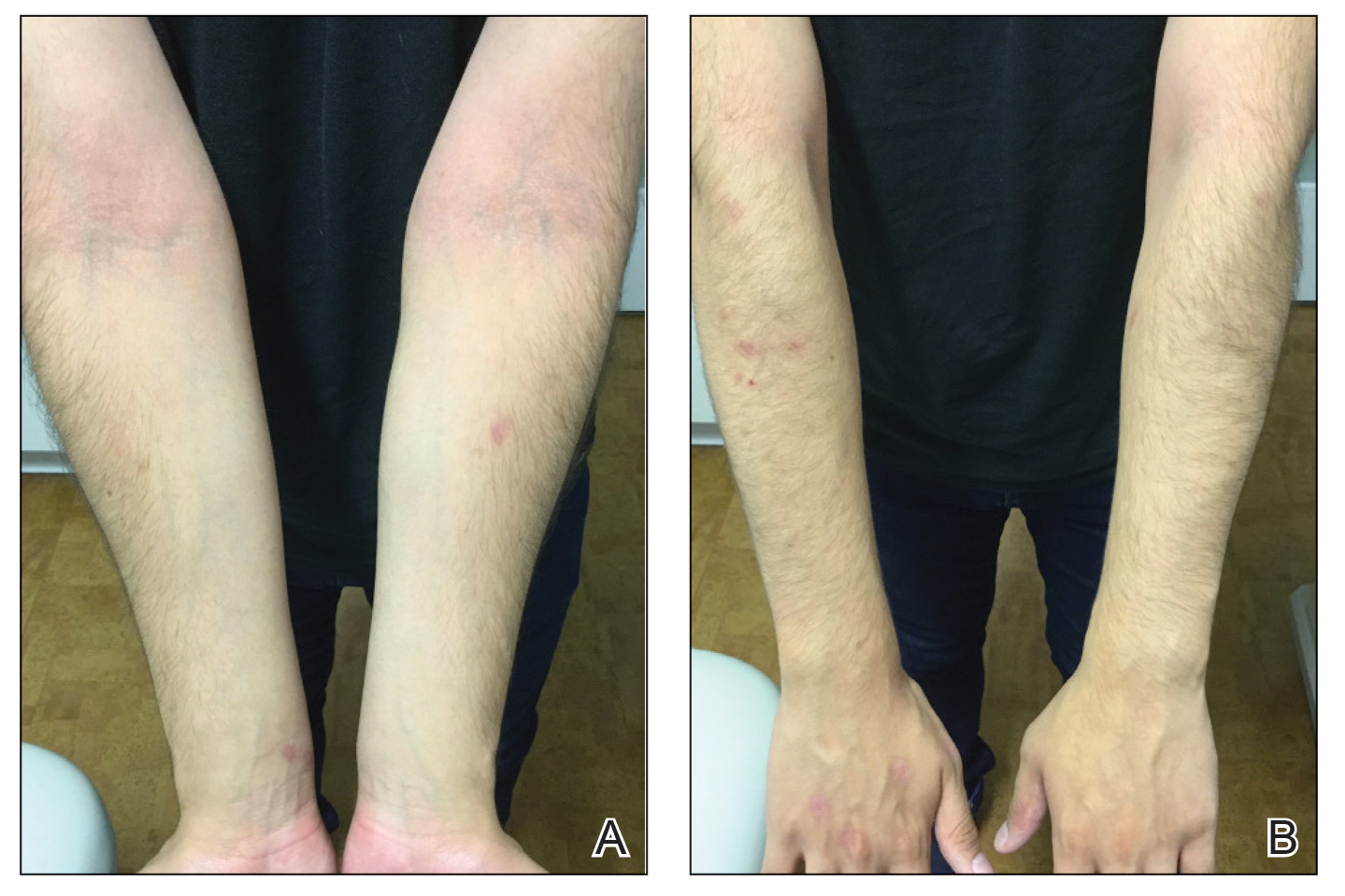
A 16-year-old adolescent boy presented to our pediatric dermatology clinic for evaluation of long-standing mild atopic dermatitis (AD) that had become severe over the last year after omalizumab was initiated for severe asthma. The patient had a history of multiple hospitalizations for severe asthma. Despite excellent control of asthma with omalizumab given every 2 weeks, he developed widespread eczematous plaques on the neck, trunk, and extremities over the course of a year. The AD often was complicated by superimposed folliculitis due to scratching from severe pruritus. Treatment with topical corticosteroids including triamcinolone ointment 0.1% to AD on the body, plus clobetasol ointment 0.05% for prurigolike lesions on the legs resulted in modest improvement; however, the AD consistently recurred within a few days after the biweekly omalizumab injection (Figure 1). When the omalizumab injections were delayed, the flares temporarily improved, and when injections were decreased to once monthly, the exacerbations subsided partially but not fully.
Because omalizumab resulted in dramatic improvement in the patient’s asthma, there was hesitation to discontinue it initially; however, the patient and his parents in conjunction with the dermatology and pulmonary teams decided to transition to dupilumab. The patient reported vast improvement of AD 1 month after initiation of dupilumab (Figure 2), which remained well controlled more than 1 year later. Mid-potency topical corticosteroids for the treatment of occasional mild eczematous flares on the extremities were used. The patient’s asthma has remained well controlled on dupilumab without any exacerbations.
Omalizumab is a recombinant DNA-derived humanized monoclonal antibody that binds both circulating and membrane-bound IgE. It has been proposed as a possible treatment for severe and/or recalcitrant AD, with mixed treatment results.1 A case series and review of 174 patients demonstrated a moderate to complete AD response to treatment with omalizumab in 74.1% of patients.2 The Atopic Dermatitis Anti-IgE Pediatric Trial (ADAPT) showed a statistically significant reduction in the Scoring Atopic Dermatitis (SCORAD) index (P=.01), along with improved quality of life in children treated with omalizumab vs those treated with placebo.3 However, a prior randomized, placebo-controlled, double-blind study did not show a significant difference in clinical disease parameters in patients treated with omalizumab.4
The humanized monoclonal antibody dupilumab, an anti–IL-4/IL-13 agent, has demonstrated more consistent efficacy for the treatment of AD in children and adults.1 Dupilumab is effective for both intrinsic and extrinsic AD1 because its clinical efficacy is unrelated to circulating levels of IgE in the bloodstream. Although IgE may have a role in childhood AD, our case demonstrated a different pathophysiologic mechanism independent of IgE. Our patient’s AD flares occurred within a few days of omalizumab injection, which may have resulted in a paradoxical increase in basophil sensitivity to other cytokines such as IL-335 and led to an increase in IL-4/IL-13 production within the skin. In our patient, this increase was successfully blocked by dupilumab. Furthermore, omalizumab has been shown to modulate helper T cell (TH2) cytokine response such as thymic stromal lymphopoietin.6 A cytokine imbalance could have exacerbated AD in our case.
Although additional work to clarify the pathogenesis of AD is needed, it is important to recognize the potential for the occurrence of paradoxical AD flares in patients treated with omalizumab, which is analogous to the well-documented entity of tumor necrosis factor α inhibitor–induced psoriasis. It is equally important to recognize the potential benefit for patients treated with dupilumab.